Abstract
Low vitamin D status is associated with an increased risk of Th1 mediated autoimmune diseases like inflammatory bowel disease. 1,25(OH)2D3 treatments have been shown to suppress Th1 mediated immunity and protect animals from experimental autoimmunity. Th1 mediated immunity is important for clearance of a number of different infectious diseases. For tuberculosis 1,25(OH)2D3 treatment is associated with decreased Th1 mediated immunity but increased bactericidal activity. Systemic candidiasis is unaffected by 1,25(OH)2D3 treatment. The seemingly paradoxical effects of 1,25(OH)2D3 and vitamin D on Th1 mediated autoimmunity versus infectious immunity point to a broad array of vitamin D targets in the immune system. The interplay of these vitamin D targets and their impact on the host-immune response then dictate the outcome.
Keywords: Vitamin D, inflammatory bowel disease, infectious immunity, immune regulation
1. Introduction
The classical role of vitamin D is in the regulation of calcium homeostasis. Recently it has become clear that vitamin D regulates immune function. In particular, poor vitamin D status has been linked to the development of a number of different type-one (Th1) mediated autoimmune diseases including type-1 diabetes, multiple sclerosis (MS), and inflammatory bowel diseases (IBD) (Cantorna et al. 2004). In addition the active form of vitamin D (1,25(OH)2D3) has been shown to completely block the development of experimental models of these autoimmune diseases (Cantorna et al. 1996; Cantorna et al. 2000; Zella et al. 2003). Th1 mediated immunity is critical for the ability of the host to mount a protective immune response to many different infections. Paradoxically, 1,25(OH)2D3 treatments did not compromise the ability of infected mice to fight a fungal infection that depends on Th1 mediated immunity (Cantorna et al. 1998; Kaposzta et al. 1998). Furthermore, vitamin D status and 1,25(OH)2D3 have been suggested to be protective against tuberculosis (TB) where host immunity depends on Th1 production of the cytokine IFN-γ. Clouding the picture further is the evidence that patients with Th1 driven diseases like IBD (Crohn’s disease), sarcoidosis and tuberculosis show hypercalcemia and calcification of granulomatous lesions and suggest that the local immune response may produce 1,25(OH)2D3 (Yang et al. 2000; Tuohy et al. 2005; Volpicelli et al. 2005; Falk et al. 2007). How can 1,25(OH)2D3 selectively regulate the immune system to suppress autoimmune disease without compromising the host's ability to fight infection? Furthermore, is the local production of 1,25(OH)2D3 in granulomatous lesions protective or pathogenic? The effects of vitamin D, 1,25(OH)2D3 and vitamin D metabolism on the susceptibility and resistance to Th1 driven immune responses will be discussed in order to clarify the seemingly paradoxical effects of vitamin D.
2. Vitamin D and the hygiene hypothesis
The incidence of immune mediated diseases like IBD, MS, allergies and asthma have all increased in developed countries over the last 50 years. This rapid increase in disease incidence cannot be explained genetically. In addition, the concordance rate between identical twins is only between 20–50% for IBD and MS (Cantorna 2006). Therefore, environmental factors impact the development of autoimmunity. The prevailing theory about the environmental cause of the increase in immune mediated diseases has been called the hygiene hypothesis (Christen et al. 2005). The hygiene hypothesis states that because of vaccination, improved sanitation, and reduced rates of infection the immune system is missing important signals that prevent over-activation and disease (Christen et al. 2005). If the hygiene hypothesis is relevant to diseases from IBD to asthma there is likely going to be common mechanisms of infectious disease stimulated protection in these very different diseases.
The hygiene hypothesis for IBD centers around the critical findings that uncontrolled immune responses to the bacterial flora in the gut result in disease development. Changes in the normal bacterial flora and or the decreased incidence of intestinal parasites including worm infections are cited as the basis for the hygiene hypothesis in IBD (Khan et al. 2002; Strober et al. 2007; Koloski et al. 2008). Experimental evidence shows that gastrointestinal worm infections can suppress IBD in experimental animals and humans (Khan et al. 2002; Strober et al. 2007; Koloski et al. 2008). In addition, approaches that aim to alter the types of bacteria in the gastrointestinal track including the use of probiotics to replace the existing bacteria with “good” bacteria have been successful in some circumstances (Rolfe et al. 2006). However, there are also infections that are associated with disease induction and therefore it is likely that other environmental factors including pollution, diet etc. affect the development of these complex diseases.
We propose a modification to the hygiene hypothesis that states that vitamin D is one of the environmental factors that affects the development of immune mediated diseases. Current research in the vitamin D field shows that people in developed countries especially those that experience winter have large fluctuations in their vitamin D status. A major source of vitamin D results from it being manufactured via a photolysis reaction in the skin and vitamin D availability from sunlight exposure is significantly less in northern climates, and is especially low during the winter (Clemens et al. 1982; DeLuca 1993). The fluctuations in vitamin D are a direct result of decreased outdoor activity, changes in season, increased pollution and diets that lack adequate vitamin D levels. It seems possible that vitamin D exposure especially early in life may be an environmental factor that affects the development of the immune system. Vitamin D exposure and exposure to other environmental elements would determine which genetically susceptible individuals develop autoimmunity.
3. Autoimmunity, IBD and Th1 driven immune responses
The etiology of autoimmune diseases and asthma are very different and cells that are pathogenic for one disease are beneficial for the other. For most forms of autoimmunity the immune response is characterized by overproduction of type-1 helper cell (Th1) associated cytokines including interferon (IFN)- γ and tumor necrosis factor (TNF)-α (Fig. 1). Th1 mediated immune responses are induced when a combination of circumstances occur. Antigen specific T cells that are primed in the presence of interleukin (IL)-12 and little IL-4 result in the development of Th1 cells (Fig. 1). Th1 mediated responses are inhibited by regulatory T (T reg) and NKT cells that produce IL-4, IL-10 and transforming growth factor (TGF)-β (Fig. 1). The cell types that are involved include macrophage and dendritic cells (DC) that act as antigen presenting cells (APC), Th1 cells, T reg and NKT cells. Type 2 helper cells (Th2) produce IL-4 and IL-10 that inhibit Th1 development and are the pathogenic cells in allergic asthma (Fig. 1). The pathogenesis of asthma and autoimmune diseases are complex and although autoimmune diseases can be classified as Th1 driven diseases other cells including Th17 cells are pathogenic (Fig. 1) (Iwakura et al. 2006). For asthma the NKT cells are required for disease pathology since mice that are NKT cell deficient fail to develop asthma (Akbari et al. 2003). NKT cells are early innate regulatory cells that can suppress autoimmunity (Taniguchi et al. 2003). Induction of T reg cells that produce IL-10 and TGF-β are protective for both Th1 and Th2 driven diseases including experimental asthma and IBD (Akbari et al. 2003; Maul et al. 2005).
Fig. 1.
CD4+ T helper cell subsets and functions. The black arrows (→) indicate cytokines that induce differentiation and the black lines (
 ) indicate cytokines that inhibit Th cell development. The grey arrows and lines indicate induction (
) indicate cytokines that inhibit Th cell development. The grey arrows and lines indicate induction (
 ) or suppression (
) or suppression (
 ) by 1,25(OH)2D3 treatment. Th1 immune responses are inhibited by Th2 and T reg cells and are important for protection from intracellular infections, cancer immunity and participate in autoimmune disease. Th2 cell immune responses are also important for fighting infections, can be suppressed by T reg cells and are pathogenic in asthma and allergies. Regulatory NKT cells can be either pathogenic or protective for autoimmune responses and are required for experimental asthma disease induction. All T cells that have been tested express the VDR. 1,25(OH)2D3 suppresses Th1 driven cytokine responses, induces T reg cells, induces IL-4 production and enhances NKT cell function.
) by 1,25(OH)2D3 treatment. Th1 immune responses are inhibited by Th2 and T reg cells and are important for protection from intracellular infections, cancer immunity and participate in autoimmune disease. Th2 cell immune responses are also important for fighting infections, can be suppressed by T reg cells and are pathogenic in asthma and allergies. Regulatory NKT cells can be either pathogenic or protective for autoimmune responses and are required for experimental asthma disease induction. All T cells that have been tested express the VDR. 1,25(OH)2D3 suppresses Th1 driven cytokine responses, induces T reg cells, induces IL-4 production and enhances NKT cell function.
IBD are immune mediated diseases of unknown etiology affecting the gastrointestinal tract. In North America and Europe about 1 in 1000 people are affected with IBD (Podolsky 1991; Podolsky 1991). There are at least two distinct forms of IBD, ulcerative colitis and Crohn’s disease. IBD are chronic recurring illnesses most commonly involving inflammation of the terminal ileum and colon, although these diseases can also affect many sites throughout the alimentary tract. Crohn’s disease is a Th1 mediated disease and ulcerative colitis has more of a mixed Th1/Th2 phenotype. Both genetic and environmental factors predispose individuals to develop IBD. Cells from Crohn’s disease patients have been shown to secrete high amounts of IFN-γ and TNF-α among other cytokines (Fig. 2) (Neissner et al. 1995). There is no cure for Crohn’s disease, although there are some very good treatments available that mainly suppress inflammation. TNF-α blocking drugs have been shown to be beneficial for Crohn’s disease patients. Experimental approaches to treating Crohn’s disease include induction of T reg cells (Fig. 2). However, there is no cure and repeated treatments and maintenance drugs are required to keep Crohn’s patients healthy long-term. The major side effect associated with all therapies is an increased risk of infection and cancer as a result of the immunosuppression.
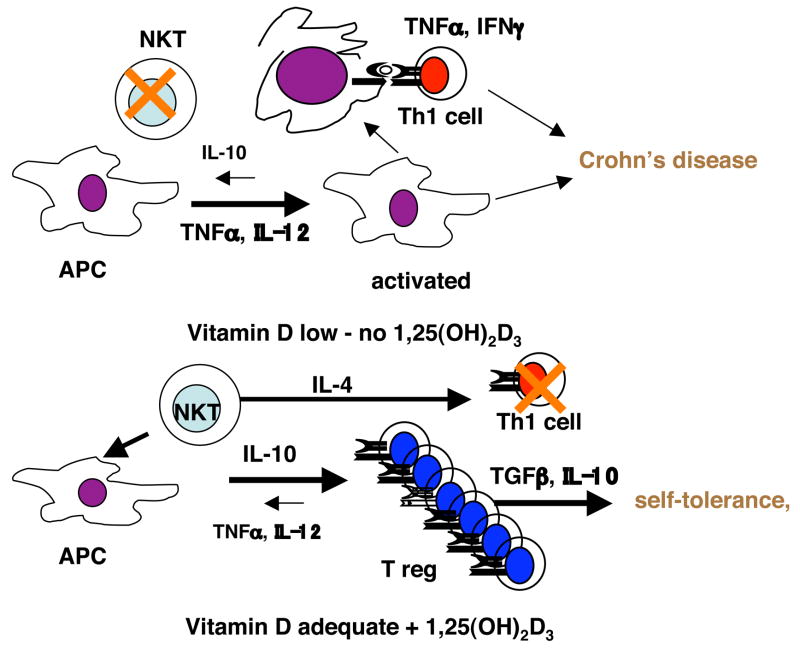
Fig. 2.
Model of the effects of vitamin D status and 1,25(OH)2D3 treatment on Chron’s disease. When vitamin D is low NKT cells and T reg cells fail to develop, Th1 cells are overactive and the incidence of Th1 mediated diseases increase. Vitamin D regulates the APC such that Th1 cell responses are inhibited, T reg cell function is induced and NKT cells develop. The net result is decreased Chron’s disease and normalization of the Th cell response.
4. Vitamin D and the immune system
The identification of the vitamin D receptor (VDR) in peripheral blood mononuclear cells sparked the early interest in vitamin D as an immune system regulator (Bhalla et al. 1983; Provvedini et al. 1983). All cells of the immune system that have been evaluated express the VDR and at least in T cells activation induces the expression of additional VDR (Veldman et al. 2000; Mahon et al. 2003). 1,25(OH)2D3 is a potent in vivo suppressor of experimental autoimmune diseases such as arthritis, type-1 diabetes, lupus, IBD and experimental MS models (reviewed in (Cantorna 2000)). Regulation of macrophage and DC function by 1,25(OH)2D3 is important for the inhibition of experimental autoimmunity. 1,25(OH)2D3 treatment of DC in vitro inhibited differentiation and maturation of DC and resulted in DC that when transferred induced in vivo suppression of T cells (Griffin et al. 2003). Similarly, 1,25(OH)2D3 induced myeloid progenitors to differentiate into macrophage and inhibited the production of IL-12 and TNF-α in vitro (Fig. 2) (Koeffler et al. 1984; Clohisy et al. 1987). In vivo the efficacy of 1,25(OH)2D3 to suppress experimental IBD has been shown to correlate with the inhibition of TNF-α and other downstream genes activated by TNF-α (Fig. 2) (Zhu et al. 2005). Overall, the effects of 1,25(OH)2D3 on the APC are to decrease production of IL-12, and TNF-α and to suppress maturation and differentiation such that the immature APC fail to prime Th1-like immune responses (Fig. 2).
T cells are also vitamin D targets and vitamin D regulates T cell function both directly and indirectly by regulating APC function. 1,25(OH)2D3 treatments have been shown to directly inhibit IFN-γ in vitro (Staeva-Vieira et al. 2002; Mahon et al. 2003). Furthermore 1,25(OH)2D3 inhibits the development of Th1 cells in vivo and the inhibition of the Th1 mediated response correlates with the protection from disease pathology (Cantorna et al. 2000). More recently 1,25(OH)2D3 has been shown to induce T reg cells directly through upregulation of FoxP3 and indirectly through APC (Barrat et al. 2002; Adorini et al. 2003). Induction of T reg and suppression of Th1 cells by 1,25(OH)2D3 has been correlated with suppression of experimental autoimmunity. In addition, the VDR deficient host is more susceptible to experimental IBD in a number of different models (Froicu et al. 2003; Froicu et al. 2007). The immune system of VDR KO mice has normal CD4+, CD8+, and NK cell numbers. However, CD4+ T cells from VDR KO mice overproduce IFN-γ, have a strong mixed lymphocyte reaction that is Th1 mediated and are lacking NKT cells (Froicu et al. 2003; Froicu et al. 2006; Yu et al. 2008). iNKT cells are early innate regulatory cells that can alter the outcome of autoimmunity (Taniguchi et al. 2003). Furthermore 1,25(OH)2D3 treatments induced NKT cell function in vitro and in vivo (Yu et al. 2008). Two types of cells are induced by 1,25(OH)2D3 the T reg and the NKT cell; induction of these regulatory cells and direct inhibition of Th1 cells are the mechanisms by which 1,25(OH)2D3 suppresses experimental autoimmunity (Fig.2).
5. Vitamin D and Th1 mediated host immunity to infection
Th1 mediated immune responses and especially the ability to produce IFN-γ is important for host immunity to a number of infectious diseases. In particular, immunity to intracellular pathogens including many viruses, bacteria such as Mycobacteria and Listeria species and parasites like Leishmania require IFN-γ as part of the host response for successful clearance. Regulatory cell induction and IL-10 production are associated with prolonged infections and chronicity with these same organisms (Scott-Browne et al. 2007). In vitro, 1,25(OH)2D3 has been shown to suppress IFN-γ and IL-12 production from human leukocytes infected with Mycobacteria tuberculosis (Vidyarani et al. 2007). In addition, it should be noted that regulatory T cell induction suppresses macrophage anti-TB effects and 1,25(OH)2D3 has been shown to induce T reg cells (Adorini et al. 2003; Hougardy et al. 2007; Scott-Browne et al. 2007). 1,25(OH)2D3 addition to murine macrophages inhibited the IFN-γ induced killing of Leishmania major in vitro (Ehrchen et al. 2007). Furthermore, mouse monocytes treated with 1,25(OH)2D3 induced the growth of Listeria organisms in bone marrow derived macrophage cultures (Helming et al. 2005). Mice that lack the VDR showed enhanced IFN-γ responses and increased clearance rates of L. major in vivo (Ehrchen et al. 2007). The evidence is consistent that in the presence of 1,25(OH)2D3 there is an inhibition of Th1 mediated immune responses to infectious organisms that includes depressed IFN-γ and IL-12 production and induction of T reg cells.
1,25(OH)2D3 has some paradoxical effects on host resistance to infections. Even though IFN-γ production is critical for survival from Candida albicans infection; 1,25(OH)2D3 treatment of mice had no effect on survival from systemic candidiasis (Cantorna et al. 1998; Kaposzta et al. 1998). For TB there is anecdotal evidence that perhaps vitamin D deficiency and reduced sunlight exposure result in more severe TB (Gibney et al. 2008). Vitamin D deficiency in mice resulted in the increased replication of M. bovis (Waters et al. 2004). In addition, 1,25(OH)2D3 has been shown to inhibit replication of TB in vitro by inducing anti-bacterial peptides (Liu et al. 2006). More work is needed to determine in vivo what the net effects of vitamin D status are on the whole organisms resistance to Th1 mediated diseases like TB. Although there has been a flurry of activity aimed at studying the anti-bacterial effects of 1,25(OH)2D3 to date no information is available to address the potential 1,25(OH)2D3 mediated effects of Th1 inhibition or T reg induction in the context of TB host resistance.
6. Extra-renal production of the 1alpha hydroxylase
Cyp27B1 is the gene encoding the 1 alpha hydroxylase that produces active 1,25(OH)2D3 and is expressed classically in the kidneys. Extra-renal production of Cyp27B1 has been implicated to occur in a number of inflammatory conditions including sarcoidosis, and Crohn’s disease and may explain hypercalcemia that occurs in these patients (Conron et al. 2000; Abreu et al. 2004). Other granulomatous diseases including TB are associated with disordered vitamin D metabolism and hypercalcemia (Yang et al. 2000). Interestingly enough the granulomatous diseases are Th1 mediated events. Although these conditions exist it is unclear whether extra-renal production of 1,25(OH)2D3 is beneficial or pathological in these diseases. Often resolution of the disease corresponds with normalization of the vitamin D and calcium status (Conron et al. 2000; Abreu et al. 2004). Immune cells including macrophage and DC express the 1alpha hydroxylase enzyme (Hewison et al. 2007). The possibility that the immune system can produce locally active 1,25(OH)2D3 is an attractive idea. In vitro induction of the Cyp27B1 enzyme in macrophage induces anti-bacterial peptides that kill TB (Liu et al. 2006). At present the in vivo relevance of immune mediated production of Cyp27B1 is not known.
One group (TG Marshall, Autoimmunity Research Foundation, Thousand Oaks, CA) has recommended and continues to recommend lowering vitamin D status to treat a number of chronic inflammatory conditions (Marshall 2008). The basis for this recommendation are the reports of production of 1,25(OH)2D3 by the immune system during disease pathology and the possibility that this induction of 1,25(OH)2D3 may be contributing to diseases from obesity to other chronic diseases including those mediated by Th1 cells like sarcoidosis and Crohn’s disease (Marshall et al. 2004; Marshall 2008). The argument is that the hormone activity of 1,25(OH)2D3 induces transcription of a large number of genes that somehow cause these diseases and that 1,25(OH)2D3 should be measured because it is the biologically active form of vitamin D that binds the receptor (Marshall 2008). Current assays for 25(OH)D3 that are used to assess vitamin D levels in the general population are inaccurate and lack standards (Glendenning et al. 2006). 1,25(OH)2D3 measurements are even more difficult to do, are not standardized, and are also inaccurate. Measurements of 1,25(OH)2D3 are meaningless in the face of these measurement problems. Furthermore, excess 1,25(OH)2D3 has been given to animals with Th1 mediated diseases and the symptoms of the disease as well as the Th1 response were significantly reduced in many experiments by many investigators (Lemire et al. 1991; Cantorna et al. 1998; Cantorna et al. 2000; Zella et al. 2003; Mathieu et al. 2004). More work is needed to determine whether immune mediated production of 1,25(OH)2D3 is biologically significant, pathogenic, or protective. At present there is not strong evidence to recommend decreasing vitamin D status in any population.
7. Conclusions
The effects of vitamin D on immunity are beginning to be appreciated. Regardless of whether Th1 mediated autoimmunity or infectious immunity is being monitored, 1,25(OH)2D3 inhibits IL-12 and IFN-γ production. Induction of regulatory T cells by 1,25(OH)2D3 is an additional mode for inhibition of immune responsiveness. In addition, regulatory NKT cells depend on vitamin D for development and are upregulated by 1,25(OH)2D3. For L. monocytogenes and L. major 1,25(OH)2D3 suppression of the Th1 immune response is associated with decreased clearance of the microorganism. Conversely, 1,25(OH)2D3 increases the clearance of TB in infected human macrophage. Furthermore, clearance of a systemic infection with C. albicans was unaffected by 1,25(OH)2D3 treatment. Vitamin D should not be labeled as broadly anti-infectious or immunosuppressive and caution should be used in interpreting studies in isolation. The in vivo effects of 1,25(OH)2D3 on immune function likely depend on the vitamin D status of the host, the local expression of the VDR and Cyp27B1 and additional factors that impact on host resistance to infection or autoimmunity. The seemingly paradoxical effects of 1,25(OH)2D3 on Th1 mediated immunity point to a role of vitamin D and 1,25(OH)2D3 on multiple aspects of immunity that are still only partially understood.
Acknowledgments
This work was supported by National Institutes of Health Grant #R01 DK070781 to MTC.
Abbreviations
- APC
antigen presenting cell
- DC
dendritic cell
- IBD
inflammatory bowel disease
- IFN
Interferon
- IL
interleukin
- MS
multiple sclerosis
- T reg
regulatory T
- TGF
transforming growth factor
- TB
tuberculosis
- TNF
tumor necrosis factor
- Th1
type 1 helper
- Th2
type 2 helper
- VDR
vitamin D receptor
- Th2
type 2 helper
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITTED
- Abreu MT, Kantorovich V, Vasiliauskas EA, Gruntmanis U, Matuk R, Daigle K, Chen S, Zehnder D, Lin YC, Yang H, Hewison M, Adams JS. Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn's disease patients with elevated 1,25-dihydroxyvitamin D and low bone mineral density. Gut. 2004;53(8):1129–36. doi: 10.1136/gut.2003.036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adorini L, Penna G, Giarratana N, Uskokovic M. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting allograft rejection and autoimmune diseases. J Cell Biochem. 2003;88(2):227–33. doi: 10.1002/jcb.10340. [DOI] [PubMed] [Google Scholar]
- Akbari O, Stock P, DeKruyff RH, Umetsu DT. Role of regulatory T cells in allergy and asthma. Curr Opin Immunol. 2003;15(6):627–33. doi: 10.1016/j.coi.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9(5):582–8. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O’Garra A. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195(5):603–16. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57(6):1308–10. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- Cantorna MT. Vitamin D and autoimmunity: is vitamin D status an environmental factor affecting autoimmune disease prevalence? Proc Soc Exp Biol Med. 2000;223(3):230–3. doi: 10.1046/j.1525-1373.2000.22333.x. [DOI] [PubMed] [Google Scholar]
- Cantorna MT. Vitamin D and its role in immunology: multiple sclerosis, and inflammatory bowel disease. Prog Biophys Mol Biol. 2006;92(1):60–4. doi: 10.1016/j.pbiomolbio.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci U S A. 1996;93(15):7861–4. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis. J Nutr. 1998;128(1):68–72. doi: 10.1093/jn/128.1.68. [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Hullett DA, Redaelli C, Brandt CR, Humpal-Winter J, Sollinger HW, Deluca HF. 1,25-Dihydroxyvitamin D3 prolongs graft survival without compromising host resistance to infection or bone mineral density. Transplantation. 1998;66(7):828–31. doi: 10.1097/00007890-199810150-00003. [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood) 2004;229(11):1136–42. doi: 10.1177/153537020422901108. [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130(11):2648–52. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- Christen U, von Herrath MG. Infections and autoimmunity--good or bad? J Immunol. 2005;174(12):7481–6. doi: 10.4049/jimmunol.174.12.7481. [DOI] [PubMed] [Google Scholar]
- Clemens TL, Adams JS, Nolan JM, Holick MF. Measurement of circulating vitamin D in man. Clin Chim Acta. 1982;121(3):301–8. doi: 10.1016/0009-8981(82)90239-x. [DOI] [PubMed] [Google Scholar]
- Clohisy DR, Bar-Shavit Z, Chappel JC, Teitelbaum SL. 1,25-Dihydroxyvitamin D3 modulates bone marrow macrophage precursor proliferation and differentiation. Up-regulation of the mannose receptor. J Biol Chem. 1987;262(33):15922–9. [PubMed] [Google Scholar]
- Conron M, Young C, Beynon HL. Calcium metabolism in sarcoidosis and its clinical implications. Rheumatology (Oxford) 2000;39(7):707–13. doi: 10.1093/rheumatology/39.7.707. [DOI] [PubMed] [Google Scholar]
- DeLuca HF. Vitamin D. Nutrition Today. 1993;28:6–11. [Google Scholar]
- Ehrchen J, Helming L, Varga G, Pasche B, Loser K, Gunzer M, Sunderkotter C, Sorg C, Roth J, Lengeling A. Vitamin D receptor signaling contributes to susceptibility to infection with Leishmania major. Faseb J. 2007;21(12):3208–18. doi: 10.1096/fj.06-7261com. [DOI] [PubMed] [Google Scholar]
- Falk S, Kratzsch J, Paschke R, Koch CA. Hypercalcemia as a result of sarcoidosis with normal serum concentrations of vitamin D. Med Sci Monit. 2007;13(11):CS133–136. [PubMed] [Google Scholar]
- Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007;8:5. doi: 10.1186/1471-2172-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17(12):2386–92. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- Froicu M, Zhu Y, Cantorna MT. Vitamin D receptor is required to control gastrointestinal immunity in IL-10 knockout mice. Immunology. 2006;117(3):310–8. doi: 10.1111/j.1365-2567.2005.02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibney KB, MacGregor L, Leder K, Torresi J, Marshall C, Ebeling PR, Biggs BA. Vitamin D deficiency is associated with tuberculosis and latent tuberculosis infection in immigrants from sub-Saharan Africa. Clin Infect Dis. 2008;46(3):443–6. doi: 10.1086/525268. [DOI] [PubMed] [Google Scholar]
- Glendenning P, Taranto M, Noble JM, Musk AA, Hammond C, Goldswain PR, Fraser WD, Vasikaran SD. Current assays overestimate 25-hydroxyvitamin D3 and underestimate 25-hydroxyvitamin D2 compared with HPLC: need for assay-specific decision limits and metabolite-specific assays. Ann Clin Biochem. 2006;43(Pt 1):23–30. doi: 10.1258/000456306775141650. [DOI] [PubMed] [Google Scholar]
- Griffin MD, Kumar R. Effects of 1alpha,25(OH)2D3 and its analogs on dendritic cell function. J Cell Biochem. 2003;88(2):323–6. doi: 10.1002/jcb.10335. [DOI] [PubMed] [Google Scholar]
- Helming L, Bose J, Ehrchen J, Schiebe S, Frahm T, Geffers R, Probst-Kepper M, Balling R, Lengeling A. 1alpha,25-Dihydroxyvitamin D3 is a potent suppressor of interferon gamma-mediated macrophage activation. Blood. 2005;106(13):4351–8. doi: 10.1182/blood-2005-03-1029. [DOI] [PubMed] [Google Scholar]
- Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103(3–5):316–21. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- Hougardy JM, Place S, Hildebrand M, Drowart A, Debrie AS, Locht C, Mascart F. Regulatory T cells depress immune responses to protective antigens in active tuberculosis. Am J Respir Crit Care Med. 2007;176(4):409–16. doi: 10.1164/rccm.200701-084OC. [DOI] [PubMed] [Google Scholar]
- Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116(5):1218–22. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaposzta R, Tree P, Marodi L, Gordon S. Characteristics of invasive candidiasis in gamma interferon- and interleukin-4-deficient mice: role of macrophages in host defense against Candida albicans. Infect Immun. 1998;66(4):1708–17. doi: 10.1128/iai.66.4.1708-1717.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan WI, Blennerhasset PA, Varghese AK, Chowdhury SK, Omsted P, Deng Y, Collins SM. Intestinal nematode infection ameliorates experimental colitis in mice. Infect Immun. 2002;70(11):5931–7. doi: 10.1128/IAI.70.11.5931-5937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeffler HP, Amatruda T, Ikekawa N, Kobayashi Y, DeLuca HF. Induction of macrophage differentiation of human normal and leukemic myeloid stem cells by 1,25-dihydroxyvitamin D3 and its fluorinated analogues. Cancer Res. 1984;44(12 Pt 1):5624–8. [PubMed] [Google Scholar]
- Koloski NA, Bret L, Radford-Smith G. Hygiene hypothesis in inflammatory bowel disease: A critical review of the literature. World J Gastroenterol. 2008;14(2):165–73. doi: 10.3748/wjg.14.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire JM, Archer DC. 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest. 1991;87(3):1103–7. doi: 10.1172/JCI115072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;89(5):922–32. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- Marshall TG. Vitamin D discovery outpaces FDA decision making. Bioessays. 2008;30(2):173–82. doi: 10.1002/bies.20708. [DOI] [PubMed] [Google Scholar]
- Marshall TG, Marshall FE. Sarcoidosis succumbs to antibiotics--implications for autoimmune disease. Autoimmun Rev. 2004;3(4):295–300. doi: 10.1016/j.autrev.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Mathieu C, van Etten E, Decallonne B, Guilietti A, Gysemans C, Bouillon R, Overbergh L. Vitamin D and 1,25-dihydroxyvitamin D3 as modulators in the immune system. J Steroid Biochem Mol Biol. 2004;89–90(1–5):449–52. doi: 10.1016/j.jsbmb.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, Zeitz M, Duchmann R. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128(7):1868–78. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- Neissner M, Volk BA. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reverse trancribed polymerase chain reaction (RT-PCR) Clin Exp Immunol. 1995;101:428–35. doi: 10.1111/j.1365-2249.1995.tb03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky DK. Inflammatory bowel disease (1) N Engl J Med. 1991;325(13):928–37. doi: 10.1056/NEJM199109263251306. [DOI] [PubMed] [Google Scholar]
- Podolsky DK. Inflammatory bowel disease (2) N Engl J Med. 1991;325(14):1008–16. doi: 10.1056/NEJM199110033251406. [DOI] [PubMed] [Google Scholar]
- Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221(4616):1181–3. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- Rolfe VE, Fortun PJ, Hawkey CJ, Bath-Hextall F. Probiotics for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2006;(4):CD004826. doi: 10.1002/14651858.CD004826.pub2. [DOI] [PubMed] [Google Scholar]
- Scott-Browne JP, Shafiani S, Tucker-Heard G, Ishida-Tsubota K, Fontenot JD, Rudensky AY, Bevan MJ, Urdahl KB. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J Exp Med. 2007;204(9):2159–69. doi: 10.1084/jem.20062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staeva-Vieira TP, Freedman LP. 1,25-dihydroxyvitamin D3 inhibits IFN-gamma and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. J Immunol. 2002;168(3):1181–9. doi: 10.4049/jimmunol.168.3.1181. [DOI] [PubMed] [Google Scholar]
- Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117(3):514–21. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- Tuohy KA, Steinman TI. Hypercalcemia due to excess 1,25-dihydroxyvitamin D in Crohn’s disease. Am J Kidney Dis. 2005;45(1):e3–6. doi: 10.1053/j.ajkd.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys. 2000;374(2):334–8. doi: 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- Vidyarani M, Selvaraj P, Jawahar MS, Narayanan PR. 1, 25 Dihydroxyvitamin D3 modulated cytokine response in pulmonary tuberculosis. Cytokine. 2007;40(2):128–34. doi: 10.1016/j.cyto.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Volpicelli G, Mussa A, Frascisco M. A case of severe hypercalcemia with acute renal failure in sarcoidosis: a diagnostic challenge for the emergency department. Eur J Emerg Med. 2005;12(6):320–1. doi: 10.1097/00063110-200512000-00015. [DOI] [PubMed] [Google Scholar]
- Waters WR, Palmer MV, Nonnecke BJ, Whipple DL, Horst RL. Mycobacterium bovis infection of vitamin D-deficient NOS2−/− mice. Microb Pathog. 2004;36(1):11–7. doi: 10.1016/j.micpath.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Yang WC, I, Chang T, Tsai BL. Hypercalcemia in renal transplant patients with tuberculosis. Transplant Proc. 2000;32(7):1882–3. doi: 10.1016/s0041-1345(00)01475-5. [DOI] [PubMed] [Google Scholar]
- Yu S, Cantorna MT. The vitamin D receptro is required for iNKT cell development. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0711558105. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zella JB, McCary LC, DeLuca HF. Oral administration of 1,25-dihydroxyvitamin D3 completely protects NOD mice from insulin-dependent diabetes mellitus. Arch Biochem Biophys. 2003;417(1):77–80. doi: 10.1016/s0003-9861(03)00338-2. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Mahon BD, Froicu M, Cantorna MT. Calcium and 1alpha,25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur J Immunol. 2005;35(1):217–24. doi: 10.1002/eji.200425491. [DOI] [PubMed] [Google Scholar]