Abstract
Background
Buprenorphine is a partial μ-opiate agonist and κ-opiate antagonist with established efficacy in the treatment of opiate dependence. Its efficacy for cocaine dependence is uncertain. This study evaluated buprenorphine for the treatment of concomitant cocaine and opiate dependence.
Methods
Two hundred outpatients currently dependent on both cocaine and opiates were randomly assigned to double-blind groups receiving a sublingual solution of buprenorphine (2, 8, or 16 mg daily, or 16 mg on alternate days, or placebo), plus weekly individual drug abuse counseling, for 13 weeks. The chief outcome measures were urine concentrations of opiate and cocaine metabolites (quantitative) and proportion of urine samples positive for opiates or cocaine (qualitative). Group differences were assessed by use of mixed regression modeling.
Results
The target dose of buprenorphine was achieved in 179 subjects. Subjects receiving 8 or 16 mg buprenorphine daily showed statistically significant decreases in urine morphine levels (P = .0135 for 8 mg and P < .001 for 16 mg) or benzoylecgonine concentrations (P = .0277 for 8 mg and P = .006 for 16 mg) during the maintenance phase of the study. For the 16-mg group, mean benzoylecgonine concentrations fell from 3715 ng/mL during baseline to 186 ng/mL during the withdrawal phase; mean morphine concentrations fell from 3311 ng/mL during baseline to 263 ng/mL during withdrawal. For the 8-mg group, mean benzoylecgonine concentrations fell from 6761 ng/mL during baseline to 676 ng/mL during withdrawal; mean morphine concentrations fell from 3890 ng/mL during baseline to 661 ng/mL during withdrawal. Qualitative urinalysis showed a similar pattern of results. Subjects receiving the highest dose showed concomitant decreases in both urine morphine and benzoylecgonine concentrations. There were no significant group differences in treatment retention or adverse events.
Conclusions
A sublingual buprenorphine solution at 16 mg daily is well tolerated and effective in reducing concomitant opiate and cocaine use. The therapeutic effect on cocaine use appears independent of that on opiate use.
Buprenorphine is a partial μ-opiate agonist and κ-opiate antagonist marketed in the United States and worldwide as a parenteral or sublingual analgesic1 and as maintenance treatment for opiate dependence.2 However, its efficacy for treatment of dually (cocaine and heroin) dependent individuals has not been established.
For the treatment of opiate dependence, sublingual doses of 8 to 16 mg daily of buprenorphine are as effective as 55 to 80 mg of oral methadone in reducing opiate use.3,4 There is some evidence that sublingual doses as low as 2 mg daily may reduce opiate use.5 Because buprenorphine has a relatively long duration of action, every-other-day and 3-times-weekly dosing have also shown efficacy.6-9
A large proportion of opiate users use cocaine, even during opiate agonist substitution treatment for opiate dependence. Cocaine use during opiate agonist maintenance treatment is associated with increased opiate use, poorer treatment outcomes, and premature dropout from treatment.10 Methadone and levomethadyl acetate (INN, levacetylmethadol) do not seem efficacious for the treatment of cocaine dependence in opiate-dependent individuals. Thus a medication that effectively reduced both opiate and cocaine use would offer a therapeutic advantage over methadone or levomethadyl acetate alone for the many patients who are using or are dependent on both opiates and cocaine.
Buprenorphine was found to have such potential in preclinical studies. It significantly reduced cocaine self-administration in monkeys without interfering with appetitive behaviors such as eating.11-14 However, attempted replications of these effects in humans have yielded inconsistent results. In some human laboratory studies, buprenorphine treatment reduced self-reported cocaine craving and cocaine self-administration; other studies have failed to replicate those results.15-18
Currently, there is no consensus in the literature about the clinical efficacy of buprenorphine for treatment of concurrent cocaine and heroin dependence. In some clinical trials with opiate-dependent subjects, buprenorphine treatment has been associated with reductions in cocaine use,19-21 whereas other trials have found no evidence of efficacy.18,22 One negative trial used buprenorphine doses of 4 mg and 12 mg/d22; the other used a mean dose of 11.2 mg.18 The positive studies have used buprenorphine doses up to 16 mg daily, with more reduction of cocaine use at higher doses.
The purpose of this study was to evaluate the efficacy of sublingual buprenorphine maintenance (10 weeks) in reducing cocaine and opiate use in dually (cocaine and opiate) dependent outpatients.
METHODS
Study design
This 4-group, randomized, double-blind study was approved by the Johns Hopkins Bayview Institutional Review Board and conducted in 200 opiate- and cocaine-dependent subjects (Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised [DSM-IIIR] criteria) in the outpatient clinic of the National Institute on Drug Abuse Intramural Research Program (NIDA IRP), Baltimore, Md. A sample size of 44 per group was necessary for 80% power to detect a medium effect size (f = 0.25) for a 4-group ANOVA having an α of .05.23 Fifty subjects were recruited per group to allow for subject dropout. Subjects were randomly assigned to 1 of 4 buprenorphine medication groups (2 mg daily, 8 mg daily, 16 mg daily, or 16 mg/0 mg on alternate days). Prospective stratification by gender and age (21−35 years and 36−50 years) was used because of the suggested influence of these characteristics on response to opiate agonist treatment.24-26 Allocation was done by the NIDA IRP pharmacy at the time of subject consent by use of a table of random numbers and masked from all study personnel. The primary outcome measures for the study were opiate and cocaine use determined by urine toxicologic studies and retention time in treatment.
Subjects
Subject inclusion criteria were as follows: age 21 to 50 years, current cocaine and opiate dependence (based on DSM-IIIR criteria), self-reported use of cocaine and opiates within the past 14 days, use of at least $50 of heroin per day and $100 of cocaine per week at some time over the past month, 2 urine samples positive for opiates and for cocaine during the screening process, and not currently in drug abuse treatment elsewhere. Subjects discharged from a methadone maintenance program were eligible only if their dose did not exceed 30 mg/d over the 7-day period before initiation of buprenorphine (to prevent potential buprenorphine-induced withdrawal symptoms). Excluded were individuals unable to understand and fill out questionnaires, those with other current psychoactive substance dependence (except nicotine or caffeine), those with a current psychiatric or unstable medical disorder, and pregnant or nursing women. Human immunodeficiency virus (HIV)–infected individuals with CD4 T-cell count lower than 200/mL were also excluded, because of the risk that their impaired immune status might interfere with study participation.
Procedures
Applicants underwent a thorough medical and psychosocial evaluation in two 4-hour sessions no more than 2 weeks apart. This included a medical history and physical examination, clinical laboratory tests, 12-lead electrocardiogram, tuberculosis skin test (chest radiograph when indicated), urine toxicology, and pregnancy test. HIV antibody testing by enzyme-linked immunosorbent assay (ELISA) (with positive findings confirmed by Western blot) was offered with appropriate pretest and posttest counseling but was not mandatory. CD4 T-cell counts were obtained from subjects who had HIV-positive test results or self-reported as HIV-positive. Psychosocial evaluation included the Addiction Severity Index,27 Diagnostic Interview Schedule,28 and Symptom Checklist 90R.29 After qualification, all subjects signed the consent form approved by the Johns Hopkins Bayview Medical Center Institutional Review Board. Subjects then had their first clinic visit within 1 week.
Subjects participated in a 91-day treatment program that required daily clinic visits. At each visit, they ingested a medication dose under direct observation of nursing staff, had blood pressure and pulse checked, reported any adverse effects, and provided self-report data on outcome measures. At 3 visits each week (usually Monday, Wednesday, and Friday), they provided a urine sample (for drug assay) under staff observation. Subjects were discharged for missing 3 consecutive medication doses or 6 counseling sessions.
Urine samples were stored at −20°C and later assayed semiquantitatively by use of the Abuscreen On-Line DAT immunoassay (Roche Diagnostics Corporation, Indianapolis, Ind) for morphine and benzoylecgonine, both with a lower limit of quantification of 100 ng/mL. Qualitative urine toxicology results were available to the subjects’ counselors, but the treatment program had no systematic contingencies based on these results. Venous blood samples were obtained every 4 weeks to monitor complete blood cell count, electrolytes, and kidney and liver function.
Treatment
Nursing staff administered buprenorphine sublingually as 1 mL of a 40% aqueous ethanol solution according to a 3-phase dosing schedule. All groups started at 2 mg daily. Doses escalated to the targeted dose by day 5 (dose-escalation phase), then remained at the target for 9 weeks 2 days (until day 70) (maintenance phase). During the last 20 days, doses were decreased to 0 mg (withdrawal phase, days 71−91). Matching buprenorphine placebo (40% ethanol) was given on days of 0-mg buprenorphine dosing. Dosage could be halved, or held completely, at the physician's discretion if the subject was unable to tolerate the dose. Subjects who discontinued medication were offered 21-day methadone detoxification plus counseling.
Subjects received weekly individual standardized drug abuse counseling, based on interpersonal psychotherapy,30 from manual-trained master's level counselors. The therapy had 4 phases, as follows: (1) review of personal history, formulation of problems and goals, and development of a therapeutic alliance; (2) development of strategies to achieve treatment goals and control drug use; (3) strengthening of strategies and skills that prevent drug use, as well as learning to use available support resources; and (4) resolution of separation and termination issues.
Statistical analysis
Baseline characteristics among medication groups (Table I) were compared by chi-square test for categoric variables and ANOVA for continuous variables. Retention in treatment was evaluated via nonparametric survival analysis methods (log-rank and Wilcoxon tests).
Table I.
Sociodemographic characteristics, drug use history, treatment experience, and medication adherence of 179 opiate- and cocaine-dependent outpatients by buprenorphine treatment group
| 16 mg daily (n = 46) | 16 mg every other day (n = 43) | 8 mg daily (n = 44) | 2 mg daily (n = 46) | Statistic, P value | |
|---|---|---|---|---|---|
| Male (%) | 65.2 | 62.8 | 68.2 | 69.6 | χ32 = 0.55, P = .91 |
| Black (%) | 73.9 | 72.1 | 77.3 | 76.1 | χ32 = 0.37, P = .95 |
| Aged 21−35 y (%) | 52.2 | 58.1 | 63.6 | 58.7 | χ32 = 1.22, P = .75 |
| Age (mean, SD) | 34.2 (7.25) | 34.6 (5.31) | 33.1 (5.51) | 34.3 (6.45) | F3,175 = 0.48, P = .70 |
| Single (%) | 56.5 | 53.5 | 56.8 | 64.4 | χ32 = 1.19, P = .76 |
| Unemployed (%) | 39.1 | 37.2 | 52.3 | 32.6 | χ32 = 3.95, P = .27 |
| High school diploma (%) | 65.2 | 55.8 | 68.2 | 76.1 | χ32 = 4.19, P = .24 |
| Education (y) (mean, SD) | 11.7 (1.62) | 11.3 (1.68) | 11.5 (2.53) | 12.1 (1.59) | F3,175 = 1.47, P = .22 |
| HIV-positive (%) | 10.9 | 2.3 | 9.1 | 4.4 | P = .63 (Fisher exact test) |
| HIV-negative (%) | 65.2 | 79.1 | 65.9 | 73.9 | |
| Declined testing (%) | 23.9 | 18.6 | 25.0 | 21.7 | |
| Baseline daily use | |||||
| Cocaine (g) | 1.56 (1.68) | 1.20 (1.02) | 1.33 (1.53) | 1.93 (2.51) | F3,166 = 1.39, P = .25 |
| Opiates (mg) | 33.4 (24.0) | 30.7 (30.7) | 24.1 (23.9) | 31.3 (32.5) | F3,166 = 0.89, P = .45 |
| Age first used (y) (mean, SD) | |||||
| Cocaine | 23.2 (7.43) | 24.0 (4.98) | 20.6 (5.71) | 25.4 (8.39) | F3, 35 = 0.87, P = .46 |
| Opiate | 20.1 (3.14) | 27.2 (6.59) | 21.0 (6.20) | 19.7 (3.47) | F3, 35 = 3.57, P = .024 |
| Use other drugs (%) | 58.7 | 72.1 | 63.6 | 54.4 | χ32 = 3.27, P = .35 |
| Prior drug treatments (No.) (mean, SD) | 2.00 (3.76) | 1.44 (2.32) | 1.32 (1.80) | 1.20 (2.15) | F3,174 = 0.82, P = .48 |
| ASI scores (mean, SD) | |||||
| Medical | 0.41 (0.83) | 0.72 (1.24) | 0.72 (1.12) | 0.86 (1.50) | F3, 172 = 1.14, P = .33 |
| Employment | 1.96 (2.03) | 2.88 (1.88) | 2.56 (1.96) | 2.69 (1.77) | F3, 172 = 1.95, P = .12 |
| Alcohol | 0.64 (1.35) | 0.86 (2.05) | 0.75 (1.67) | 0.67 (1.43) | F3, 171 = 0.15, P = .93 |
| Drug | 6.13 (1.86) | 6.16 (1.57) | 6.11 (1.67) | 5.89 (1.89) | F3, 174 = 0.23, P = .88 |
| Legal | 0.60 (1.12) | 0.88 (1.61) | 1.27 (1.82) | 0.98 (1.45) | F3, 172 = 1.48, P = .22 |
| Family | 1.98 (2.19) | 2.30 (2.10) | 2.41 (2.39) | 1.95 (2.22) | F3, 175 = 0.47, P = .70 |
| Psychiatric | 0.71 (1.24) | 0.48 (0.89) | 0.75 (1.31) | 0.86 (1.52) | F3, 171 = 0.71, P = .55 |
| Completed maintenance (%) | 47.8 | 60.5 | 45.5 | 47.8 | χ32 = 2.42, P = .49 |
| Proportion counseling appointments kept | 59.8 (36.4) | 51.9 (28.7) | 56.9 (32.5) | 53.1 (28.5) | F3, 173 = 0.58, P = .63 |
| Patients with adverse events (%) | 39.1 | 51.2 | 34.1 | 45.7 | χ32 = 3.00, P = .39 |
| Time in study (d) (median) | 60 | 84 | 64 | 57 | Log rank = −1.4, P = .91 |
HIV, Human immunodeficiency virus; ASI, Addiction Severity Index.
Urine drug metabolite data were analyzed by use of repeated-measures linear mixed regression, which uses data from subjects with incomplete follow-up, thus obviating the need to delete subjects with missing observations or impute missing values, either of which can bias results.31 Although mixed regression models do not eliminate dropout bias, they reduce the bias in comparison with more conventional alternatives.32 Piecewise linear regression models were fit by use of a linear spline technique so that a separate slope was estimated for each study phase (maintenance and withdrawal) with both lines passing through a common intercept.33 The dose-induction phase was excluded from regression analyses because it was short (4 days), so that few urine specimens were available per participant. The dependent variable in these regression equations was urine metabolite concentration (benzoylecgonine or morphine); the independent variable was urine specimen collection day. Thus the regression models provided estimates of change in urine metabolite levels per day.
The regression modeling procedure was conducted separately for each treatment group so that changes in urine metabolite levels over time for different treatment groups could be compared. Within-subject correlation was modeled by use of a first-order autoregressive covariance structure.34 Urine metabolite concentrations were log-transformed before statistical analysis to normalize their right-skewed distributions. To assess the presence of main effects (medication dose and study phase) on log-transformed urine metabolite levels, mixed regression models that consisted of only 2 independent class variables, dose and study phase, were fit.
The primary outcome measure was quantitative urinalysis (drug metabolite concentration) rather than qualitative urinalysis (metabolite present or absent) because drug metabolite levels have been shown to have good discriminative validity, correlating well with self-reported drug use,35 and to confer additional statistical power for detecting a treatment effect.36 To determine whether comparable results would be obtained from qualitative urinalysis, untransformed levels of both metabolites were dichotomized as positive or negative for drug by use of a 300-ng/mL cutoff. This is the cutoff required by the US government for workplace urine drug testing and is used by most drug treatment programs. Piecewise logistic regression models were then fit to these data.
The effect of buprenorphine dose on urine metabolite concentrations was evaluated by considering urine benzoylecgonine and morphine both independently and jointly. Urine benzoylecgonine and morphine levels were considered independently: Was buprenorphine dose associated with downward trends over time in either urine benzoylecgonine or morphine levels? Next, we considered changes in urine benzoylecgonine and urine morphine levels jointly: The piecewise regression models of urine benzoylecgonine on time were rerun with concurrent urine morphine concentration added as a time-varying covariate, and the piecewise regression models of morphine on time were rerun with concurrent urine benzoylecgonine concentration added as a time-varying covariate. Finally, a multivariate mixed regression model was fit to determine the effect of time during the maintenance phase on concomitant drug metabolite levels by buprenorphine dose (ie, do both urine benzoylecgonine and urine morphine levels decrease during treatment?).
Adverse event data were analyzed by comparing incidence densities of adverse events with use of the normal approximation to the binomial test when the numbers of events and person-weeks of medication exposure were sufficient for the normal approximation to be valid; otherwise, the exact binomial test was used. All statistical analyses used SAS version 8 (SAS Institute, Cary, NC), with a 2-tailed α of .05.
RESULTS
A total of 360 applicants were screened to obtain 200 subjects who met study criteria and signed the consent form. Of these, 179 subjects were included in data analyses (8 did not keep their first clinic appointment and 13 were discharged before their target buprenorphine dose was achieved on day 5). The proportion of evaluable subjects did not differ significantly among treatment groups: 46 of 50 (92%) at 2 mg daily, 44 of 48 (92%) at 8 mg daily, 43 of 50 (86%) at 16 mg every other day, and 46 of 52 (86%) at 16 mg daily (χ2 = 1.28, df = 3, P = .73). Evaluable subjects were significantly older than the nonevaluable subjects (34.0 ± 0.46 years versus 30.2 ± 1.43 years; F = 7.23, df = 1, 198, P = .0078) but otherwise did not differ significantly (all P values > .05) with regard to any baseline subject characteristics listed in Table I.
Subject characteristics
The sociodemographic and drug use characteristics of all 4 groups were very similar (Table I). As expected, the prospective stratification generated equivalent age and gender distributions across the groups. Given the study's exclusion of subjects with current psychiatric disorders, there was a low frequency of lifetime psychiatric comorbidity (DSM-IIIR criteria) with no significant differences across groups (data not shown). The only significant difference in drug-use history was an older age at initiation of opiate use in the group randomized to 16 mg every other day (F = 3.57, df = 3, P = .024) (Table I). Because age at initiation of opiate use was not correlated with study outcome measures, it was not included as a covariate in analyses assessing treatment efficacy. Of the 200 subjects offered HIV antibody testing, 44 (22%) declined testing, 144 (72%) had negative test results , and 12 (6%) had positive test results. There was no significant difference among the 4 medication groups in proportion of HIV-positive subjects (F = 3.84, df = 3, P = .28) (Table I).
Retention and treatment compliance
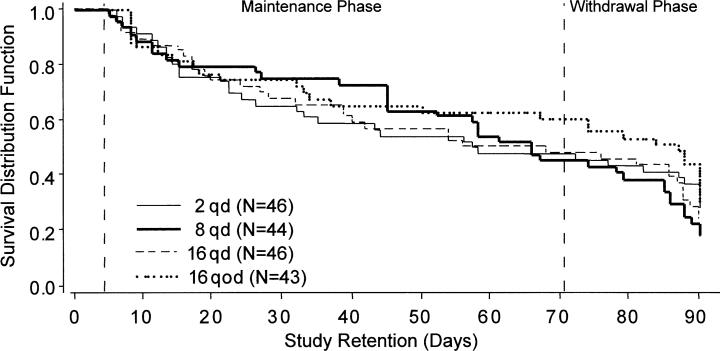
Of the 179 evaluable subjects, 90 (50%) completed the maintenance phase (70 days) and 45 (25%) completed the withdrawal phase. Treatment retention was not significantly different among medication groups (Fig 1) (log-rank test, P = .91; proportion completing 70 days: χ2 = 2.42, df = 3, P = .49). Counseling attendance was similar among groups (F = 0.58, df = 3, P = .63) (Table I). Twenty-four subjects entered a 21-day methadone detoxification program during the maintenance phase (at a similar frequency from each treatment group), as follows: 7 from the group receiving 2 mg daily, 6 each from the groups receiving 8 mg daily and 16 mg every other day, and 5 from the group receiving 16 mg daily. Five subjects entered a metha-done detoxification program during the withdrawal phase, as follows: 2 from the group receiving 2 mg daily and 1 each from the other 3 medication groups. HIV antibody status was unrelated to number of days in treatment (log-rank test, P = .53) and proportion of counseling visits kept (F = 2.17, df = 2, 174, P = .12).
Fig 1.
Retention in treatment by medication group among 179 cocaine- and opiate-dependent outpatients participating for at least 5 days. Subjects received assigned dose of sublingual buprenorphine for 10 weeks (maintenance phase) and then doses tapering to 0 over a 3-week period (withdrawal phase). qd, Daily; qod, every other day.
Urine toxicology results
Opiates
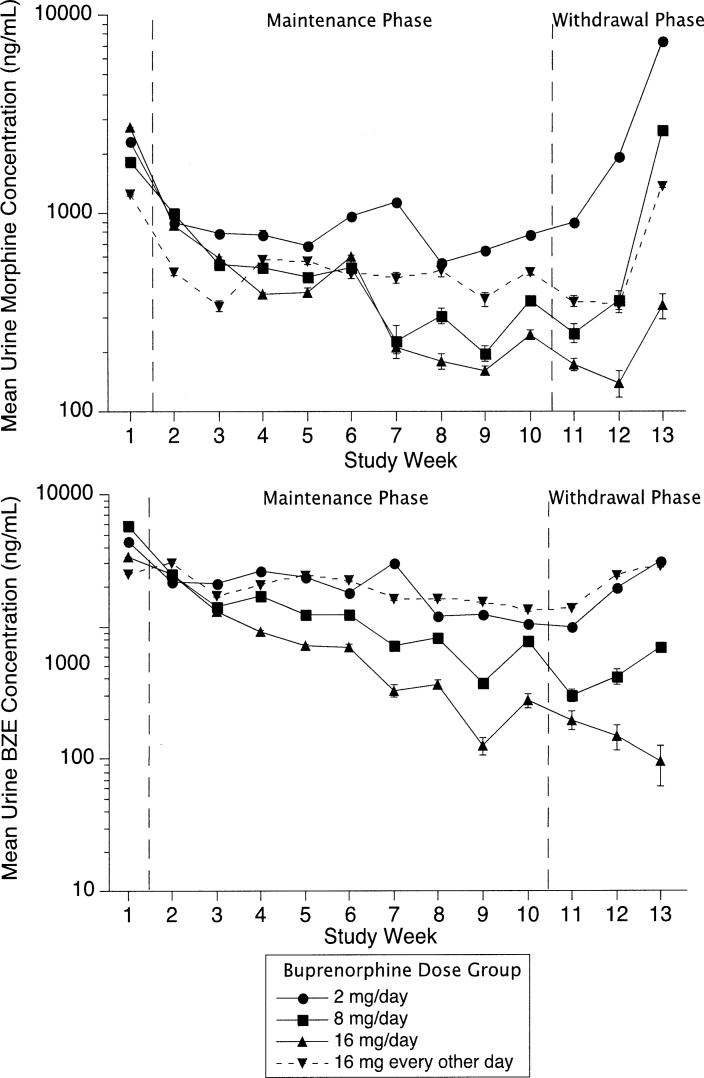
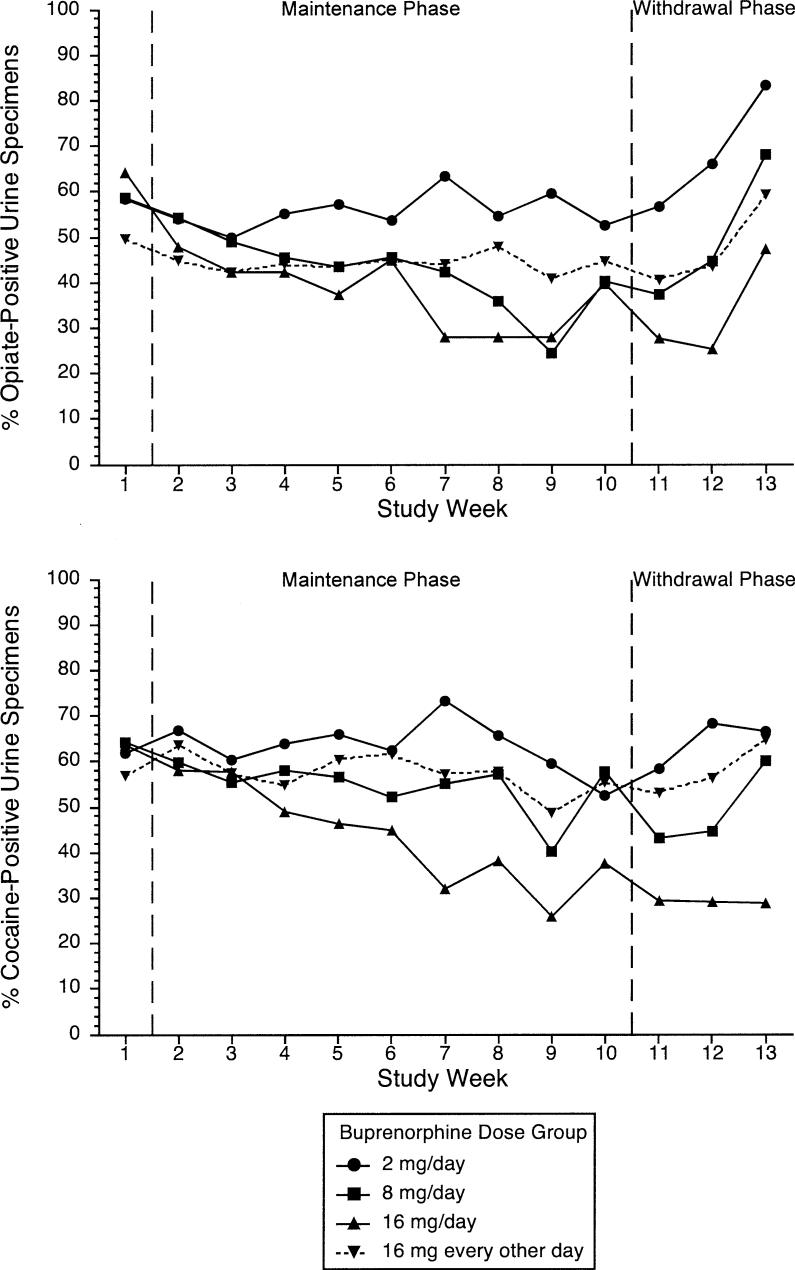
The buprenorphine doses of 8 mg daily and 16 mg daily were associated with statistically significant decreases in urine morphine concentration during the maintenance phase (P = .014 for 8 mg and P < .001 for 16 mg), with no significant changes in the other 2 groups (P = .43 for both) (Fig 2 and Table II). All treatment groups except the group receiving 16 mg daily showed increases in urine morphine concentration during the withdrawal phase (P = .013 for 2 mg daily, P = .0028 for 8 mg daily, P = .033 for 16 mg every other day, and P = .15 for 16 mg daily) (Table II), suggesting that beneficial effects on opiate use may be lost during buprenorphine withdrawal at doses of less than 16 mg/d. A similar pattern of findings was noted in the qualitative urinalysis results. The doses of 8 mg daily and 16 mg daily were associated with statistically significant decreases in the odds of opiate-positive urine samples during the maintenance phase (P = .0029 for 8 mg and P = .0003 for 16 mg), but only the dose of 8 mg daily was associated with a statistically significant increase in the odds of opiate-positive urine samples during the withdrawal phase (P = .011) (Table III and Fig 3). Further regression analysis to assess main effects of medication dose and study phase on quantitative urinalysis (log-transformed urine morphine levels) showed the effect of dose as a trend toward statistical significance (F3,175 = 2.62, P = .052), whereas the effect of phase was nonsignificant (F1,89 = 1.17, P = .28).
Fig 2.
Results of quantitative urine assays by medication group among 179 cocaine- and opiate-dependent outpatients participating for at least 5 days. Subjects received assigned dose of sublingual buprenorphine for 10 weeks (maintenance phase) and then doses tapering to 0 over a 3-week period (withdrawal phase). Sample size varied by study week; see Fig 1 to derive number of subjects included at each study week. Vertical bars represent SD (where bars are absent, SD is less than height of symbol). Top, Opiate use measured as mean of log morphine concentration over 3 urine specimens collected each week. Bottom, Cocaine use measured as mean of log benzoylecgonine (BZE) concentration over 3 urine specimens collected each week.
Table II.
Effect of buprenorphine dosing on urine drug metabolite levels in 179 opiate- and cocaine-dependent outpatients
| 16 mg daily | 16 mg every other day | 8 mg daily | 2 mg daily | |||||
|---|---|---|---|---|---|---|---|---|
| Urine log BZE | ||||||||
| Baseline (mean, SEM)* | 3.57 (0.14) | 3.67 (0.17) | 3.83 (0.14) | 3.69 (0.16) | ||||
| Maintenance (mean, SEM) | 3.31 (0.19) | 3.50 (0.16) | 3.44 (0.19) | 3.52 (0.16) | ||||
| Withdrawal (mean, SEM) | 2.27 (0.30) | 3.38 (0.24) | 2.83 (0.33) | 3.22 (0.28) | ||||
| Daily change in urine log BZE | Unadjusted† | Adjusted for urine morphine | Unadjusted | Adjusted for urine morphine | Unadjusted | Adjusted for urine morphine | Unadjusted | Adjusted for urine morphine |
| Maintenance (mean, 95% CI) | −0.014‡ (−0.025, −0.0041) |
−0.010‡ (−0.020, −0.0006) |
−0.0040 (−0.013, 0.0049) |
−0.0015 (−0.0087, 0.0056) |
−0.011‡ (−0.021, −0.0013) |
−0.0083 (−0.017, 0.00060) |
−0.0077 (−0.016, 0.0083) |
0.0068 (−0.015, 0.0014) |
| Withdrawal (mean, 95% CI) | −0.011 (−0.050, 0.028) |
−0.023 (−0.059, 0.014) |
0.012 (−0.021, 0.045) |
−0.00089 (−0.29, 0.027) |
0.0040 (−0.037, 0.045) |
−0.019 (−0.056, 0.018) |
0.023‡ (−0.0094, 0.055) |
0.0065 (−0.024, 0.037) |
| Urine log morphine | ||||||||
| Baseline (mean, SEM) | 3.52 (0.16) | 3.37 (0.16) | 3.59 (0.13) | 3.53 (0.19) | ||||
| Maintenance (mean, SEM) | 2.89 (0.14) | 2.89 (0.17) | 2.85 (0.17) | 3.15 (0.16) | ||||
| Withdrawal (mean, SEM) | 2.42 (0.24) | 2.86 (0.24) | 2.82 (0.29) | 3.25 (0.23) | ||||
| Daily change in urine log morphine | Unadjusted | Adjusted for urine BZE | Unadjusted | Adjusted for urine BZE | Unadjusted | Adjusted for urine BZE | Unadjusted | Adjusted for urine BZE |
| Maintenance (mean, 95% CI) | −0.015‡ (−0.023, −0.0075) |
−0.0095‡ (−0.016, −0.0025) |
−0.0036 (−0.013, 0.0053) |
−0.0015 (−0.0091, 0.0061) |
−0.011‡ (−0.020, 0.0024) |
−0.0059 (−0.014, 0.0018) |
−0.0035 (−0.012, 0.0052) |
0.00033 (−0.0077, 0.0084) |
| Withdrawal (mean, 95% CI) | 0.024 (−0.0091, 0.058) |
0.033‡ (0.0019, 0.063) |
0.036‡ (0.0030, 0.070) |
0.028 (−0.00081, 0.057) |
0.063‡ (0.022, 0.10) |
0.062‡ (0.026, 0.097) |
0.043‡ (0.0092, 0.078) |
0.032‡ (0.0064, 0.064) |
BZE, Benzoylecgonine; CI, confidence interval.
Baseline phase, days 1 to 4 (dose increased to target); maintenance phase, days 5 to 70 (target dose administered); withdrawal phase, days 71 to 91 (target dose tapered to zero).
Unadjusted parameters are interpreted as the change in outcome variable (eg, log BZE) per study day. A minus sign indicates decrease; all other values showed an increase. Adjusted parameter estimates have a similar interpretation and reflect the change in outcome variable per study day, with levels of the adjusting variable held constant.
Parameter significantly different from 0 (P < .05).
Table III.
Effect of buprenorphine dosing on drug-positive urinalysis in 179 opiate- and cocaine-dependent outpatients
| 16 mg daily | 16 mg every other day | 8 mg daily | 2 mg daily | |||||
|---|---|---|---|---|---|---|---|---|
| Cocaine-positive (%) | ||||||||
| Baseline (mean, SEM)* | 70.5 (5.82) | 60.5 (5.74) | 70.2 (5.48) | 70.5 (5.87) | ||||
| Maintenance (mean, SEM) | 60.0 (5.57) | 66.4 (5.26) | 63.7 (5.72) | 69.5 (4.97) | ||||
| Withdrawal (mean, SEM) | 33.4 (7.87) | 67.0 (7.39) | 55.0 (9.38) | 63.4 (8.30) | ||||
| Daily change in log OR cocaine-positive† | Unadjusted‡ | Adjusted for urine opiate-positive† | Unadjusted | Adjusted for urine opiate-positive | Unadjusted | Adjusted for urine opiate-positive | Unadjusted | Adjusted for urine opiate-positive |
| Maintenance (mean, 95% CI) | −0.027§ (−0.040, −0.013) |
−0.023§ (−0.037, −0.010) |
−0.0051 (−0.019, 0.0084) |
−0.0028 (−0.015, 0.0098) |
−0.009 (−0.023, 0.0052) |
−0.0056 (−0.019, 0.0079) |
−0.0083 (−0.022, 0.0056) |
0.075 (−0.021, 0.0065) |
| Withdrawal (mean, 95% CI) | 0.020 (−0.038, 0.077) |
0.0089 (−0.050, 0.066) |
0.028 (−0.028, 0.083) |
0.022 (−0.035, 0.078) |
−0.0013 (−0.061, 0.058) |
−0.023 (−0.082, 0.036) |
0.019 (−0.036, 0.075) |
0.0037 (−0.052, 0.060) |
| Opiate-positive (%) | ||||||||
| Baseline (mean, SEM) | 67.5 (6.21) | 56.6 (6.05) | 70.2 (4.95) | 67.4 (5.65) | ||||
| Maintenance (mean, SEM) | 52.2 (5.23) | 52.0 (5.51) | 52.0 (5.49) | 62.1 (5.00) | ||||
| Withdrawal (mean, SEM) | 38.5 (8.12) | 56.2 (7.61) | 49.3 (9.10) | 64.9 (8.69) | ||||
| Daily change in urine log OR opiate-positive | Unadjusted | Adjusted for urine cocaine | Unadjusted | Adjusted for urine cocaine | Unadjusted | Adjusted for urine cocaine-positive | Unadjusted | Adjusted for urine cocaine positive |
| Maintenance (mean, 95% CI) | −0.024§ (−0.036, −0.011) |
−0.018§ (−0.031, −0.0053) |
−0.0015 (−0.014, 0.011) |
0.00052 (−0.011, 0.012) |
−0.020§ (−0.032, −0.0067) |
−0.019§ (−0.031, −0.0064) |
−0.026§ (−0.015, 0.097) |
−0.00079§ (−0.013, 0.012) |
| Withdrawal (mean, 95% CI) | 0.051 (−0.0046, 0.11) |
0.060§ (0.0022, 0.12) | 0.043 (−0.0094, 0.095) |
0.035 (−0.016, 0.087) |
0.083§ (0.019, 0.15) | 0.099§ (0.035, 0.16) | 0.054 (−0.0051, 0.11) |
0.058 (−0.0043, 0.12) |
OR, Odds ratio.
Baseline phase, days 1 to 4 (dose increased to target); maintenance phase, days 5 to 70 (target dose administered); withdrawal phase, days 71 to 91 (target dose tapered to zero).
Adjusted parameter estimates reflect the change in outcome variable with levels of the adjusting variable held constant.
In logistic regression, regression coefficients are log ORs. The ORs in this analysis are odds of drug-positive urine on each study day divided by the odds of drug-positive urine on the previous day. For log OR a minus sign indicates a decrease (decreasing urine-positive results over time); all other values showed an increase (increasing urine-positive results over time).
Parameter significantly different from 0 (P < .05).
Fig 3.
Results of qualitative urine assays by medication group among 179 cocaine- and opiate-dependent outpatients participating for at least 5 days. Subjects received assigned dose of sublingual buprenorphine for 10 weeks (maintenance phase) and then doses tapering to 0 over a 3-week period (withdrawal phase). Sample size varied by study week; see Fig 1 to derive number of subjects included at each study week. Top, Opiate use measured as proportion of urine specimens having morphine concentration at least 300 ng/mL. Bottom, Cocaine use measured as proportion of urine specimens having benzoylecgonine concentration at least 300 ng/mL.
Opiate use was associated with cocaine use during the study; urine morphine concentrations were more highly correlated with urine benzoylecgonine concentrations at the same visit (t = 23.0, P < .001) than with those at the previous visit (t = 1.32, P = .19) when both terms were included in a repeated-measures linear regression model. Adding concurrent urine benzoylecgonine concentration as a time-varying covariate to the piecewise linear regressions described earlier yielded similar conclusions regarding the relationship between buprenorphine dose and urine morphine changes over time. After adjustment for urine benzoylecgonine, only the group receiving 16 mg daily showed a significant decline in urine morphine concentration during the maintenance phase (P = .0079). When opiate-positive urine test results were used as the outcome and cocaine-positive urine test results were used as a covariate, both 8 mg daily and 16 mg daily showed statistically significant decreases during the maintenance phase (P = .0031 for 8 mg and P = .0059 for 16 mg), with statistically significant increases during the withdrawal phase (P = .0024 for 8 mg and P = .042 for 16 mg) (Table III).
Cocaine
The buprenorphine doses of 8 mg daily and 16 mg daily were associated with statistically significant decreases in urine benzoylecgonine concentrations during the maintenance phase (P = .028 for 8 mg and P = .006 for 16 mg), with no significant changes in the other groups (P = .077 for 2 mg daily and P = .37 for 16 mg every other day) (Fig 2, Table II). Urine benzoylecgonine concentrations did not increase significantly during the withdrawal phase (P = .16 for 2 mg daily, P = .85 for 8 mg daily, P = .48 for 16 mg every other day, and P = .59 for 16 mg daily) (Table II), suggesting that beneficial effects on cocaine use may be maintained during buprenorphine withdrawal. When cocaine-positive urine samples were used as outcome, only 16 mg daily showed significant decreases during the maintenance phase (P < .001); no significant changes during the withdrawal phase were noted (Fig 3, Table III). Further regression analysis to assess the main effects of medication dose and study phase on quantitative urinalysis (log-transformed urine benzoylecgonine concentrations) showed statistically significant main effects of both dose (F3, 175 = 2.91, P = .036) and phase (F1, 89 = 5.83, P = .018).
As was true for urine morphine concentrations, urine benzoylecgonine concentrations were more highly correlated with urine morphine concentrations at the same visit (t = 22.0, P < .001) than at the previous visit (t = −0.06, P = .95) when both were included in the repeated-measures regression model. Adding concurrent urine morphine concentration as a time-varying covariate to the piecewise linear regressions did not change the conclusions, with one exception: The downward trend in urine benzoylecgonine during the maintenance phase for the group receiving 8 mg daily was no longer statistically significant after adjustment for urine morphine (P = .068) (Table II). Adding opiate-positive urine as a time-varying covariate did not alter the results when qualitative cocaine tests were used as outcome: Only 16 mg daily was associated with significant declines in cocaine-positive urine samples during the maintenance phase (P < .001), and there were no significant changes in cocaine-positive urine samples during the withdrawal phase (Table III).
Concomitant opiates and cocaine
There were similar findings when urine benzoylecgonine and morphine levels were examined jointly (multivariate linear mixed regression models). The group receiving 16 mg daily showed a significant decrease over time (slope = −0.013, SE = 0.0048, P = .0089), whereas the group receiving 8 mg daily showed a trend toward a decrease (slope = −0.0093, SE = 0.0053, P = .079). The other 2 groups showed no significant decreases in metabolite levels over time (2 mg daily: slope = −0.0026, SE = 0.0049, P = .59; 16 mg every other day: slope = −0.0016, SE = 0.0045, P = .72).
Adverse events
In 76 subjects (42.5% of 179) a total of 137 adverse events were reported, as follows: opiate withdrawal (n = 13), constipation (n = 9), gastrointestinal other than constipation (n = 13), genitourinary (n = 5), ear, nose, and throat or respiratory (n = 34), musculoskeletal (n = 22), headache (n = 14), dermal (n = 8), dental (n = 11), and other (n = 8). There were no apparent idiosyncratic or allergic reactions to study medication. No adverse event required breaking the medication blind.
The group receiving 16 mg every other day showed a trend toward a higher incidence density of adverse events (47 events in 383 person-weeks = 0.123 events/person-week) than was seen for the other 3 medication groups (2 mg daily, 0.083 events/person-week; 8 mg daily, 0.081 events/person-week; and 16 mg daily, 0.083 events/person-week) (z = 1.59, P = .055). Much of this difference came from an increased incidence of gastrointestinal events other than constipation (incidence density, 0.0261 versus 0.0028 in the group receiving 2 mg daily [P = .025 by exact binomial test]). There were no other significant medication group differences in incidence of adverse events. There was no significant association between adverse event incidence and subjects’ age or HIV serostatus.
It was often not possible to accurately determine whether adverse events were related to study medication. Many manifestations of opiate agonist action (eg, headache, constipation) or opiate withdrawal (eg, diarrhea, rhinorrhea, muscle aches) resemble signs and symptoms associated with common intercurrent illnesses (eg, viral syndromes). Regardless of etiology, most adverse events were of mild severity: 26.3% resulted in no action being taken. Only 2 subjects (both receiving 16 mg daily) had a serious adverse event (resulting in hospitalization). Neither was considered to be study-related.
Six subjects had their medication dose lowered or held because of adverse events. Two subjects had their dose lowered, 1 in the group receiving 8 mg daily (sore throat) and 1 in the group receiving 16 mg daily (wheezing). Four subjects had their dose held for at least 1 day: 1 in the group receiving 2 mg daily (upper respiratory infection) and 3 in the group receiving 16 mg daily (constipation, headache, back and neck pain).
Concomitant benzodiazepine use during buprenorphine treatment has been claimed to be associated with serious adverse events.37 In this study qualitative urine toxicology assays for benzodiazepines performed on 3-times-weekly urine specimens showed that 39 of 179 study participants (21.8%) had positive test results for benzodiazepines at some point during the 13-week study period. The proportion of participants who used benzodiazepines was comparable for the 4 treatment groups (χ2 = 0.19, df = 3, P = .98).
Laboratory tests
None of the clinical laboratory parameters (complete blood cell count, electrolyte levels, kidney and liver function) showed any consistent or clinically significant pattern of abnormalities that might be associated with the study medication.
Of subjects, 38% (46 of 122 with available transaminase data) had serum transaminase levels (either ALT or AST [or both]) elevated above normal (ALT, >40 IU/L; AST, >37 IU/L) during the maintenance phase. Only 10 of these subjects had elevations greater than twice normal. There was no significant difference in frequency of elevated transaminase levels during the maintenance phase among the 4 medication groups (7 in the group receiving 2 mg daily, 17 in the group receiving 8 mg daily, 13 in the group receiving 16 mg every other day, and 9 in the 16 mg daily group; χ2 = 7.23, df = 3, P = .065). Three subjects had elevated plasma bilirubin concentrations during the maintenance phase; none of them had accompanying symptoms or elevations in ALT or AST levels. More than half (29/46 [63%]) of subjects having elevated serum transaminase levels during maintenance also had elevated ALT or AST levels at baseline, suggesting that mild transaminase abnormalities preceded buprenorphine exposure in some subjects.
DISCUSSION
This study found sublingual buprenorphine solution, in combination with weekly individual drug abuse counseling, to be effective and well tolerated at higher doses for the maintenance treatment of outpatients with concurrent dependence on opiates and cocaine. Daily doses of 8 and 16 mg showed reductions in opiate and cocaine use individually, and a daily dose of 16 mg showed concomitant reductions in both opiate and cocaine use. This is the first study to demonstrate the efficacy of buprenorphine in reducing cocaine use among opiate-dependent subjects also meeting criteria for cocaine dependence in which a double-blind, controlled clinical trial design and strict eligibility criteria were used. The highest dose tested (16 mg daily) showed significant reductions in cocaine use after adjustment for concurrent opiate use. These reductions were maintained through the withdrawal phase of the study. Our results support the findings of several prior clinical trials suggesting that buprenorphine might be effective in reducing cocaine use among opiate-dependent outpatients who used or abused cocaine.20,21,38
Previous studies testing the use of buprenorphine for treatment of cocaine dependence have shown contrasting results. These contrasting results may result from differences in subject characteristics that may affect treatment outcome (eg, differences in cocaine use or in comorbid psychiatric disorders) or differences in study methods. The reported clinical trials that found buprenorphine treatment to be associated with reductions in cocaine use19-21,39 used higher buprenorphine doses (8−12 mg daily) than the trials that found no evidence of efficacy.18,22,40 The apparent persistence of the reduction in cocaine use during the withdrawal phase raises the possibility that high-dose buprenorphine engendered some change relevant to cocaine addiction that may persist even after medication intake is decreased or stopped. However, the relatively small number of urine toxicology tests obtained during the withdrawal phase precludes any strong conclusions regarding the persistent effects of the maintenance dose. Further studies are needed to determine the optimal doses of buprenorphine and schedule of buprenorphine withdrawal, with evaluation of withdrawal periods longer than 3 weeks.
The lowest dose of buprenorphine (2 mg daily) was not associated with reductions in opiate use, which fails to confirm an earlier suggestion that this low dose might be effective for the treatment of opiate dependence.5 The lack of significant effect from 16 mg every other day is consistent with a recent controlled trial comparing daily (8 mg) versus 3-times-weekly buprenorphine41 but fails to confirm earlier studies of smaller samples that indicated every-other-day dosing could reduce opiate use.6,42 In this study every-other-day dosing was not coupled with reduced clinic attendance requirements, which ensured comparable clinic contact by all groups. Reduced clinic attendance requirements in prior studies could have improved treatment compliance and outcome.
The beneficial effect of 8 mg daily buprenorphine on opiate use observed during maintenance treatment (days 5−70) faded as the buprenorphine dose was reduced (withdrawal phase, days 71−91), although opiate use for the group receiving 16 mg daily and cocaine use for all medication groups (as measured by urine toxicology studies) remained stable during medication withdrawal. These findings indicate that 9 weeks of lower-dose agonist maintenance treatment is not sufficient to substantially alter the course of opiate dependence, a pattern similar to that observed in studies of methadone maintenance treatment.43 As noted for cocaine use, the small number of urine toxicology tests performed during the withdrawal phase precludes any strong conclusions about the persistent effects of the maintenance dose.
Buprenorphine was well tolerated at all doses. Although almost half (40.2%) of the subjects reported adverse events, only 2 were clinically serious and these were not medication-related. Most adverse events appeared to be related to concurrent illnesses common in this population or to opiate withdrawal or other discomforts related to drug use rather than to direct side effects of the medication.
HIV infection, which is common among injecting drug users, was less common in our population, most likely representing a sampling bias among subjects interested in research participation. HIV infection did not appear to be associated with the occurrence of adverse events. This study did not address the interaction of buprenorphine with HAART (highly active antiretroviral therapy) because, at the time the study was conducted, this therapy was not available. The lack of immunologic or virologic analysis in our study precludes drawing firm conclusions about the effects of buprenorphine treatment on the health of this special population. Nonetheless, these findings are consistent with a previous report from a small study sample.44 The safety of buprenorphine in this population was confirmed in a recent study that showed no significant influence of buprenorphine maintenance treatment on CD4 counts or HIV viral load in 20 HIV-infected subjects receiving highly active antiretroviral treatment.45
The buprenorphine in this study, as in almost all previously published US studies, was administered as a sublingual alcoholic solution. The buprenorphine formulations approved by the Food and Drug Administration for opiate dependence treatment are 2-mg and 8-mg tablets to be placed under the tongue for dissolution. They are available as buprenorphine alone and as a combination product with naloxone in a 4:1 ratio to discourage intravenous misuse. The tablet may have up to 50% less bioavailability than the solution, resulting in significantly lower mean and peak plasma buprenorphine concentrations.46-49 This raises the possibility that doses of the buprenorphine tablet may have to be higher than the 8 or 16 mg daily of buprenorphine solution used in this study to achieve comparable effects on cocaine use.
One limitation of this study is the high dropout rate, with fewer than half of the subjects remaining by the end of the maintenance phase (week 10) (Fig 1). Comparably high dropout rates have been reported in other published clinical trials of buprenorphine treatment.18,21,22,50-52 Early dropout may have been a consequence of the intensive and lengthy nature of treatment monitoring during this study, which may have discouraged some subjects from remaining in treatment. However, this level of monitoring was necessary to ensure the validity of the study results and to adequately assess subject safety. The similar dropout rates in all 4 medication groups support valid between-group comparisons, although the high overall dropout rate may limit the external validity (generalizability) of the findings. Another potential limitation is the use of drug metabolite levels that were not adjusted for urine creatinine concentration; the amount of metabolite in urine depends not only on recent drug intake but also on recent fluid intake. A further limitation of this study is that it was conducted in a treatment research environment that may differ from “real-life” drug abuse treatment settings.53 However, subjects did not receive any special inducements or compensation for treatment compliance or improvement.
The strengths of this study include its large sample size, the use of prospective stratification by age and gender, and the use of quantitative urine results for cocaine and opiates as outcome measures. In addition, data analytic methods that used all data points from all subjects were used, thus reducing the potential for bias from missing data.
In summary, this study demonstrated for the first time in a well-controlled, prospectively stratified clinical trial that high-dose (16 mg daily sublingually) buprenorphine maintenance treatment is well tolerated and can significantly reduce cocaine use among dually (cocaine and opiate) dependent outpatients, while confirming its previously demonstrated efficacy in reducing opiate use. The good tolerability of buprenorphine, even in HIV-infected subjects, suggests that it would be a very useful therapy with which to address the dual epidemic of cocaine and opiate addiction. The recent approval of buprenorphine in the United States for maintenance treatment of opiate dependence, along with its availability for prescribing by office-based physicians rather than in tightly regulated clinics, offers promise that it will be increasingly available to a large number of patients who could benefit. Further studies are needed to clarify the optimal dose of buprenorphine for treatment of dual dependency, as well as to identify prognostic variables that will allow optimal patient-treatment matching.
Acknowledgments
Study funded by National Institute on Drug Abuse Intramural Research Program.
Footnotes
Preliminary results from this study were presented at the College on Problems of Drug Dependence Annual Meeting, Scottsdale, Ariz, June 15, 1995, and the American Society of Addiction Medicine Annual Meeting, New York, NY, April 30, 1999.
Dr Johnson is currently employed by Reckitt Benckiser Pharmaceuticals, Richmond, Va, the manufacturer of buprenorphine. None of the other authors have any financial or personal relationships that could potentially be perceived as influencing the research.
References
- 1.Ohtani M, Kotaki H, Sawada Y, Iga T. Comparative analysis of buprenorphine- and norbuprenorphine-induced analgesic effects based on pharmacokinetic-pharmacodynamic modeling. J Pharmacol Exp Ther. 1995;272:505–10. [PubMed] [Google Scholar]
- 2.FDA Talk Paper, T02−38. Subutex and Suboxone approved to treat opiate dependence. Food and Drug Administration (US); Rockville (MD): 2002. Available from: URL: www.fda.gov/bbs/topics/ANSWERS/2002/ANS01165.html. [Google Scholar]
- 3.Johnson RE, Jaffe JH, Fudala PJ. A controlled trial of buprenorphine treatment for opioid dependence. JAMA. 1992;267:2750–5. [PubMed] [Google Scholar]
- 4.Schottenfeld RS, Pakes JR, Oliveto A, Ziedonis D, Kosten TR. Buprenorphine vs methadone maintenance treatment for concurrent opioid dependence and cocaine abuse. Arch Gen Psychiatry. 1997;54:713–20. doi: 10.1001/archpsyc.1997.01830200041006. [DOI] [PubMed] [Google Scholar]
- 5.Kosten TR, Schottenfeld R, Ziedonis D, Falcioni J. Buprenorphine versus methadone maintenance for opioid dependence. J Nerv Ment Dis. 1993;181:358–64. doi: 10.1097/00005053-199306000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Amass L, Kamien JB, Mikulich SK. Efficacy of daily and alternate-day dosing regimens with the combination buprenorphine-naloxone tablet. Drug Alcohol Depend. 2000;58:143–52. doi: 10.1016/s0376-8716(99)00074-5. [DOI] [PubMed] [Google Scholar]
- 7.Eissenberg T, Johnson RE, Bigelow GE, Walsh SL, Liebson IA, Strain EC, et al. Controlled opioid withdrawal evaluation during 72 h dose omission in buprenorphine-maintained patients. Drug Alcohol Depend. 1997;45:81–91. doi: 10.1016/s0376-8716(97)01347-1. [DOI] [PubMed] [Google Scholar]
- 8.Johnson RE, Eissenberg T, Stitzer ML, Strain EC, Liebson IA, Bigelow GE. Buprenorphine treatment of opioid dependence: clinical trial of daily versus alternate-day dosing. Drug Alcohol Depend. 1995;40:27–35. doi: 10.1016/0376-8716(95)01189-7. [DOI] [PubMed] [Google Scholar]
- 9.O'Connor PG, Oliveto AH, Shi JM, Triffleman EG, Carroll KM, Kosten TR, et al. A randomized trial of buprenorphine maintenance for heroin dependence in a primary care clinic for substance users versus a metha-done clinic. Am J Med. 1998;105:100–5. doi: 10.1016/s0002-9343(98)00194-6. [DOI] [PubMed] [Google Scholar]
- 10.Leri F, Bruneau J, Stewart J. Understanding polydrug use: review of heroin and cocaine co-use. Addiction. 2003;98:7–22. doi: 10.1046/j.1360-0443.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- 11.Mello NK, Mendelson JH, Bree MP, Lukas SE. Buprenorphine suppresses cocaine self-administration by rhesus monkeys. Science. 1989;245:859–62. doi: 10.1126/science.2772637. [DOI] [PubMed] [Google Scholar]
- 12.Mello NK, Lukas SE, Kamien JB, Mendelson JH, Drieze J, Cone EJ. The effects of chronic buprenorphine treatment on cocaine and food self-administration by rhesus monkeys. J Pharmacol Exp Ther. 1992;260:1185–93. [PubMed] [Google Scholar]
- 13.Carroll ME, Lac ST. Effects of buprenorphine on self-administration of cocaine and a nondrug reinforcer in rats. Psychopharmacology. 1992;106:439–46. doi: 10.1007/BF02244812. [DOI] [PubMed] [Google Scholar]
- 14.Mello NK, Negus SS. The effects of buprenorphine on self-administration of cocaine and heroin “speedball” combinations and heroin alone by rhesus monkeys. J Pharmacol Exp Ther. 1998;285:444–56. [PubMed] [Google Scholar]
- 15.Mendelson JH, Teoh SK, Mello NK, Ellingboe J. Buprenorphine attenuates the effects of cocaine on adrenocorticotropin (ACTH) secretion and mood states in man. Neuropsychopharmacology. 1992;7:157–62. [PubMed] [Google Scholar]
- 16.Kouri EM, Lukas SE, Mendelson JH. P300 assessment of opiate and cocaine users: effects of detoxification and buprenorphine treatment. Biol Psychiatry. 1996;40:617–28. doi: 10.1016/0006-3223(95)00468-8. [DOI] [PubMed] [Google Scholar]
- 17.Foltin RW, Fischman MW. Effects of methadone or buprenorphine maintenance on the subjective and reinforcing effects of intravenous cocaine in humans. J Pharmacol Exp Ther. 1996;278:1153–64. [PubMed] [Google Scholar]
- 18.Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Buprenorphine versus methadone in the treatment of opioid-dependent cocaine users. Psychopharmacology (Berl) 1994;116:401–6. doi: 10.1007/BF02247469. [DOI] [PubMed] [Google Scholar]
- 19.Kosten TR, Kleber HD, Morgan C. Role of opioid antagonists in treating intravenous cocaine abuse. Life Sci. 1989;44:887–92. doi: 10.1016/0024-3205(89)90589-4. [DOI] [PubMed] [Google Scholar]
- 20.Kosten TR, Kleber HD, Morgan C. Treatment of cocaine abuse with buprenorphine. Biol Psychiatry. 1989;26:637–9. doi: 10.1016/0006-3223(89)90090-5. [DOI] [PubMed] [Google Scholar]
- 21.Schottenfeld RS, Pakes J, Ziedonis D, Kosten TR. Buprenorphine: dose-related effects on cocaine and opioid use in cocaine-abusing opioid-dependent humans. Biol Psychiatry. 1993;34:66–74. doi: 10.1016/0006-3223(93)90258-f. [DOI] [PubMed] [Google Scholar]
- 22.Schottenfeld RS, Pakes JR, Oliveto A, Ziedonis D, Kosten TR. Buprenorphine vs methadone maintenance treatment for concurrent opioid dependence and cocaine abuse. Arch Gen Psychiatry. 1997;54:713–20. doi: 10.1001/archpsyc.1997.01830200041006. [DOI] [PubMed] [Google Scholar]
- 23.Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates; Hillsdale (NJ): 2002. [Google Scholar]
- 24.Babst DV, Chambers CD, Warner A. Patient characteristics associated with retention in a methadone maintenance program. Br J Addict Alcohol Other Drugs. 1971;66:195–204. doi: 10.1111/j.1360-0443.1971.tb02386.x. [DOI] [PubMed] [Google Scholar]
- 25.Brown BS, DuPont RL, Bass UF, III, Brewster GW, Glendinning ST, Kozel NJ, et al. Impact of a large-scale narcotics treatment program: a six month experience. Int J Addict. 1973;8:49–57. doi: 10.3109/10826087309048763. [DOI] [PubMed] [Google Scholar]
- 26.Hser YI, Anglin MD, Liu Y. A survival analysis of gender and ethnic differences in responsiveness to methadone maintenance treatment. Int J Addict. 1990;25:1295–315. doi: 10.3109/10826089009068465. [DOI] [PubMed] [Google Scholar]
- 27.McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Robins LN, Helzer JE, Ratcliff KS, Seyfried W. Validity of the diagnostic interview schedule, version II: DSM-III diagnoses. Psychol Med. 1982;12:855–70. doi: 10.1017/s0033291700049151. [DOI] [PubMed] [Google Scholar]
- 29.Derogatis LR, Cleary PA. Factorial invariance across gender for the primary symptom dimensions of the SCL-90. Br J Soc Clin Psychol. 1977;16:347–56. doi: 10.1111/j.2044-8260.1977.tb00241.x. [DOI] [PubMed] [Google Scholar]
- 30.Rounsaville BJ, Gawin F, Kleber H. Interpersonal psychotherapy adapted for ambulatory cocaine abusers. Am J Drug Alcohol Abuse. 1985;11:171–91. doi: 10.3109/00952998509016860. [DOI] [PubMed] [Google Scholar]
- 31.Nich C, Carroll K. Now you see it, now you don't: a comparison of traditional versus random-effects regression models in the analysis of longitudinal follow-up data from a clinical trial. J Consult Clin Psychol. 1997;65:252–61. doi: 10.1037//0022-006x.65.2.252. [DOI] [PubMed] [Google Scholar]
- 32.Mallinckrodt CH, Clark WS, David SR. Accounting for dropout bias using mixed-effects models. J Biopharm Stat. 2001;11:9–21. doi: 10.1081/BIP-100104194. [DOI] [PubMed] [Google Scholar]
- 33.Naumova EN, Must A, Laird NM. Tutorial in biostatistics: evaluating the impact of ‘critical periods’ in longitudinal studies of growth using piecewise mixed effects models. Int J Epidemiol. 2001;30:1332–41. doi: 10.1093/ije/30.6.1332. [DOI] [PubMed] [Google Scholar]
- 34.Diggle PJ, Liang KY, Zeger SL. Analysis of longitudinal data. Clarendon; Oxford (United Kingdom): 1994. [Google Scholar]
- 35.Delucchi KL, Jones RT, Batki SL. Measurement properties of quantitative urine benzoylecgonine in clinical trials research. Addiction. 1997;92:297–302. [PubMed] [Google Scholar]
- 36.Batki SL, Manfredi LB, Jacob P, III, Jones RT. Fluoxetine for cocaine dependence in methadone maintenance: quantitative plasma and urine cocaine/benzoylecgonine concentrations. J Clin Psychopharmacol. 1993;13:243–50. [PubMed] [Google Scholar]
- 37.Auriacombe M, Franques P, Tignol J. Deaths attributable to methadone vs buprenorphine in France [letter]. JAMA. 2001;285:45. doi: 10.1001/jama.285.1.45. [DOI] [PubMed] [Google Scholar]
- 38.Kosten TR, Kleber HD, Morgan C. Role of opioid antagonists in treating intravenous cocaine abuse. Life Sci. 1989;44:887–92. doi: 10.1016/0024-3205(89)90589-4. [DOI] [PubMed] [Google Scholar]
- 39.Oliveto AH, Feingold A, Schottenfeld R, Jatlow P, Kosten TR. Desipramine in opioid-dependent cocaine abusers maintained on buprenorphine vs methadone. Arch Gen Psychiatry. 2001;56:812–20. doi: 10.1001/archpsyc.56.9.812. [DOI] [PubMed] [Google Scholar]
- 40.Oliveto A, Kosten T, Schottenfeld R, Ziedonis D. Cocaine abuse among methadone-maintained patients [letter]. Am J Psychiatry. 1993;150:1755. doi: 10.1176/ajp.150.11.1755a. [DOI] [PubMed] [Google Scholar]
- 41.Perez de los Cobos J, Martin S, Etcheberrigaray A, Trujols J, Batlle F, Tejero A, et al. A controlled trial of daily versus thrice-weekly buprenorphine administration for the treatment of opioid dependence. Drug Alcohol Depend. 2000;59:223–33. doi: 10.1016/s0376-8716(99)00122-2. [DOI] [PubMed] [Google Scholar]
- 42.Amass L, Bickel WK, Crean JP, Blake J, Higgins ST. Alternate-day buprenorphine dosing is preferred to daily dosing by opioid-dependent humans. Psychopharmacology (Berl) 1998;136:217–25. doi: 10.1007/s002130050559. [DOI] [PubMed] [Google Scholar]
- 43.Magura S, Rosenblum A. Leaving methadone treatment: lessons learned, lessons forgotten, lessons ignored. Mt Sinai J Med. 2001;68:62–74. [PubMed] [Google Scholar]
- 44.Montoya ID, Umbricht A, Preston KL. Buprenorphine for human immunovirus-positive opiate-dependent patients [letter]. Biol Psychiatry. 1995;38:135–6. doi: 10.1016/0006-3223(95)00071-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carrieri MP, Vlahov D, Dellamonica P, Gallais H, Lepeu G, Spire B, et al. Use of buprenorphine in HIV-infected injection drug users: negligible impact on virologic response to HAART. The Manif-2000 Study Group. Drug Alcohol Depend. 2000;60:51–4. doi: 10.1016/s0376-8716(99)00136-2. [DOI] [PubMed] [Google Scholar]
- 46.Chawarski MC, Schottenfeld RS, O'Connor PG, Pakes J. Plasma concentrations of buprenorphine 24 to 72 hours after dosing. Drug Alcohol Depend. 1999;55:157–63. doi: 10.1016/s0376-8716(98)00192-6. [DOI] [PubMed] [Google Scholar]
- 47.Mendelson J, Jones RT, Fernandez I, Welm S, Melby AK, Baggott MJ. Buprenorphine and naloxone interactions in opiate-dependent volunteers. Clin Pharmacol Ther. 1996;60:105–14. doi: 10.1016/S0009-9236(96)90173-3. [DOI] [PubMed] [Google Scholar]
- 48.Nath RP, Upton RA, Everhart ET, Cheung P, Shwonek P, Jones RT, et al. Buprenorphine pharmacokinetics: relative bioavailability of sublingual tablet and liquid formulations. J Clin Pharmacol. 1999;39:619–23. doi: 10.1177/00912709922008236. [DOI] [PubMed] [Google Scholar]
- 49.Schuh KJ, Johanson CE. Pharmacokinetic comparison of the buprenorphine sublingual liquid and tablet. Drug Alcohol Depend. 1999;56:55–60. doi: 10.1016/s0376-8716(99)00012-5. [DOI] [PubMed] [Google Scholar]
- 50.Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343:1290–7. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- 51.Ling W, Charuvastra C, Collins JF, Batki S, Brown LS, Jr, Kintaudi P, et al. Buprenorphine maintenance treatment of opiate dependence: a multicenter, randomized clinical trial. Addiction. 1998;93:475–86. doi: 10.1046/j.1360-0443.1998.9344753.x. [DOI] [PubMed] [Google Scholar]
- 52.Petitjean S, Stohler R, Deglon JJ, Livoti S, Waldvogel D, Uehlinger C, et al. Double-blind randomized trial of buprenorphine and methadone in opiate dependence. Drug Alcohol Depend. 2001;62:97–104. doi: 10.1016/s0376-8716(00)00163-0. [DOI] [PubMed] [Google Scholar]
- 53.Lamb SJ, Greenlick MR, McCarty D. Bridging the gap between practice and research: forging partnerships with community-based drug and alcohol treatment. National Academy Press; Washington: 1998. [PubMed] [Google Scholar]