Abstract
This study was conducted to determine the effectiveness of carbamazepine (CBZ) for treatment of cocaine dependence. Sixty-two (CBZ = 28, placebo = 34) cocaine-dependent (DSM-III-R criteria) volunteers consented to be treated for eight weeks with standardized outpatient individual counseling twice a week plus double-blind CBZ or inactive placebo. During the 8-week trial, both groups showed increased number of urine samples negative for cocaine, significantly (P < 0.01) decreased self-reported cocaine use (money spent and grams used), and decreased Beck Depression Inventory and Symptom Check List-90-Revised (SCL-90-R) total scores. However, there were no significant differences between CBZ and placebo. This study does not support the effectiveness of CBZ for outpatient treatment of cocaine dependence.
Keywords: Cocaine, Dependence, Carbamazepine
1. Introduction
The anti-convulsant carbamazepine (CBZ) has been used for treatment of cocaine dependence, although its effectiveness has not been established. The rationale for using CBZ is based on pharmacological kindling (Post et al., 1975; Post and Kopanda, 1976; Post, 1977) proposed to mediate cocaine craving (Halikas et al., 1989; Browne et al., 1990). Chronic treatment with CBZ almost completely blocks the development of cocaine-induced kindling (Post and Kopanda, 1976; Post, 1977; Weiss et al., 1989; Weiss et al., 1990), decreases cocaine-self administration, and food reinforced behavior in rats (Carroll et al., 1990; Sharpe et al., 1992). The mechanism of action by which CBZ prevents the development of kindling may be by blocking the activation of the type II sodium channels in neurons that are firing rapidly (MacDonald et al., 1985; Willow et al., 1985; McLean and MacDonald, 1986) or possibly effects on dopaminergic systems (Kowalik et al., 1984; Barros et al., 1986). Since the effect of CBZ in rats is to prevent the development but not the expression of cocaine-induced kindling, it might be hypothesized that CBZ would be more effective for prevention of, rather than treatment of already developed, cocaine dependence. However, it was for treatment, rather than prevention, that several studies of CBZ were conducted.
The clinical efficacy of CBZ in controlling either cocaine-induced kindling or cocaine use in humans has not been established. Case reports from cocaine-dependent patients indicated that CBZ suppressed the ‘rush’ (Sherer et al., 1990) and craving (Halikas et al., 1989) induced by cocaine administration. Although a double-blind cocaine challenge experiment did not replicate this effect (Hatsukami et al., 1991), two small scale open-label outpatient studies found that CBZ decreased the number of days of cocaine use (Halikas et al., 1992; Montoya et al., 1992). These results were confirmed in one short-term (10-day cross-over) double-blind study (Halikas et al., 1991a). Media reports of these findings led to substantial publicity for CBZ as a treatment for cocaine dependence, resulting in widespread CBZ prescribing by clinicians (Halikas et al., 1993).
We present here the first reported double-blind, placebo-controlled, random assignment, parallel group clinical trial to investigate the clinical safety and efficacy of CBZ for outpatient treatment of cocaine dependent individuals receiving counseling. Determining the efficacy of CBZ for treatment of cocaine dependence has great clinical and public health importance to improve treatment, reduce the risks associated with cocaine use, and establish the proper use of this widely prescribed medication for treatment of cocaine dependence.
2. Patients and methods
2.1. Patients
Research volunteers (n = 81) seeking treatment for cocaine dependence were recruited through newspaper and radio advertisements, word-of-mouth, and referrals from treatment agencies. Patients were screened and treated at the Intramural Research Program of the National Institute on Drug Abuse (NIDA-IRP). Patient inclusion criteria were: (i) males or females ages 21–50 years; (ii) current cocaine dependence by DSM-III-R (American Psychiatric Association, 1987) criteria, using the Diagnostic Interview Schedule (Helzer et al., 1981) and clinical interview; and (iii) at least 14 g of self-reported cocaine use in the prior 3 months. Patient exclusion criteria were: (i) concurrent dependence on other psychoactive substances except for caffeine or tobacco, by DSM-III-R criteria; (ii) current institutional residence (e.g., jail, half-way house); (iii) illiteracy; (iv) history of seizure disorder, glaucoma, renal failure, asthma, bone marrow suppression, liver disease, lupus erythematosus, or any other current severe or uncontrolled psychiatric or medical disorders which in the judgement of the investigators would impair the ability of the patient to safely participate in this study; (v) known allergy to a tricyclic antidepressant or CBZ; (vi) pregnancy, lactation, or women of child bearing potential not using a medically accepted method of birth control; or (viii) diastolic blood pressure greater than 95 mmHg measured at least twice on two separate examinations. Patients were offered HIV testing in conjunction with pre- and post-test counseling. Willingness to be tested or to learn the results of the test did not influence the acceptance of the patient into this study. All patients signed a written consent form approved by an Institutional Review Board. Since patients received free treatment, they were not paid any additional form of compensation for participation in this study.
All patients were mentally and physically healthy as determined by intake medical and psychological evaluation, including physical examination, electrocardiogram, complete blood cell count (CBC), chemistry profile, and urinalysis. Psychosocial intake evaluation included the Shipley Institute of Living Scale (SILS; Shipley, 1940), Cocaine Use Questionnaire (CUQ), Diagnostic Interview Schedule (DIS; Helzer et al., 1981), and Addiction Severity Index (ASI; McLellan et al., 1986).
2.2. Treatment
Patients who were eligible and consented to participate were randomly assigned to receive either CBZ or matching inactive placebo tablets (Ciba-Geigy). Patients in the CBZ group were treated according to the following dosing schedule: days 1–7, 200 mg/day; days 8–14, 400 mg/day; days 15–21, 600 mg/day; days 22–28, 800 mg/day; days 29–35, 600 mg/day; days 36–42, 400 mg/day; days 43–49, 200 mg/day; and days 50–56, matching placebo. An equivalent number of matching tablets were dispensed for the placebo group. The rationale for this medication schedule was to achieve the CBZ dose (600 mg/day) and plasma level (>4 mcg/ml) previously reported (Halikas et al., 1992) effective for treatment of cocaine dependence, and test a higher dose (800 mg/day) for 1 week while adjusting doses in a step-wise manner to limit the CBZ side-effects to which cocaine addicts had been reported to be sensitive (Halikas et al., 1989; Halikas et al., 1992). Previous studies had suggested that 7–9 days of CBZ at the dose of 600 mg/day was sufficient to obtain a therapeutic effect (Halikas et al., 1989; Halikas et al., 1991a; Halikas et al., 1992).
Patients reported to the clinic 3 days each week. At each clinic visit, they provided urine samples under staff observation for drug testing, ingested 1 medication dose in front of the dispensing nurse, received additional medication to last until the next scheduled visit, had vital signs checked, and completed self-reported drug use and craving questionnaires. Twice a week patients had cognitive/behavioral individual counseling lasting approximately 60 min. Counseling was standardized using a manual developed at the NIDA-IRP (Covi et al., 1993).
Blood was drawn every 2 weeks to assess medication blood levels, CBC (because of the known association of CBZ with cytopenia), and liver function (because of the known association of CBZ with liver toxicity). If the total white blood cell (WBC) count of a patient dropped by greater than 15%, the test was repeated at the next visit. If the WBC count continued to drop, the medication was discontinued and the patient was referred for medical follow-up. If there was a greater than four-fold increase above baseline levels in liver transaminase levels, medication was discontinued and, if indicated, the patient was referred for medical follow-up. If a patient presented with mild to moderate side-effects he/she was evaluated by a NIDA-IRP physician who determined whether the medication dose should be reduced. Medication could be discontinued prematurely if a patient: (i) presented with side-effects; (ii) became pregnant; (iii) missed 3 consecutive or 6 non-consecutive scheduled visits for medication; or (iv) violated clinic regulations. If a patient had his/her medication discontinued for medical reasons he/she was allowed to complete the 8 weeks of outpatient counseling.
At the end of the 8-week medication administration period or at termination from the study, all patients were scheduled for a termination medical and psychological evaluation (exit interview). The exit interview consisted of the ASI, evaluation of the treatment by the patient using a standard questionnaire developed at the IRP, complete physical examination and clinical laboratory tests, and referral for continued treatment in the community, as appropriate.
2.3. Outcome measures
The primary outcome measure was cocaine use as determined by assay of urine samples. Secondary outcome measures were the report of drug use by interview with a research technician, the result of the question about weekly changes in cocaine craving from the Minnesota Cocaine Craving Scale (MCCS; Halikas et al., 1991c), and the Beck Depression Inventory (BDI; Beck et al., 1961) and Symptom Check List-90-Revised (SCL-90R) total scores (Derogatis, 1977).
Urine samples were assayed for the cocaine metabolite benzoylecgonine using the enzyme-multiplied immunotechnique (Syva Corp, Palo Alto, Calif). The cut-off for positivity was set at 300 ng/ml. For purposes of data analysis, missing samples were considered positive for cocaine unless both the previous and subsequent samples were negative.
2.4. Statistical methods
Patients were considered evaluable if they stayed in treatment for one week or more, since previous studies suggested therapeutic effects of CBZ after 7–9 days (Halikas et al., 1989; Halikas et al., 1991a; Halikas et al., 1992). Patients were considered completers if they finished the 7 weeks of active medication or equivalent placebo.
The following two subject groupings were analyzed independently: (i) 62 evaluable patients, and (ii) 19 completers. Analyses were done for (i) pre-treatment versus post-treatment, where the post-treatment measure is defined as the last measure obtained from the subject while in treatment regardless of time of discharge; (ii) subjects grouped by the results of urine toxicology for cocaine on the first day of treatment; and (iii) the remaining subjects at each timepoint. These separate analyses were done to evaluate possible bias due to differential drop-out rates and to distinguish possible effects on initiation of abstinence (patients cocaine-positive on first day), from effects on relapse prevention (patients cocaine-negative on first day).
Comparisons between treatment groups at baseline for socio-demographic and drug use/treatment history variables were performed using the χ2 statistic or Fisher’s exact test (as appropriate with regard to contingency table cell sizes) for categorical and dichotomous data, or t-tests for continuous data. Retention time in treatment was analyzed using the log rank test of the survival distribution functions. With respect to other study measures, continuous variables were analyzed using a two-factor (group, time) repeated measures ANOVA. Categorical variables and dichotomous data were analyzed using the χ2 statistic or Fisher’s exact test, as appropriate. All statistical tests were considered significant at P < 0.05.
3. Results
Eighty-one applicants consented to participate in the study, of whom 9 did not appear at the first clinic visit. The remaining 72 subjects were randomly assigned and received the first dose of CBZ or placebo. Ten subjects started treatment but discontinued their participation before completing one week of treatment (none of them reported side-effects). The 19 non-evaluable patients did not differ significantly from the 62 evaluable ones on any of the intake characteristics (data not shown).
Results from the 62 subjects (CBZ, n = 28; placebo, n = 34) who received medication for 1 week or more showed no significant differences in socio-demographic characteristics, drug use history, or psychiatric co-morbidity between the CBZ and placebo groups (Table 1). The most frequent psychiatric disorders were antisocial personality (n = 18), phobic disorders (n = 16), and post-traumatic stress disorder (n = 8).
Table 1.
Socio-demographic characteristics and drug use history of patients on medication ≥ 1 week
| CBZ (n = 28) | Placebo (n = 34) | Total (n = 62) | |
|---|---|---|---|
| Age (mean) | 33.2 ± 5.4 | 33.3 ± 5.4 | 33.2 ± 5.4 |
| Males | 24 (85.7%) | 25 (73.5%) | 49 (79.0%) |
| Females | 4 (14.3%) | 9 (26.5%) | 13 (21.0%) |
| Black | 16 (57.1%) | 26 (76.5%) | 42 (67.7%) |
| White | 12 (42.9%) | 7 (20.6%) | 19 (30.7%) |
| Native American | 0 | 1 (2.9%) | 1 (1.6%) |
| Education completed (years) | 12.8 ± 1.7 | 12.5 ± 1.8 | 12.7 ± 1.8 |
| Attended college | 15 (53.6%) | 12 (35.3%) | 27 (43.6%) |
| Employment | |||
| Full-time | 25 (89.3%) | 24 (71.8%) | 49 (79.0%) |
| Part-time | 1 (3.6%) | 5 (14.7%) | 6 (9.7%) |
| Student | 1 (3.6%) | 0 (0.00%) | 1 (1.6%) |
| Unemployed | 1 (3.6%) | 5 (14.7%) | 6 (9.7%) |
| Days used cocaine in the past 30 days | 14.1 ± 8.1 | 17.5 ± 9.6 | 16.0 ± 9.0 |
| Cocaine negative urine on first day of treatment (%) | 12 | 8 | 10 |
| Amount of cocaine use in the past 30 days | |||
| Grams | 16.1 ± 25.1 | 11.4 ± 9.4 | 13.3 ± 17.6 |
| Dollars | 1139 ± 1560 | 621 ± 546 | 835 ± 1101 |
| Previous drug treatment | |||
| Never treated | 9 (32.1%) | 14 (43.8%) | 23 (38.3%) |
| Prior treatment | 19 (67.9%) | 18 (56.2%) | 37 (61.7%) |
There were no significant differences for socio-demographic characteristics or drug use history between patients treated with CBZ or placebo.
All patients attended most of the scheduled standardized individual counseling sessions during the time they participated in treatment. Bi-weekly CBZ plasma level monitoring showed a mean (S.D.) CBZ plasma level of 3.63 (2.52) μg/ml during week 2, 5.61 (3.54) μg/ml during week 4, and 3.57 (2.57) μg/ml during week 6 of treatment. There was no difference (χ2 = 0.653, df = 1) between the number of patients in either group who reported at the exit interview that they thought they had received a particular treatment.
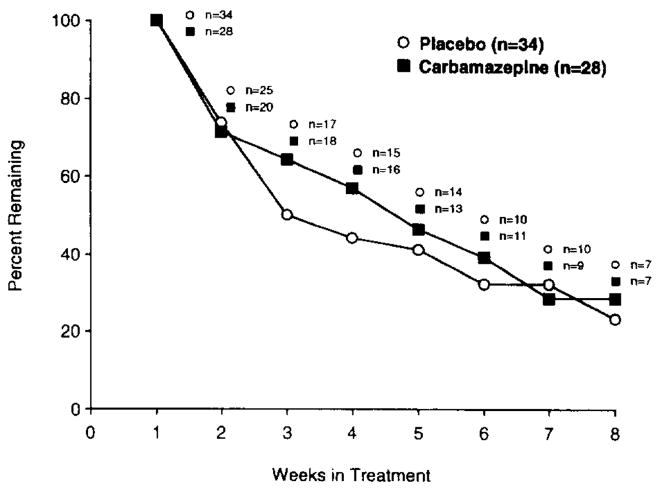
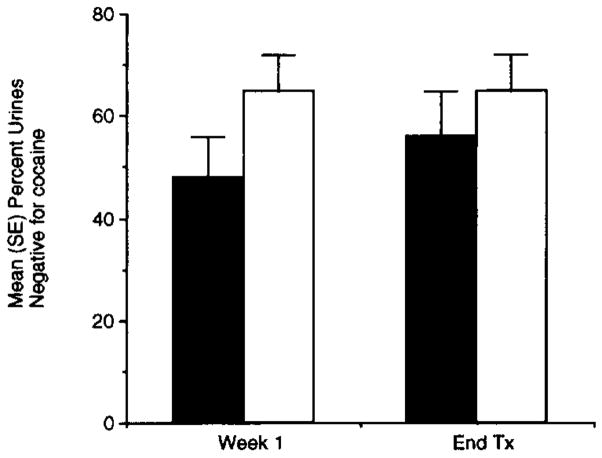
Mean (S.D.) retention time in the study was 30.6 (18.2) days. This estimate of retention time in the study is necessarily biased since it excludes patients participating less than one week and the maximum retention time of 8 weeks was fixed by the study design. There were no significant differences between the two groups for retention rates χ2 = 0.27, P = 0.60) or for the number of patients who completed the 8-week study (χ2 = 0.2, P = 0.65) (Fig. 1). For all subjects as a whole, there were significant decreases between intake and treatment termination in the self-reported drug outcome variables, including cocaine use in grams (F = 8.79; df = 1,58; p = < 0.005) and dollars spent (F = 8.18; df = 1,59; P = < 0.006) in the past 24 h, and cocaine craving (F = 9.08; df = 1,48; P < 0.005). However, these decreases were not associated with significant decreases in the percentage of urines negative for cocaine (F = 0.44; df = 1,60; p = 0.51) (Fig. 2).
Fig. 1.
There were no significant differences between CBZ (■, n = 28) and placebo (○, n = 34) for retention in treatment for cocaine dependence (62 patients completing one week of treatment).
Fig. 2.
Mean (SE) percentage of urines negative for cocaine at week 1 of and discharge from (End Tx) treatment. There were no significant differences between CBZ (■, n = 28) and placebo (□, n = 34).
For the 62 evaluable subjects, the first method of analysis (comparing pre-treatment versus post-treatment) showed no significant differences between CBZ and placebo on self-reported cocaine used in grams (F = 2.44; df = 1,58; P = 0.12) or dollars (F = 2.54; df = 1,59; P = 0.12), self-reported cocaine craving (F= 0.4; df = 1,48; P = 0.5) or percentage of urines negative for cocaine (F = 2.01; df= 1,60; P = 0.16). There were no significant group by time interactions for self-reported cocaine use in grams (F = 1.57; df = 1,58; P = 0.22) or dollars (F = 1.37; df = 1,59; P = 0.25), self- reported cocaine craving (F = 0.40; df = 1,48; P = 0.52), or percent of urines negative for cocaine(F = 0.44; df = 1,60; P = 0.51).
During the 8 weeks of treatment, there was a significant decrease in BDI (F = 21.7; df = 1,48; P < 0.0001) and SCL-90R total (F= 31.14; df = 1,56; P < 0.0001) scores for both groups, with no significant differences between CBZ and placebo groups (F = 0.4; df = 1,48; P = 0.53; and F = 0.16; df = 1,56; P = 0.69 respectively) (data not shown). There were no significant group by time interactions for the BDI (F = 0; df = 1,48; P = 0.98) or SCL-90R scores (F =0.57; df = 1,56; P = 0.45).
The second method of analysis (comparing CBZ and placebo groups stratified by urine toxicology result for cocaine on the first day of treatment), showed no significant differences between the CBZ and placebo groups for retention in treatment, urine toxicology results, or cocaine use as measured by amount in grams or money spent.
Post hoc weekly comparisons using the third method of analysis (remaining subjects at each timepoint) showed that the only significant group difference (Fisher’s exact test, P < 0.01) was lower self-reported weekly cocaine craving in the MCCS for the CBZ group at week 5. There were no other significant treatment outcome differences between CBZ and placebo (data not shown). The sample of 19 completers showed no significant differences between CBZ and placebo groups on any outcome measure (data not shown).
None of the patients treated with CBZ had severe or medically serious side-effects. Ten (16.1%) patients had mild or moderate side-effects. The most frequent (10%) was increased blood pressure. There were no significant differences between CBZ and placebo groups for frequency or severity of symptoms (Fisher’s exact test = 0.47). CBZ was discontinued in 3 patients due to side-effects (2 patients had high blood pressure and 1 had skin rash and leukopenia); these patients continued receiving counseling for the remainder of the 8-week trial. All side-effects resolved without hospitalization or permanent sequelae.
4. Comment
This is the first published report of a double-blind, random assignment, placebo-controlled, clinical trial on the use of CBZ for treatment of cocaine-dependent patients who did not meet criteria for other psychoactive substance dependencies (except nicotine) nor were methadone maintained. The results showed that CBZ was not better than placebo for patients receiving standardized counseling twice a week in an 8-week treatment program at doses and blood levels previously reported effective (Halikas et al., 1992). During treatment both groups showed similar significant decreases in self-reported cocaine use (money spent and grams used), cocaine craving, and depression and psychopathology scores. No significant change in qualitative urine toxicology results for cocaine was observed.
The only significant medication group difference was lower cocaine craving for the CBZ group at one time-point. This may be a spurious result of the multiple statistical analyses. However, since this result corresponded with the week after the peak CBZ dose, it may also be interpreted as a CBZ dose-related effect. It is possible that higher doses of CBZ would be more effective in controlling cocaine craving, although potential side-effects (Rall and Schleifer, 1990) might limit their use.
There are limitations of the study that should be considered when interpreting the results, (i) The drop-out rate, although similar for both groups, may limit the validity of the analysis done for the remaining patients who stayed in treatment at each week, (ii) It is possible that CBZ could be effective at higher doses or plasma levels, or when given for longer duration. However, even for the time that previously reported effective plasma levels (Halikas et al., 1992) were achieved in this study, there were no significant differences between the groups for the outcome measures, (iii) It is possible that CBZ shows some differential effect on cocaine-dependent patients with abnormal EEG (Montoya et al., 1992). Although patients in this study were excluded for a history of seizures, they were not screened for EEG abnormalities. However, the consistency of the results obtained from several methods of data analysis (pre-treatment versus post-treatment, initial cocaine urine positive versus negative, and completers versus non-completers) and the good medication compliance support the validity of the conclusion that CBZ is not effective for the treatment of cocaine dependence.
The high attrition rate for cocaine dependence treatment is usually a limitation for this type of study. By achieving comparable numbers of patients exposed to at least 1 week of treatment we partly controlled for the potential bias that attrition may create and also gave reasonable duration for the medication to produce its effects. The attrition rate in this study (76% over 8 weeks, based on 81 subjects who signed the consent form) is comparable to that observed in other outpatient medication trials (Weddington et al., 1991; Montoya et al., 1994) in cocaine-dependent subjects not also maintained on methadone.
Use of inactive placebo in the evaluation of psychotropic medications has been criticized because the active medication can be discriminated (Fisher and Greenberg, 1993). However, this does not appear to be an issue in the present study. Results from the exit interview showed no significant ability of the patients to distinguish medication versus placebo. Also, the presentation of side-effects was similar for both groups.
CBZ has been reported to have psychotropic effects in patients treated for seizure disorders (Trimble, 1988) and to be effective for treatment of non-responsive psychosis (Neppe, 1988). These effects may relate to an anti-kindling mechanism. However, although both groups in this study showed reduction of scores for psychopathology and depression, there was no evidence of differences between CBZ and placebo. The intensity and good quality of the standardized counseling may have been stronger factors than medication in determining the reductions in psychological symptoms, overshadowing any treatment effect of CBZ.
Because CBZ has been reported to produce hematologic reactions (Sobotka et al., 1990), and to have cardiovascular side-effects (Halikas et al., 1991b), periodic cardiovascular and blood monitoring has been recommended. Furthermore, the combination of CBZ and cocaine has been postulated to worsen those complications (Hatsukami et al., 1991). In close medical monitoring of patients in the present study, no severe side-effects were observed. Thus, CBZ appears not to cause serious side-effects in this population of cocaine dependent individuals at the doses used in this study.
Despite the animal data suggesting that CBZ blocks the development of cocaine-induced kindling, the previous clinical studies (chiefly open-label) suggesting that CBZ was effective in treating cocaine dependence, its publicity and extensive prescription by clinicians (Halikas et al., 1993) for treatment of cocaine dependence, the results from this study do not support the use of CBZ for this indication. However, they do not rule out its possible therapeutic value for cocaine dependence in patients with certain comorbid psychopathology. For example, CBZ is used to treat bipolar disorders (Post, 1990) and may be effective for cocaine-dependent patients with the concurrent presence of these disorders. Further research would be required to evaluate this possibility.
Acknowledgments
We thank Judith Hess and Nancy Kreiter for research support and statistical analysis; Edward Cone and David Darwin for urine toxicology and plasma CBZ analyses; Daniel Baker, Gigi Farley, Steve Herr and Sue Ruckel for patient counseling; Lil Morgan for nursing services; Robert Lange, Carlo Contoreggi and Beverly Beall for medical monitoring; NOVA recruitment staff; and Archway Clinic staff. This study was supported through NIH-NIDA intramural research funds and was presented at the 55th annual scientific meeting of the College on Problems of Drug Dependence (CPDD), June, 1993.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders-III-Revised. American Psychiatric Association; Washington, DC: 1987. [Google Scholar]
- Barros HMT, Braz S, Leite JR. Effect of carbamazepine on dopamine release and reuptake in rat striatal slices. Epilepsia. 1986;27:534–537. doi: 10.1111/j.1528-1157.1986.tb03579.x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Browne TR, Empting L, Lydiard RB, Yerby MS. New uses and old for carbamazepine. Patient Care. 1990;24:48–79. [Google Scholar]
- Carroll ME, Lac ST, Asencio M, Halikas JA, Kragh R. Effects of carbamazepine on self-administration of intravenously delivered cocaine in rats. Pharmacol Biochem Behav. 1990;37:551–556. doi: 10.1016/0091-3057(90)90026-e. [DOI] [PubMed] [Google Scholar]
- Covi L, Hess JM, Kreiter NA. Psychotherapy dosage effects in cocaine dependence. American Psychiatric Association 146th Annual Meeting (Abstract); 1993. p. 53. [Google Scholar]
- Derogatis LR. SCL-90R: Administration, Scoring and Procedures Manual-II for the Revised Version. The Johns Hopkins University School of Medicine, Clinical Psychometrics Research; Baltimore: 1977. [Google Scholar]
- Fisher S, Greenberg RP. How sound is the double-blind design for evaluating psychotropic drugs? J. Nerv Ment Dis. 1993;181:345–350. doi: 10.1097/00005053-199306000-00002. [DOI] [PubMed] [Google Scholar]
- Halikas JA, Kemp K, Kuhn K, Carlson GA, Crea F. Carbamazepine for cocaine addition? (letter) Lancet. 1989;1:623–624. doi: 10.1016/s0140-6736(89)91660-7. [DOI] [PubMed] [Google Scholar]
- Halikas JA, Crosby RD, Carlson GA, Crea F, Graves NM, Bowers LD. Cocaine reduction in unmotivated crack users using carbamazepine versus placebo in a short term, double-blind, crossover design. Clin Pharmacol Ther. 1991a;50:81–95. doi: 10.1038/clpt.1991.107. [DOI] [PubMed] [Google Scholar]
- Halikas JA, Crosby RD, Koop LP, Crea F, Nugent SM, Carlson GA. Daily monitored cardiovascular effects of carbamazepine in chronic crack cocaine users. Psychopharmacol Bull. 1991b;27(3):345–351. [PubMed] [Google Scholar]
- Halikas JA, Kuhn K, Carlson GA, Crea F, Crosby R. The effect of carbamazepine on cocaine use. Am J Addict. 1992;1:30–39. [Google Scholar]
- Halikas JA, Kuhn KL, Crosby R, Carlson G, Crea F. The measurement of craving cocaine patients using the Minnesota Cocaine Craving Scale. Comp Psychiatry. 1991c;32:22–27. doi: 10.1016/0010-440x(91)90066-l. [DOI] [PubMed] [Google Scholar]
- Halikas JA, Nugent SM, Crosby RD, Carlson GA. 1990–1991 Survey of pharmacotherapies used in the treatment of cocaine abuse. J Addict Dis. 1993;12(2):129–139. doi: 10.1300/J069v12n02_09. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Keenan R, Halikas JA, Pentel PR, Brauer LH. Effects of carbamazepine on acute responses to smoked cocaine-base in human cocaine users. Psychopharmacology. 1991;104:120–124. doi: 10.1007/BF02244565. [DOI] [PubMed] [Google Scholar]
- Helzer J, Croughan J, Robins L, Ratcliff K. National Institute of Mental Health Diagnostic Interview Schedule: its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Kowalik S, Levitt M, Barkai A. Effects of carbamazepine and antidepressant drugs on endogenous catecolamine levels in the cerebroventricular compartment of the rat. Psychopharmacology. 1984;83:169–171. doi: 10.1007/BF00429729. [DOI] [PubMed] [Google Scholar]
- MacDonald RL, McLean MJ, Skerritt JH. Anticonvulsant drug mechanisms of action. Fed Proc. 1985;44:2634–2639. [PubMed] [Google Scholar]
- McLean MJ, MacDonald RL. Carbamazepine and 10,11-epoxycarbamazepine produce use- and voltage-dependent limitation of rapidly firing action potentials of mouse central neurons in cell culture. J Pharmacol Exp Ther. 1986;238:727–738. [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, McGahan P, O’Brien CP. Guide to the Addiction Severity Index: Background, Administration, and Field Testing Results. National Institute on Drug Abuse, Treatment Research Reports; Rockville, MD: 1986. [Google Scholar]
- Montoya ID, Llosa T, Hess JM, Gorelick DA. Open-label carbamazepine reduces cocaine use in cocaine-dependent patients with and without abnormal EEG. Biol Psychiatry. 1992;31:142A. [Google Scholar]
- Montoya ID, Preston K, Cone E, Rothman R, Gorelick D. Safety and efficacy of bupropion in combination with bromocriptine for treatment of cocaine dependence. Neuropsychopharmacology. 1994;10:129S. [Google Scholar]
- Neppe VM. Carbamazepine in non-responsive psychosis. J Clin Psychiatry. 1988;49(4 Suppl):22–28. [PubMed] [Google Scholar]
- Post RM. Progressive changes in behavior and seizures following chronic cocaine administration: relationship to kindling and psychosis. In: Ellinwood EH, kilbey MM, editors. Advances in Behavioral Biology. Plenum; New York: 1977. [Google Scholar]
- Post RM. Non-lithium treatment for bipolar disorder. J Clin Psychiatry. 1990;5:9–16. [PubMed] [Google Scholar]
- Post RM, Kopanda RT. Cocaine kindling and psychosis. Am J Psychiatry. 1976;133:627–634. doi: 10.1176/ajp.133.6.627. [DOI] [PubMed] [Google Scholar]
- Post RM, Kopanda RT, Lee A. Progressive behavioral changes during chronic lidocaine administration: relationship to kindling. Life Sci. 1975;17:943–950. doi: 10.1016/0024-3205(75)90447-6. [DOI] [PubMed] [Google Scholar]
- Rall TW, Schleifer LS. Drugs effective in the therapy of the epilepsies. In: Goodman Gilman A, Rall TW, Nies AS, Taylor P, editors. The Pharmacological Basis of Therapeutics. 8. Pergamon Press; New York: 1990. pp. 436–462. [Google Scholar]
- Sharpe LG, Jaffe JH, Katz JL. Carbamazepine produces non-specific effects on cocaine self-administration in rats. Life Sci. 1992;51:PL13–PL18. doi: 10.1016/0024-3205(92)90081-y. [DOI] [PubMed] [Google Scholar]
- Sherer MA, Kumor KM, Mapou RL. A case in which carbamazepine attenuated cocaine ‘rush’. Am J Psychiatry. 1990;147(7):950. doi: 10.1176/ajp.147.7.950b. [DOI] [PubMed] [Google Scholar]
- Shipley WC. A self-administering scale for measuring intellectual impairment and deterioration. J Psychol. 1940;9:371–377. [Google Scholar]
- Sobotka JL, Alexander B, Cook BL. A review of carbamazepine’s hematologic reactions and monitoring recommendation. DICP Ann Pharmacother. 1990;24:1214–1219. doi: 10.1177/106002809002401214. [DOI] [PubMed] [Google Scholar]
- Trimble MR. Carbamazepine and mood: evidence from patients with seizure disorders. J Clin Psychiatry. 1988;49(4 Suppl):7–11. [PubMed] [Google Scholar]
- Weddington WW, Brown BS, Haertzen CA, Hess J, Mahaffey JR, Kolar AF, Jaffe JH. Single blind, placebo-controlled comparison of amantadine and desipramine combined with psychotherapy for treatment of cocaine dependence. Am J Drug Alcohol Abuse. 1991;17:137–153. doi: 10.3109/00952999108992817. [DOI] [PubMed] [Google Scholar]
- Weiss SR, Post RM, Costello M, Nutt DJ, Tandeciarz S. Carbamazepine retards the development of cocaine-kindled seizures but not sensitization to cocaine-induced hyperactivity. Neuropsychopharmacology. 1990;3:273–281. [PubMed] [Google Scholar]
- Weiss SR, Post RM, Szele F, Woodward R, Nierenberg J. Chronic carbamazepine inhibits the development of local anesthetic seizures kindled by cocaine and lidocaine. Brain Res. 1989;497:72–79. doi: 10.1016/0006-8993(89)90971-2. [DOI] [PubMed] [Google Scholar]
- Willow M, Gonoi T, Catterall WA. Voltage clamp analysis of the inhibitory actions of diphenylhydantoin and carbamazepine on voltage-sensitive sodium channels in neuroblastoma cells. Mol Pharmacol. 1985;27:549–558. [PubMed] [Google Scholar]