Abstract
Cefepime is a ‘fourth-generation’ cephalosporin with an in vitro extended-spectrum of activity against Gram-negative and Gram-positive pathogens. Cefepime is approved for the treatment of moderate-to-severe infections, such as pneumonia, uncomplicated and complicated urinary tract infections, skin and soft-tissue infections, intra-abdominal infections and febrile neutropenia. In this article, we provide a critical review of pharmacodynamics, clinical management, pharmacokinetics, metabolism, pharmacodynamic target analyses, clinical efficacy, safety and tolerability of cefepime after more than a decade of clinical use.
Keywords: cephalosporin, ESBL, extended-spectrum β-lactamase, susceptibility
Cefepime is a ‘fourth-generation’ cephalosporin with an in vitro extended-spectrum of activity against Gram-negative and Gram-positive pathogens. Cefepime was introduced into clinical practice in 1994 and is approved for the treatment of moderate-to-severe infections, such as pneumonia, uncomplicated and complicated urinary tract infections (UTIs), skin and soft-tissue infections, intra-abdominal infections and febrile neutropenia.
In the past 15 years, we have observed an increased occurrence of multidrug-resistant Enterobacteriaceae, Pseudomonas aeruginosa, Acinetobacter baumannii and Staphylococcus aureus in the community, long-term care and hospital settings, posing serious problems in the choice of an appropriate antibiotic treatment [1,2]. In this article, we provide a critical reappraisal of cefepime after more than a decade of clinical experience.
Chemistry
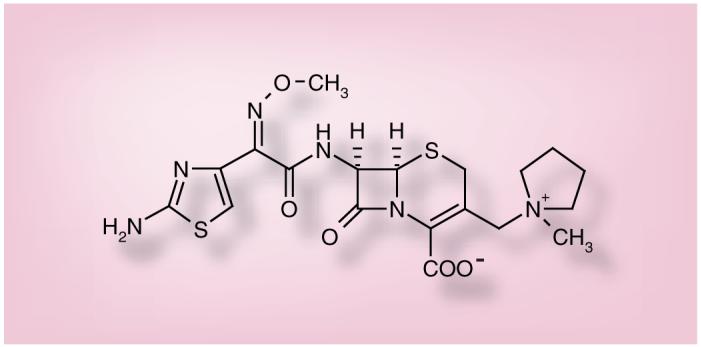
The chemical structure of cefepime is represented in Figure 1. As shown, cefepime is a zwitterionic oxymino β-lactam with an amino-thiazole side chain.
Figure 1. Cefepime.
Pharmacodynamics
Mechanism of action
Cefepime, similar to other β-lactams, inhibits bacterial cell wall biosynthesis by covalent attachment to penicillin-binding proteins (PBPs) and impedes the final transpeptidation step of peptidoglycan synthesis. Bacteria exposed to a concentration of cefepime above their MIC lyse due to the combined inhibition of PBPs and ongoing activity of cell wall autolytic enzymes (autolysis and murein hydrolases) [3].
Unlike other extended-spectrum cephalosporins, the methylpyrrolidinium group of cefepime confers a zwitterionic charge that enhances bactericidal activity by rapid penetration through the porin channels in the outer membrane of Gram-negative pathogens [4,5]. In addition, in vitro studies and clinical observations suggest that cefepime may select for resistance at a lower rate than other extended-spectrum cephalo sporins [6,7]. For example, in vitro development of resistance to cefepime versus other β-lactams (i.e., cefpirome, ceftazidime, cefotaxime, piperacillin and imipenem) in P. aeruginosa was evaluated. Stepwise resistance was assessed by serial passage of colonies located nearest to the inhibition zone. The lowest frequencies of spontaneous resistance mutations were found with cefepime and imipenem. These drugs also resulted in the slowest appearance of resistance by spontaneous mutations: cefepime-selected isolates required a mean of 30 passages to reach resistance, whereas resistance occurred more rapidly in strains selected with other cephalosporins [8].
Mechanism of resistance in Gram-negative organisms
The most frequent mechanism of resistance expressed by Gram-negative organisms against cefepime is the production of β-lactamases that are able to hydrolyze the drug [9]. Enterobacteriaceae (e.g., Escherichia coli and Klebsiella spp.) demonstrate resistance to cefepime by producing class A extended-spectrum β-lactamases (ESBLs) or, more rarely, carbapenemases of class A (e.g., Klebsiella pneumoniae carbapenemases) or class B (e.g., VIM and IMP metallo-β-lactamases) [9-12].
Resistance to cefepime in P. aeruginosa is generally mediated by the combination of hyperproduction of class C chromosomal enzymes (i.e., AmpC) and/or upregulation of efflux pumps [13]. Among P. aeruginosa, there is basal production of chromosomal AmpC β-lactamases that generally does not result in full resistance to cefepime. However, some of these strains produce high levels of AmpC β-lactamases, due to induction or complete derepression, and become fully cefepime resistant [13]. This phenotype is usually observed in isolates from intensive care unit (ICU) patients and patients with cystic fibrosis (CF) who frequently receive multiple treatment courses of expanded-spectrum β-lactam antibiotics for prolonged periods [13,14]. A phenotype commonly observed among P. aeruginosa is resistance to cefepime and susceptibility to ceftazidime; this is due to over-expression of the MexXY-OprM efflux system [15]. In certain geographic regions, production of metallo-β-lactamases is also an important mechanism of resistance that may be found in P. aeruginosa [10,13,16].
Cefepime resistance is demonstrated in A. baumannii by a combination of hyperproduction of the chromosomal OXA-51/69-like carbapenemases, activation of efflux pumps (i.e., AdeABC) and probably porin changes [17,18]. The expression of OXA-type carbapenemases in the absence of the other mechanisms of resistance does not result in high-level cefepime resistance [19]. Similarly, expression of chromosomal Acinetobacter-derived cephalosporinase by A. baumannii does not seem to confer resistance [20]. By contrast, production of certain acquired class D OXA-type carbapenemases and/or metallo-β-lactamases are important mechanisms of resistance against cefepime [10,13,16,17,21].
As shown in Table 1, Ambler class A ESBLs (e.g., TEM-, SHV- and CTX-M-type) show high affinity for cefepime, similar to that expressed against cefotaxime and ceftazidime. By contrast, cefepime has a much lower affinity for chromosomal or plasmid-mediated AmpC β-lactamases than cefotaxime or ceftazidime [9,22,23]. Cefepime is also hydrolyzed less than ceftazidime by carbapenemases of Ambler class A and class B [10,19,21,24].
Table 1.
Affinity (Km or Ki) of different class of β-lactamase enzymes for cefepime and other cephalosporins
| Enzyme class |
Km or Ki (μM) |
Ref. | ||
|---|---|---|---|---|
| Ceftazidime | Cefotaxime | Cefepime | ||
| β-lactamase | ||||
| TEM-1 | 200 | NR | 650 | [129] |
| SHV-1 | 500 | NR | 81 | [129] |
| Extended-spectrum β-lactamases | ||||
| TEM-3 | 100 | 26 | 190 | [130] |
| TEM-12 | 130 | 94 | 130 | [131] |
| TEM-24 | 180 | 25 | 90 | [132] |
| TEM-26 | 10 | 13 | 105 | [130] |
| TEM-121 | 150 | 430 | 40 | [132] |
| SHV-5 | 9.2 | 5.0 | 100 | [130] |
| SHV-18 | 47 | 8.7 | 81 | [130] |
| CTX-M-3 | >3000 | 113 | 170 | [133] |
| CTX-M-10 | NR | 40 | 110 | [130] |
| CTX-M-15 | 1760 | 54 | 1075 | [133] |
| OXA-10 | NR | NR | 230 | [129] |
| Carbapenemases | ||||
| VIM-2 | 98 | 32 | 184 | [134] |
| IMP-2 | 111 | NR | 7 | [135] |
| KPC-2 | 230 | NR | 540 | [129] |
| Ambler class C enzymes | ||||
| AmpC Pseudomonas aeruginosa | 8 | NR | 370 | [129] |
| AmpC Morganella morganii | 14 | NR | 690 | [129] |
| AmpC Providencia stuartii | 26 | NR | 780 | [129] |
| AmpC Serratia marcescens | 570 | NR | >800 | [129] |
| ADC-7 | 11.6 | 0.10 | 594 | [20] |
| CMY-9 | 560 | 0.28 | 950 | [136] |
| CMY-19 | 3.7 | 31 | 630 | [136] |
| P99 | 15 | NR | 100 | [137] |
ADC: Acinetobacter-derived cephalosporinase; KPC: Klebsiella pneumoniae carbapenemases; NR: Not reported.
Mechanism of resistance in Gram-positive organisms
The major mechanism conferring resistance to cefepime in Gram-positive bacteria is changes in the PBPs. Cefepime exhibits low affinity for PBP-2a in methicillin-resistant staphylococci and low affinity to PBP-4 and PBP-5 in Enterococcus spp. [25-27].
In vitro activity
Data regarding the in vitro activity of cefepime compared with ceftazidime and imipenem against clinical isolates of Enterobacteriaceae, Gram-negative non-Enterobacteriaceae and Gram-positive bacteria are summarized in Tables 2, 3 & 4. The majority of published antimicrobial susceptibility data are interpreted according to Clinical and Laboratory Standards Institute (CLSI) criteria, and this may underestimate resistance [28]. Notably, the European Committee on Antimicrobial Susceptibility Testing (EUCAST) asserts that resistance breakpoints should be much lower than CLSI criteria [201]. A comparison of the two criteria is shown in Table 5.
Table 2.
MICs (mg/l) of, and susceptibility to, cefepime, ceftazidime and imipenem among clinical populations of Gram-negative Enterobacteriaceae
| Organism | Region | Site of infection | Year(s) of collection | MIC (mg/l)* |
Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Cefepime |
Ceftazidime |
Imipenem |
|||||||||||
| MIC50 | MIC90 | S (%) | MIC50 | MIC90 | S (%) | MIC50 | MIC90 | S (%) | |||||
| Escherichia coli | North America | Mixture | 2007 | ≤0.12 | 0.25 | 95.9 | 0.25 | 1 | 96.6 | 0.12 | 0.25 | 100 | [138] |
| BSI, RT | 1998-2004 | ≤0.12 | ≤0.12 | 99.6 | ≤2 | ≤2 | 98.6 | ≤0.5 | ≤0.5 | 100 | [40] | ||
| UTI | 1998 | ≤0.12 | ≤0.12 | 100 | 0.25 | 0.5 | 99.9 | 0.12 | 0.25 | 100 | [139] | ||
| Latin America | BSI | 1997-2000 | ≤0.12 | 0.25 | 97.1 | 0.25 | 0.5 | 95.6 | 0.12 | 0.25 | 100 | [140] | |
| Europe | Mixture | 1997-1998 | ≤0.12 | ≤0.12 | 99.2 | ≤0.12 | 0.25 | 98.1 | 0.25 | 0.5 | 100 | [141] | |
| America and Europe | Mixture | 2005-2006 | ≤0.12 | 0.25 | 96.0 | ≤1 | ≤1 | 95.0 | ≤0.12 | 0.25 | 100 | [31] | |
| Asia-Pacific | UTI | 1998-1999 | ≤0.12 | 0.25 | 98.6 | 0.25 | 0.5 | 96.7 | 0.12 | 0.25 | 100 | [142] | |
| E. coli ESBL+ | North America | Mixture | 1998-2003 | 0.25 | 4 | 93.8 | 8 | >16 | 60.6 | ≤0.5 | ≤0.5 | 100 | [35] |
| America and Europe | Mixture | 2005-2006 | 16 | >16 | 39.6 | 16 | >16 | 39.6 | 0.25 | 0.5 | 100 | [31] | |
| Klebsiella spp. | North America | Mixture | 2007 | ≤0.12 | 16 | 89.9 | 0.25 | >16 | 81.7 | 0.25 | 2 | 90.9 | [138] |
| BSI, RT | 1998-2004 | ≤0.12 | 0.25 | 99.0 | ≤2 | ≤2 | 95.1 | ≤0.5 | ≤0.5 | 99.7 | [40] | ||
| RT | 2000 | ≤0.12 | 0.25 | 99.5 | 0.25 | 8 | NR | ≤0.06 | 0.25 | 87.8 | [143] | ||
| UTI | 1998 | ≤0.12 | 0.25 | 99.4 | 0.25 | 0.5 | 95.8 | 0.25 | 0.5 | 100 | [139] | ||
| Latin America | BSI | 1997-2000 | 0.25 | >16 | 74.8 | 1 | >16 | 64.0 | 0.25 | 0.5 | 99.8 | [140] | |
| RT | 1997-2000 | <0.12 | >16 | 76.1 | 0.5 | >16 | 63.1 | 0.25 | 0.5 | 99.6 | [144] | ||
| Europe | Mixture | 1997-1998 | <0.12 | 8 | ≤0.12 | >16 | 81.3 | 0.5 | 1 | 99.9 | [141] | ||
| America and Europe | Mixture | 2005-2006 | ≤0.12 | >16 | 86.5 | ≤1 | >16 | 81.7 | 0.25 | 0.5 | 97.2 | [31] | |
| Asia-Pacific | UTI | 1998-1999 | ≤0.12 | 4 | 98.6 | 0.25 | >16 | 87.3 | 0.25 | 0.5 | 100 | [142] | |
| Klebsiella spp. ESBL+ | North America | Mixture | 1998-2003 | 1 | 8 | 92.5 | >16 | >16 | 36.9 | ≤0.5 | ≤0.5 | 99.3 | [35] |
| America and Europe | Mixture | 2005-2006 | 8 | >16 | 51.4 | >16 | >16 | 33.0 | 0.25 | 0.5 | 99.3 | [31] | |
| Enterobacter spp. | North America | Mixture | 2007 | ≤0.12 | 2 | 95.3 | 0.25 | >16 | 78.8 | 0.5 | 2 | 97.6 | [138] |
| BSI, RT | 1998-2004 | ≤0.12 | 2 | 99.3 | ≤1 | >16 | 78.4 | ≤0.5 | 1 | 100 | [40] | ||
| Latin America | BSI | 1997-2000 | ≤0.12 | 8 | 91.5 | 0.5 | >16 | 68.4 | 0.5 | 1 | 99.7 | [140] | |
| RT | 1997-2000 | 0.12 | >16 | 61.2 | 0.5 | >16 | 63.4 | 0.5 | 2 | 100 | [144] | ||
| Europe | Mixture | 1997-1998 | ≤0.12 | 4 | 95.2 | 0.25 | >16 | 78.4 | 0.5 | 2 | 99.6 | [141] | |
| America and Europe | Mixture | 2005-2006 | ≤0.12 | 4 | 93.4 | ≤1 | >16 | 71.1 | 0.5 | 2 | 98.4 | [31] | |
| Asia-Pacific | UTI | 1998-1999 | 0.25 | 8 | 95.3 | 4 | >16 | 51.2 | 0.5 | 2 | 100 | [142] | |
| Proteus mirabilis | North America | Mixture | 2007 | ≤0.12 | ≤0.12 | 100 | ≤0.12 | ≤0.12 | 100 | 1 | 2 | 99.2 | [138] |
| Europe | Mixture | 1997-1998 | ≤0.12 | 0.50 | 96.3 | ≤0.12 | 1 | 95.3 | 1 | 2 | 99.5 | [141] | |
| America and Europe | Mixture | 2005-2006 | ≤0.12 | 0.25 | 94.3 | ≤1 | ≤1 | 98.3 | 1 | 2 | 99.8 | [31] | |
| Providencia spp. | Europe and Australia | Mixture | 1999-2000 | 0.03 | 1 | 99.0 | NR | NR | NR | 2 | 8 | 98.0 | [36] |
| Morganella morganii | Europe and Australia | Mixture | 1999-2000 | 0.03 | 2 | 100 | NR | NR | NR | 4 | 8 | 92.0 | [36] |
| Salmonella spp. | America and Europe | Mixture | 2005-2006 | ≤0.12 | ≤0.12 | 100 | ≤1 | ≤1 | 98.1 | 0.25 | 0.5 | 100 | [31] |
| Citrobacter spp. | America and Europe | Mixture | 2005-2006 | ≤0.12 | 1 | 98.3 | ≤1 | >16 | 75.9 | 0.5 | 1 | 99.6 | [31] |
| Serratia spp. | America and Europe | Mixture | 2005-2006 | ≤0.12 | 0.5 | 97.5 | ≤1 | 2 | 95.2 | 1 | 2 | 99.8 | [31] |
Results were interpreted according to Clinical and Laboratory Standards Institute criteria.
BSI: Bloodstream infection; ESBL+: Extended-spectrum β-lactamase-producing organisms; NR: Not reported; RT: Respiratory tract infection; S: Susceptibility; UTI: Urinary tract infection.
Table 3.
MICs (mg/l) of, and susceptibility to, cefepime, ceftazidime and imipenem among clinical populations of Gram-negative non-Enterobacteriaceae
| Organism | Region | Site of infection | Year(s) of collection | MIC (mg/l)* |
Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Cefepime |
Ceftazidime (Ceftriaxone)‡ |
Imipenem |
|||||||||||
| MIC50 | MIC90 | S (%) | MIC50 | MIC90 | S (%) | MIC50 | MIC90 | S (%) | |||||
| Acinetobacter spp. | North America | Mixture | 2007 | 4 | 16 | 85.7 | 2 | >16 | 83.3 | 2 | 16 | 77.5 | [138] |
| Latin America | Mixture | 1997-2001 | >16 | >16 | 35.2 | >16 | >16 | 28.5 | 1 | >8 | 86.9 | [145] | |
| BSI | 1997-2000 | 16 | >16 | 45.4 | >16 | >16 | 39.0 | 0.5 | 8 | 89.1 | [140] | ||
| RT | 1997-2000 | >16 | >16 | 26.4 | >16 | >16 | 16.7 | 1 | >8 | 84.9 | [144] | ||
| America and Europe | Mixture | 2005-2006 | 16 | >16 | 44.1 | >16 | >16 | 34.8 | 1 | >8 | 72.4 | [30] | |
| Pseudomonas aeruginosa | North America | Mixture | 2007 | 4 | 16 | 85.7 | 2 | >16 | 83.3 | 2 | 16 | 77.5 | [138] |
| BSI, RT | 1998-2004 | 2 | 8 | 90.8 | 2 | 16 | 87.3 | 1 | 2 | 94.4 | [40] | ||
| RT | 2000 | 4 | 16 | 80.5 | 4 | >16 | 78.3 | 1 | 8 | 85.6 | [143] | ||
| UTI | 1998 | 4 | 8 | 91.2 | 4 | >16 | 85.8 | 0.25 | 0.5 | 100 | [139] | ||
| Mixture§ | 2001 | 4 | 16 | 80.1 | ≤2 | >16 | 77.0 | 1 | >8 | 78.3 | [146] | ||
| RT¶ | 1996-2001 | 2 | 16 | 84.1 | 2 | 32 | 84.1 | 1 | 16 | 86.6 | [147] | ||
| RT# | NR | 64 | 256 | 15.0 | 64 | >512 | 27.0 | 32 | 128 | 30.0 | [148] | ||
| RT** | NR | 64 | 256 | 10.0 | 128 | >512 | 16.0 | 32 | 64 | 21.0 | [148] | ||
| Latin America | BSI | 1997-2000 | 4 | >16 | 72.1 | 4 | >16 | 69.4 | 1 | >8 | 84.5 | [140] | |
| RT | 1997-2000 | 8 | >16 | 61.2 | 4 | >16 | 61.2 | 2 | >8 | 69.2 | [144] | ||
| Mixture§ | 2002 | 4 | >256 | 55.4 | >192 | >256 | 31.4 | 2 | >32 | 57.3 | [149] | ||
| America and Europe | Mixture | 2005-2006 | 4 | >16 | 79.4 | 2 | >16 | 75.5 | 1 | >8 | 75.8 | [31] | |
| Asia-Pacific | UTI | 1998-1999 | 4 | 16 | 76.4 | 4 | >16 | 77.4 | 2 | 8 | 88.7 | [142] | |
| Burkholderia cepacia | America and Europe | Mixture | 2003 | 8 | 16 | 85.0 | 4 | 4 | 90.0 | 4 | 8 | 65.0 | [150] |
| RT¶ | 1996-2001 | 16 | 128 | 47.0 | 4 | 16 | 82.4 | 4 | 16 | 50.0 | [147] | ||
| RT | NR | 256 | >256 | 10.0 | 16 | >512 | 43.0 | 32 | 128 | 9.0 | [148] | ||
| Stenotrophomonas maltophilia | World | Mixture | 1997-2003 | 16 | >16 | 27.6 | 8 | >16 | 52.9 | >8 | >8 | 100 | [151] |
| America and Europe | Mixture | 2004 | >16 | >16 | 9.4 | 16 | >16 | 45.3 | >8 | >8 | 1.9 | [152] | |
| Haemophilus influenzae | North America | BSI, RT | 1998-2004 | ≤0.06 | 0.12 | 100 | ≤0.25 | 1 | 93.7 | NR | NR | NR | [40] |
| Latin America | RT | 1998-2004 | ≤0.06 | 0.12 | 100 | ≤0.06 | ≤0.06 | 100 | NR | NR | NR | [153] | |
| America and Europe | Mixture | 2005-2006 | ≤0.12 | ≤0.12 | 100 | ≤0.25 | ≤0.25 | 100 | 0.5 | 1 | 100 | [31] | |
| Moraxella catarrhalis | North America | BSI, RT | 1998-2004 | 0.5 | 2 | NR | ≤0.25 | 0.5 | NR | NR | NR | NR | [40] |
| Neisseria gonorrhoeae | Asia | STDs | 2003 | 0.12 | 0.5 | NR | 0.03 | 0.06 | 100 | NR | NR | NR | [154] |
| Neisseria meningitidis | Europe and Australia | Mixture | 1999-2000 | 0.03 | 0.06 | NR | 0.06 | 0.06 | NR | <0.06 | 0.12 | NR | [36] |
Results were interpreted according to Clinical and Laboratory Standards Institute criteria.
For H. influenzae ceftriaxone MICs were shown.
Intensive care unit patients.
Cystic fibrosis patients.
Mucoid isolates.
Nonmucoid isolates.
BSI: Bloodstream infection; NR: Not reported; RT: Respiratory tract infection; S: Susceptibility; STD: Sexually transmitted disease; UTI: Urinary tract infection.
Table 4.
MICs (mg/l) of, and susceptibility to, cefepime, ceftazidime and imipenem among clinical populations of Gram-positive organisms
| Organism | Region | Site of infection | Year(s) of collection | MIC (mg/l)* |
Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Cefepime |
Ceftazidime (Ceftriaxone)‡ |
Imipenem |
|||||||||||
| MIC50 | MIC90 | S (%) | MIC50 | MIC90 | S (%) | MIC50 | MIC90 | S (%) | |||||
| Staphylococcus aureus |
North America | Mixture | 2007 | 2 | 4 | 100§ | 8 | 16 | 89.3 | 0.03 | 0.06 | 100 | [138] |
| BSI, RT | 1998-1904 | 2 | 4 | 100§ | 8 | 16 | 86.7 | NR | NR | NR | [40] | ||
| SSTI | 2000 | 4 | >16 | 83.9¶ | 8 | >16 | 75.0 | ≤0.06 | 4 | 92.1 | [155] | ||
| Latin America | RT | 1997-1900 | >16 | >16 | 46.7¶ | NR | NR | NR | NR | NR | NR | [144] | |
| BSI | 1997-1900 | 4 | >16 | 69.4¶ | 8 | >16 | 56.4 | ≤0.06 | >8 | 69.4 | [140] | ||
| America and Europe | Mixture | 2005-2006 | 2 | 4 | 99.9 | 8 | 8 | 96.5 | ≤0.12 | ≤0.12 | >99.9 | [31]§ | |
| Coagulase-negative Staphylococcus spp. |
North America | Mixture | 2007 | 1 | 2 | 100§ | 4 | 16 | 88.2 | 2 | 4 | 98.1 | [138] |
| BSI, RT | 1998-1904 | 1 | 2 | 100§ | 8 | 8 | 94.0 | NR | NR | NR | [40] | ||
| Latin America | BSI | 1997-1900 | 4 | >16 | 24.7¶ | >16 | >16 | 24.7 | 0.5 | >8 | 24.7 | [140] | |
| Streptococcus pyogenes |
Europe and Australia | Mixture | 1999-1900 | 0.03 | 0.06 | 100 | 0.06 | 0.06 | 100 | 0.008 | 0.008 | 100 | [36] |
| β-hemolytic streptococci |
North America | BSI, RT | 1998-1904 | ≤0.12 | ≤0.12 | 100 | ≤0.25 | ≤0.25 | 100 | NR | NR | NR | [40] |
|
Viridans streptococci |
North America | Mixture | 1998-1903 | ≤0.12 | 1 | 94.1 | 0.12 | 0.5 | 93.6 | NR | NR | NR | [35] |
| America and Europe | Mixture | 2005-2006 | 0.5 | 2 | 99.9 | 4 | 8 | 96.8 | ≤0.12 | ≤0.12 | 100 | [31]§ | |
| Streptococcus pneumoniae |
North America | Mixture | 2007 | ≤0.12 | 1 | 96.0 | ≤0.25 | 1 | 95.2 | ≤0.15 | 0.25 | 88.1 | [138] |
| Mixture | 2001-02 | ≤0.12 | 1 | 96.6 | 0.03 | 0.03 | 99.8 | ≤0.06 | ≤0.06 | NR | [42] | ||
| 0.25 | 1 | 99.2 | 0.25 | 1 | 98.7 | ≤0.06 | 0.12 | NR | [42]# | ||||
| 1 | 2 | 81.9 | 1 | 2 | 79.7 | 0.25 | 0.5 | NR | [41]** | ||||
| BSI, RT | 1998-04 | ≤0.12 | 1 | 93.7 | ≤0.25 | 1 | 93.7 | NR | NR | NR | [40] | ||
| Latin America | RT | 1998-04 | ≤0.06 | 0.5 | 99.2 | 0.03 | 0.5 | 99.5 | NR | NR | NR | [153] | |
| America and Europe | Mixture | 2005-06 | ≤0.12 | 1 | 96.3 | ≤0.25 | 1 | 97.5 | NR | NR | NR | [31] | |
| Europe | RT | 1999-03 | ≤0.06 | 1 | 98.4 | 0.03 | 1 | 99.1 | NR | NR | NR | [39] | |
Results were interpreted according to Clinical and Laboratory Standards Institute criteria.
For all streptococci, ceftriaxone MICs were shown.
All strains were oxacillin-susceptible.
Susceptibility was predicted by the oxacillin results.
Considering only penicillin-intermediate strains.
Considering only penicillin-resistant strains.
BSI: Bloodstream infection; NR: Not reported; RT: Respiratory tract infection; S: Susceptibility; SSTI: Skin and soft tissue infection.
Table 5.
Cefepime MICs interpretative breakpoints: comparison between CLSI and EUCAST criteria
| Organism | CLSI (MIC, mg/l) |
EUCAST (MIC, mg/l) |
||||
|---|---|---|---|---|---|---|
| S | I | R | S | I | R | |
| Gram-negative | ||||||
| Acinetobacter spp. | ≤8 | 16 | ≥32 | IU | ||
| Burkholderia cepacia | NA | IU | ||||
| Enterobacteriaceae* | ≤8 | 16 | ≥32 | ≤1 | 2-8 | ≥16 |
| Haemophilus spp. | ≤2 | ≤0.25 | ≥0.5 | |||
| Moraxella catarrhalis | NA | ≤0.25 | ≥0.5 | |||
| Neisseria gonorrhoeae | ≤0.5 | IU | ||||
| Neisseria meningitidis | NA | IU | ||||
| Pseudomonas spp. | ≤8 | 16 | ≥32 | ≤8 | ≥16 | |
| Stenotrophomonas maltophilia | NA | IU | ||||
| Gram-positive | ||||||
| Enterococcus spp. | NA | IU | ||||
| Staphylococcus spp. | ≤8 | 16 | ≥32 | ‡ | ||
| Streptococcus pneumoniae | ||||||
| Nonmeningitis | ≤1 | 2 | ≥4 | ≤1 | 2 | ≥4 |
| Meningitis | ≤0.5 | 1 | ≥2 | |||
| Streptococcus spp. | ||||||
| β-hemolytic group | ≤0.5 | ≤0.5 | ≥1 | |||
| Viridans group | ≤1 | 2 | ≥4 | ≤0.5 | ≥1 | |
According to CLSI criteria, confirmed E. coli, Klebsiella spp. and P. mirabilis ESBL producers should be reported as resistant to all cephalosporins and aztreonam independently of MIC values [28]. Notably, criteria for the remaining Enterobacteriaceae have not been established.
Susceptibility to cefepime, as with other cephalosporins, is inferred from the methicillin susceptibility.
CLSI: Clinical and Laboratory Standards Institute; EUCAST: European Committee on Antimicrobial Susceptibility Testing; I: Intermediate; IU: Inappropriate use; NA: Not available; R: Resistant; S: Susceptible.
Clinical isolates of Enterobacteriaceae are frequently susceptible to cefepime (Table 2). Cefepime’s activity is similar to that of ceftazidime against organisms such as Klebsiella spp. and Proteus mirabilis, which do not possess AmpC chromosomally mediated β-lactamases. By contrast, Enterobacteriaceae that harbor inducible chromosomal AmpC β-lactamases, such as Citrobacter spp., Enterobacter spp., Hafnia spp., Morganella morganii, Proteus vulgaris, Providencia spp. and Serratia marcescens are typically resistant to other extended-spectrum cephalosporins, while cefepime maintains its activity (Table 2). Even in the case of high-level production of AmpC β-lactamases, ceftazidime-resistant Enterobacteriaceae may still be in the susceptible range for cefepime. For example, only 5% of clinical Enterobacter spp. strains show resistance (Table 2). Consequently, cefepime is an attractive clinical choice for the treatment of infections casued by these organisms, even in the presence of increased production of the AmpC β-lactamases [29]. As shown by EUCAST guidelines (Table 5), Enterobacteriaceae producing AmpC enzymes often have slightly elevated MICs for cefepime (e.g., between 2 and 8 mg/l). This phenomenon might affect the clinical outcome of patients with serious infections, such as bacteremia [30]. To date, clinical studies analyzing the outcome of patients treated with cefepime compared with other cephalosporins are very limited.
Recent data from Europe and America show that ESBL-producing E. coli and K. pneumoniae isolates tested susceptible to cefepime in 39.6 and 51.4% of cases, respectively (Table 2) [31]. In the Asia-Pacific region, among organisms expressing an ESBL phenotype, susceptibility to cefepime ranged from 33 to 93% for E. coli, from 25 to 100% for K. pneumoniae, from 33 to 100% for E. cloacae and from 0 to 57% for P. mirabilis [32,33]. In a European survey conducted in 2004, the susceptibility of cefepime against ESBL-producing E. coli and Klebsiella spp. isolates was 25.6 and 63.6%, respectively [34]. Although many in vitro studies have shown that ESBL-producing Enterobacteriaceae are susceptible to cefepime (i.e., MIC ≤ 8 mg/l), it is important to note that these isolates must be reported by clinical laboratories as resistant to cefepime (e.g., all penicillins and cephalosporins), according to CLSI criteria [28]. For ESBL-producing enterobacteria, the EUCAST criteria suggest reporting any susceptible results as intermediate and any intermediate results as resistant [202].
Approximately 10-35% of P. aeruginosa isolates are resistant to cefepime (Table 3). The activity of cefepime against other Pseudomonas spp. is variable: P. putida, P. fluorescens and P. oryzihabitans show susceptibilities of 80-85%, whereas resistant P. stutzeri isolates are extremely rare [35].
As shown in Table 3, clinical isolates of A. baumannii are frequently resistant to cefepime (i.e., >45% of isolates). Interestingly, this may be largely dependent upon clones that are prevalent in certain geographic locations (e.g., 85% susceptible in North America). The majority of strains of Stenotrophomonas maltophilia and Burkholderia cepacia complex show MICs of greater than 8 mg/l (Table 3). Notably, EUCAST does not report susceptibility breakpoints for cefepime against Acinetobacter spp., S. maltophilia and B. cepacia, as most isolates have MICs of greater than 8 mg/l (Table 5).
Clinical isolates of Haemophilus influenzae appear fully susceptible to cefepime (Table 3). With regard to Moraxella catarrhalis, high values of MIC50/90 were recorded among clinical isolates (Table 3). Data regarding cefepime susceptibility in clinical strains of Neisseria meningitidis and N. gonorrhoeae are very limited (Table 3).
Although criteria of interpretation have not been established, the activity of cefepime appears poor against Gram-negative anaerobes such as Bacteroides spp., Prevotella spp. and Fusobacterium spp. [36-38].
Cefepime MIC values for clinical isolates of Streptococcus pneumoniae are comparable to those of ceftriaxone. In these strains, the MICs of cefepime are consistently lower than the MICs of penicillin G [39,40]. At least 95% of penicillin-intermediate and 80% of penicillin-resistant isolates remain susceptible to cefepime (Table 4) [41].
Oxacillin-susceptible S. aureus and coagulase-negative staphylococci, β-hemolytic streptococci and viridans group streptococci are susceptible to cefepime (Table 4). As is the case with other cephalosporins, methicillin-resistant staphylococci and Enterococcus spp. are resistant to cefepime [28].
Clinical management & dosage
Cefepime is usually administered by the intravenous or intramuscular routes in doses of 0.5, 1 and 2 g. Each intravenous dose is usually diluted in 50 or 100 ml of compatible intravenous fluid, which is then administered as a 30-min infusion.
According to data from clinical trials, the following dosing schedules are recommended:
Moderately severe infections such as pneumonia due to S. pneumoniae and/or Gram-negative organisms: 1-2 g intravenously every 12 h for 10 days;
Mild/moderate uncomplicated or complicated UTIs, including pyelonephritis, due to Enterobacteriaceae: 0.5-2 g intravenously every 12 h for 7-10 days;
Moderate/severe skin and soft-tissue infections due to S. aureus or Streptococcus pyogenes: 2 g intravenously every 12 h for 10 days;
Intra-abdominal infections: 2 g intravenously every 12 h for 7-10 days.
In neutropenic fever, the suggested dosing is 2 g intravenously every 8 h for at least 7 days. Notably, many clinicians use cefepime in suspected or confirmed Gram-negative infections of the CNS. Although not approved for this, the 2 g intravenously every 8 h dosing regimen is followed [42]. The usual dosage in pediatric patients (2 months up to 16 years) is 50 mg/kg every 12 h (50 mg/kg every 8 h for febrile neutropenic patients).
Pharmacokinetics & metabolism
Serum levels
Pharmacokinetic parameters after intravenous and intramuscular administration of cefepime in healthy adults and patients with different levels of renal function are shown in Table 6.
Table 6.
Pharmacokinetic parameters of cefepime in healthy adults and patients with different renal insufficiency levels
| Patient | Dose (g) | Administration | Cmax (mg/l) | Half-life (h) | AUC (mg h/l) | ClT (ml/min) | ClR (ml/min) | %Xu | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Healthy adults | 0.25 | iv. 30 min | 16.3 ± 2.4 | 1.9 ± 0.2 | 34 ± 3* | 122 ± 10 | 96 ± 19 | 78.4 ± 11.3 | [47]‡ |
| Healthy adults | 0.5 | iv. 30 min | 31.6 ± 2.9 | 1.8 ± 0.2 | 62 ± 6* | 136 ± 13 | 116 ± 11 | 85.6 ± 2.9 | [47]‡ |
| Healthy adults | 1 | iv. 30 min | 66.9 ± 4.6 | 1.9 ± 0.2 | 137 ± 9* | 122 ± 8 | 103 ± 5 | 84.2 ± 2.2 | [47]‡ |
| Healthy adults | 2 | iv. 30 min | 133 ± 35.5 | 1.8 ± 0.2 | 263 ± 33* | 128 ± 16 | 104 ± 12 | 81.3 ± 6.9 | [47]‡ |
| Healthy adults | 0.5 | im. | 13.9 ± 3.4 | 1.4 ± 0.9 | 60.0 ± 8.0 | NR | NR | NR | [156]§ |
| Healthy adults | 1 | im. | 29.6 ± 4.4 | 1.6 ± 0.4 | 137.0 ± 11.0 | NR | NR | NR | [156]§ |
| Healthy adults | 2 | im. | 57.5 ± 9.5 | 1.5 ± 0.4 | 262.0 ± 23.0 | NR | NR | NR | [156]§ |
| Infected patients: ClCr > 100 ml/min | 2 every 12 h | iv. 30 min | 259 ± 287 | 3.1 ± 2.6 | 734 ± 344¶ | 117 ± 72 | NR | NR | [58]§ |
| Infected patients: ClCr 60-100 ml/min | 2 every 12 h | iv. 30 min | 167 ± 124 | 7.6 ± 5.2 | 1138 ± 540¶ | 73 ± 37 | NR | NR | [58]§ |
| Infected patients: ClCr < 60 ml/min | 1-2 every 24 h | iv. 30 min | 207 ± 295 | 12.1 ± 6.3 | 845 ± 296¶ | 43 ± 18 | NR | NR | [58]§ |
AUC0-∞.
Data obtained after the 30-min infusion.
Data obtained at the steady state.
AUC24.
%Xu: Percentage of dose recovered in urine; ClR: Renal clearance; ClT: Total body clearance; im.: Intramusular administration; iv.: Intravenous administration; NR: Not reported.
In pediatric patients, following single and multiple doses of 50 mg/kg every 8-12 h, the average peak serum level (Cmax) ranged from 130 to 180 mg/l, whereas, for a dose of 30 mg/kg every 8-12 h, it was 114 mg/l. After 8 h from the first intravenous or intramuscular dose of 50 mg/kg, the serum concentration was approximately 5 mg/l in both cases. The mean elimination half-life was 1.3-1.9 h and the average steady state Cmax of the 50-mg/kg intravenous regimen was 177 mg/l for every 12 h and 188 mg/l for every 8 h [43].
The pharmacokinetics of cefepime 2 g intravenously every 12 h was measured in critically ill patients with normal serum creatinine [44]. The mean plasma cefepime concentration after 12 h from the first dose was 3.2 mg/l and the mean half-life was 2.5 h. There was a large variation in plasma drug concentrations among patients, and a number of patients had very low plasma cefepime levels (median: 1.9 mg/l). Trough levels after multiple doses were low (median: 1.7-1.8 mg/l). The clinical consequences of these low levels may be important if they involve inadequate bacterial killing or the development of resistance. The large scatter in trough levels could be accounted for by the variability in creatinine clearance (ClCr) of patients who were critically ill and, therefore, probably had renal dysfunction [44].
In burn patients receiving intravenous cefepime 2 g every 12 h, the Cmax after infusion ranged from 89 to 146 mg/l at day 1 and from 72 to 243 mg/l at day 3. The average cefepime concentrations 12 h after the beginning of infusion were 2.1 and 2.4 mg/l, respectively, at day 1 and 3. The mean half-life was 2.5 h on day 1 and 2.6 h on day 3 [45].
Distribution in body sites
The average steady-state volume of distribution of cefepime is 18.0 ± 2.0 l. The serum protein binding is approximately 20% [46]. As shown in Table 6, total body clearance (ClT) ranges from 122 to 136 ml/min within doses of 0.25-2 g. The mean renal clearance (ClR) of 105 ml/min is nearly the same as that of ClCr in normal adults [47].
In adult patients with external ventricular drains who received cefepime (2g intravenously every 12 h) for the treatment of pneumonia, penetration into the CNS was variable (4-34%), but appeared similar to that reported for other cephalosporins administered to treat meningitis. In the CNS, the Cmin attained by most patients at the steady state was 2 mg/l [48]. In children, after the third dose (50 mg/kg intravenously every 8 h), concentrations in the cerebrospinal fluid (CSF) were 3.3 mg/l at 0.5 h and 5.7 mg/l at 8 h (9 and 67% of plasma concentration, respectively) [49].
In patients undergoing diagnostic bronchoscopy, after a single 2-g dose of cefepime, the mean bronchial mucosal concentration of the drug was 24 μg per gram of tissue and the mean serum concentration was 40.4 mg/l [50]. The concentration of cefepime in the epithelial lining fluid of critically ill patients with bacterial pneumonia was determined at steady state after 48 h of therapy, using 2 g intravenously, followed by a continuous infusion of 4 g over 24 h. The mean epithelial lining fluid concentration was 14 mg/l [51]. Pharmacokinetic parameters were also studied at the steady state in patients subjected to lung surgery for lung cancer. Cefepime was administered in a dose of 2 g every 12 h for 2 days with a final dose of drug administered 0.5-12 h before pulmonary artery clamping. The mean concentration into the lung was 120 μg/g at 0.5 h, 12 μg/g at 8 h and 9 μg/g at 12 h (94, 108 and 112% of plasma concentration, respectively) [52].
In patients undergoing vitreous surgery, a single 1- or 2-g intravenous dose of cefepime before the intervention resulted in a mean peak vitreous concentration of 1.9 and 2.9 mg/l, respectively. The level of cefepime in the vitreous at 12 h was 0.9 and 1.0 mg/l, respectively [53].
Cefepime concentration in the peritoneal fluid and bile was evaluated in patients undergoing elective cholecystectomy receiving 2 g intravenously every 12 h. After approximately 8.5 h the values for plasma, peritoneal fluid, bile and gall bladder tissue concentrations were 8, 6 and 16 mg/l and 5 mg/g, respectively. There was a correlation between plasma concentration and peritoneal fluid or gall bladder tissue concentrations. By contrast, a correlation was not found between bile and plasma concentrations [54]. In one study of patients with extra-hepatic biliary disease, cefepime concentrations in bile and gall bladder tissue were 20 and 48 mg/l (20 and 48% of serum level, respectively). Also in this case, a correlation between serum and bile concentrations of the drug was not observed. Notably, in patients with nonfunctioning gall bladder, very low tissue levels of cefepime (<0.2 mg/l) were measured [55].
After a 2-g intravenous dose, the concentration of cefepime in pancreatic pseudocyst fluid and pancreatic tissue was 27 mg/l (120-200 min after the end of infusion). Mean pancreatic cefepime concentration was 6 mg/l in pseudocyst and 11 mg/l in pancreatic tissue [56].
Bone tissue penetration of cefepime at a dose of 2 g intravenously was evaluated in patients undergoing total hip replacement. The mean plasma concentration of cefepime at the time of bone removal was 73 mg/l, whereas the mean concentrations in cancellous and cortical bone tissues were 74 and 68 mg/l, respectively [57].
Excretion & inactivation
Cefepime is primarily eliminated via the kidney as the unchanged active drug. Urinary recovery of intact cefepime is approximately 80% of the administered dose (Table 6) [47,58]. The urinary recovery of intact cefepime was consistent among the various dosages (0.25-2 g) every 8 h during the course of treatment. Differences in the percentage of cefepime excretion on day 9 among the dose groups were not found [46]. After administration of a low-dose of 0.25 g intravenously, cefepime concentrations in urine were 190 mg/l during the first 2 h and 90 mg/l at 8 h [47]. In pediatric patients, the renal excretion was 60% for treatments of 50 mg/kg every 8 h and 80% for regimens every 12 h [43]. Cefepime is excreted in human milk: an infant receiving 1 l/day of milk receives approximately 0.5 mg of the drug.
Approximately 10-20% of the administered dose of cefepime is metabolized to N-methylpyrrolidine (NMP) and rapidly converted to the N-oxide (NMP-N-oxide). Less than 1% of the administered dose is recovered from urine as NMP, approximately 7% is recovered as NMP-N-oxide and 2-3% is recovered as an epimer of cefepime [59].
Special populations
Elderly patients
Due to the progressive decline of the glomerular filtration rate, the normal cefepime terminal half-life of 2 h observed in young volunteers is prolonged to approximately 3 h in the elderly (>65 years of age). However, the magnitude of age-related changes in the pharmacokinetics of cefepime is not significant enough to recommend dosage adjustment in elderly patients with normal kidney function [60].
Renal insufficiency
A dosage reduction is necessary in patients with renal failure. If there is renal impairment, but ClCr is still greater than 30 ml/min, a dose of 1 g every 12 h is recommended. If ClCr is 10-30 ml/min, the dosage is 0.5 g every 24 h, but if ClCr is less than 10 ml/min, the suggested dosage is 0.25 g every 24 h. If patients are treated with hemodialysis, a supplementary dose of 0.25 g is recommended after each hemodialysis session [61]. In patients undergoing continuous ambulatory peritoneal dialysis, an intravenous cefepime dose of 1 or 2 g every 48 h would maintain the antibiotic levels in plasma and peritoneal fluid above the MIC90 values of common enteric bacteria responsible for intraperitoneal infections [46]. Pharmacokinetic models suggest that most automated peritoneal dialysis patients would achieve adequate serum cefepime concentrations if treated with standard doses of 1 g every 24 h administered intraperitoneally [62].
The pharmacokinetics of drug removal in critically ill patients receiving continuous renal replacement therapy is affected by multiple variables influencing clearance [63]. However, if the goal in critically ill patients is to maintain a therapeutic concentration of cefepime for the entire dosing interval, a normal, unadjusted dose may be used. In fact, a cefepime dosage of 1 g every 12 h is appropriate for most patients receiving continuous venovenous hemofiltration and a dosage of up to 2 g every 12 h is appropriate for patients receiving continuous venovenous hemo dialysis or receiving continuous venovenous hemodiafiltration [64,65].
Children
In neonates with severe nosocomial infection, the dose regimen should take in to consideration the ClCr and the body surface area. Cefepime therapy using 250 mg/m2 every 12 h is adequate to achieve plasma concentrations of at least 8 mg/l during more than 60% of the dosing interval and is expected to be effective in the treatment of bloodstream infections caused by most Gram-negative organisms. A dose of 550 mg/m2 would be required for the treatment of infections caused by Pseudomonas spp. [66].
Cystic fibrosis
The elimination half-life of cefepime in patients with CF is 11% shorter than in other patients, and the clearance is almost 40% higher. However, the difference in pharmacokinetics is minor and changes in dosage are not usually recommended. P. aeruginosa isolates with high MICs against cefepime are responsible for the majority of respiratory infections in CF patients; therefore, a dosage of 2 g every 6 h or 50 mg/kg every 8 h is recommended to maintain adequate serum levels [67,68].
Burn patients
After 12 h following a dose of 1 g, the cefepime concentrations measured in burned skin were 33 ± 41.6 μg/g [45]. In another study, a single 2-g dose of cefepime was evaluated in patients with thermal burn injury and infection. Simulated steady-state serum concentrations obtained by using the patients’ pharmacokinetic parameters exceeded the susceptibility interpretive standard of cefepime for at least 60% of the dosing interval, with dosing regimens of 1 g every 8 h, 2 g every 8 h and 2 g every 12 h. It appeared that these dosing regimens were clinically adequate [69].
Pharmacodynamic target analyses
It is well documented that clinical success with cefepime correlates with the percentage of time (%T) that the concentration of the antibiotic in serum exceeds the MIC (%T > MIC) for the infecting organism [70]. In animal models of infection caused by Enterobacteriaceae and streptococci, maximal efficacy for cephalosporins is obtained when %T > MIC occurs during 60-70% of the dosing interval. For staphylococci, efficacy is demonstrated when %T > MIC occurs during 40-50% of the dosing interval. The recommended dose of cefepime is sufficient to attain these goals for susceptible organisms [71]. For example, cefepime intravenous dosing regimens of 1 g every 8 h, 2 g every 8 h and 2 g every 12 h have the greatest likelihood of achieving this pharmacodynamic target against isolates of fully susceptible (i.e., MICs < 1 mg/l) Enterobacteriaceae [72,73].
By contrast, there is concern that standard treatments of 2 g every 8-12 h are not able to attain such targets and eradicate serious infections due to ESBL-producing Enterobacteriaceae, P. aeruginosa and A. baumannii isolates. Furthermore, cefepime continuous monotherapy infusion (i.e., 4 g infused for a 24-h period) seems unable to achieve these targets against P. aeruginosa and A. baumannii [72,73]. Patients infected with ESBL-producing Enterobacteriaceae, P. aeruginosa and A. baumannii have a much lower %T > MIC than patients with fully susceptible organisms. This is due to the significantly greater MIC values of those organisms, even when still considered ‘susceptible’. To illustrate, standard dosing recommendations resulted in a Cmin that was greater than the MIC in more than 80% of the simulated P. aeruginosa strains with a maximum MIC of 2 mg/l. By contrast, the same regimens were suboptimal for less-susceptible P. aeruginosa isolates (e.g., those with MIC ≥ 4 mg/l) [58].
It has been observed that, in order to achieve a probability of 80 and 90% ‘microbiological success’, it would be necessary for serum cefepime concentration to be above 4.3-times the MIC for 83% and 95% of the dosing interval, respectively [74]. The pharmacodynamic target (i.e., 4.3 × MIC for 83% of the dosing interval) was adopted and involved in a simulation investigating P. aeruginosa isolates expressing different MIC values against cefepime [75]. The standard recommended dosage had less than 80% probability of attaining the pharmadynamic target for all MICs when ClCr was 120 ml/min, but the probability was achievable for MIC values lower than 1 mg/l when ClCr was 90 ml/min and for MIC values of at least 2 mg/l when ClCr was 60 ml/min. Overall, the probability could be improved by increasing the dose to 2 g intravenously every 8 h, or by extending the infusion time to 6 h (2 g every 12 h). In this study, continuous infusion of 4 g over 24 h offered the most promising chance of attaining this target [75].
The same strategies to achieve pharmacodynamic targets by using a higher dose of cefepime and extending the infusion time have been adopted for other bacterial species. Higher doses of 4-6 g administered as a continuous infusion or 2 g administerd every 6-8 h with prolonged infusion are required to optimize therapy against ESBL-producing Klebsiella spp. or E. coli. Cefepime, however, fails to achieve bactericidal targets against A. baumannii isolates, even when administered at such high doses or with prolonged infusion [76-79].
An additional consideration in achieving targets of therapy is the limited penetration of antibiotic into certain body compartments, such as the CNS. The study of the pharmacodynamic profile of cefepime in the CSF of patients with ventricular drains revealed that neither cefepime doses of 2 g every 8 -12 h nor cefepime in continuous infusion could achieve the set target (50 and 100% T > MIC for >90% patients for MICs ≥ 1 mg/l and for >80% patients for MICs ≥ 0.5 mg/l). The clinical implications of this finding are concerning, especially considering that CSF penetration was, on average, only 10% (reflecting noninflamed meninges) and that the precise pharmacodynamic target for CNS infections in humans is unknown [80].
Similar pharmacodynamic analyses were performed under the Optimizing Pharmacodynamic Target Attainment program, using Monte Carlo simulations based upon MICs for different susceptible Gram-negative organisms obtained from the Meropenem Yearly Susceptibility Test Information Collection database. Cefepime 2 g every 12 h demonstrated a higher probability of attaining targets for bactericidal activity when used empirically for the treatment of bloodstream infections, nosocomial pneumonia (provided methicillin-resistant S. aureus prevalence was low) and secondary peritonitis (in combination with metronidazole) [81-83]. When the analysis was performed for specific nosocomial pathogens isolated from centers in North America, a standard regimen of cefepime had a high probability of attaining targets for Enterobacteriaceae, but not for P. aeruginosa and A. baumannii [84,85].
Expected probabilities of target attainment for cefepime (%T > MIC for 40% of dosing interval) were evaluated using a Monte Carlo simulation against a large collection of Staphylococcus spp. isolates detected during the SENTRY study in 1998-2004. Using the existing CLSI breakpoints, cefepime showed an advantage over ceftazidime (four- to eightfold) and superiority at the usual dosing over ceftriaxone for oxacillin-susceptible staphylococci. Probabilities of target attainment remained at a minimum of 90% to a breakpoint of 8 mg/l using 1 g every 8 -12 h. It seems that, when used at doses of at least 3 g/day, cefepime assures maximal coverage of oxacillin-susceptible staphylococci [86]. The pharmacodynamics in the CSF of cefepime against S. pneumoniae (MIC50 ≤ 0.06 mg/l) were compared with those of ceftriaxone. The probabilities of achieving targets of therapy (50 and 100% T > MIC) were superior for cefepime (91.8 and 82%, respectively) than for ceftriaxone (76 and 65%, respectively) [87].
The integration of pharmacokinetic and pharmacodynamic data to predict the efficacy of an antibiotic regimen provides tremendous insights. However, these analyses have limitations. They represent mathematical models that incorporate changing parameters (for instance, the exact %T > MIC that correlates with bactericidal vs bacteriostatic activity), which vary with location and time (MICs for different bacteria) and that may vary widely in different patient populations (pharmacokinetic data). Furthermore, these studies simplify by design and are unable to incorporate other clinical and host factors that have an impact on the course of an infection. Therefore, these studies complement but do not replace the systematic and experimental evaluation of clinical outcomes.
Clinical efficacy
Serious infections
In a retrospective analysis, cefepime treatment was analyzed in 13 ICU patients with different sites of infection due to ESBL-producing K. pneumoniae/E. coli isolates. Overall, 77% of cases were clinically cured. However, it is important to note that MICs for cefepime were no greater than 1 mg/l for ten cases, 2 mg/l for two cases and greater than 64 mg/l for the remaining one [88]. The efficacy of treatment with cefepime (2 g every 8 h) versus carbapenems (0.5 g every 6 h) was also retrospectively evaluated among ICU patients infected with a clonal TEM-24 ESBL-producing E. aerogenes isolate. Treatment failure was observed in 38% of patients treated with cefepime versus 30% of those receiving carbapenems [89]. In another retrospective study performed to analyze the outcome of cephalosporin treatment for serious infections due to Enterobacteriaceae-producing ESBLs, the overall failure of cefepime treatment was 83% [90]. In a retrospective study of nonurine ESBL-producing Klebsiella spp. or E. coli infections, patients receiving cefepime monotherapy (1 g every 12 h or 1 g every 24 h) were compared with cases receiving cefepime for infections due to non-ESBL producers. Patients receiving cefepime for an infection caused by ESBL producers were approximately ten-times as likely to have an unsuccessful clinical response compared with those with non-ESBL producers. In particular, among patients with infections due to ESBL producers, treatment failure was observed in 50% of cases when the MIC for cefepime was a maximum of 4 mg/l and in 75% of cases when the MIC was at least 16 mg/l [91].
In a recent analysis, clinical outcomes of 204 cases of bacteremia due to Gram-negative organisms have been retrospectively analyzed by Bhat et al. to establish whether current cefepime breakpoints need to be revised [30]. Mortality at 28 days varied according to the cefepime MIC of the pathogens: 23% for a MIC of up to 1 mg/l, 28% for a MIC of 2 mg/l, 27% for a MIC of 4 mg/l, 56% for a MIC of 8 mg/l and 53% for a MIC of at least 16 mg/l. Patients infected with organisms with a MIC of at least 8 mg/l had twice or greater mortality compared with those patients infected with bacteria with a cefepime of MIC less than 8 mg/l (55 vs 24%, respectively; p = 0.001). Mortality was higher for P. aeruginosa cases treated with cefepime when isolates had a MIC of at least 8 mg/l (59%) versus those with MIC values no higher than 4 mg/l (21%) [30].
In the cases of pneumonia due to S. pneumoniae and H. influenzae, satisfactory outcomes were obtained using intravenous cefepime 1 g every 12 h [92,93]. In a study analyzing ICU patients affected by pneumonia due to P. aeruginosa, clinical success rates and microbiological eradication were 78 and 77%, respectively [94]. Severe nosocomial pneumonia in ICUs has been successfully treated with cefepime 2 g intravenously every 12 h, plus amikacin 7.5 mg/kg every 12 h. The main pathogens in this analysis were S. aureus, P. aeruginosa, Klebsiella spp., Enterobacter spp. and Serratia spp. [95]. A randomized evaluator blind trial compared cefepime (2 g every 8 h) with imipenem (0.5 g every 6 h) for the treatment of nosocomial pneumonia among ICU patients. Pneumonia therapy caused by ESBL producers failed in 31% of patients in the cefepime group (the MIC values for these cases ranged from 2 to 4 mg/l), while all patients in the imipenem group were cured [96].
A recent meta-analysis evaluating the safety and efficacy of cefepime versus other β-lactams [97] (see later) included a subgroup analysis of patients with community-acquired and hospital-acquired pneumonia. The difference in mortality in these subgroups did not reach statistical significance (Table 7). Rather, it was observed that the overall increased mortality among patients treated with cefepime was driven by the subgroup of patients with febrile neutropenia (relative risk [RR] = 1.42; p = 0.009).
Table 7.
Summary of results of a systematic review and meta-analysis of 57 clinical trials evaluating efficacy and safety of cefepime
| Outcome index | Statistical comparison (number of clinical trials) | Cefepime (n/N patients) | Comparator (n/N patients) | RR (95% CI) | p-value |
|---|---|---|---|---|---|
| All-cause mortality by comparator drug | Total (n = 41) | 309/3931 | 220/3457 | 1.26 (1.08-1.49) | 0.005 |
| Cefepime vs ceftazidime (n = 23) | 167/2006 | 116/1625 | 1.20 (0.96-1.50) | 0.11 | |
| Cefepime vs piperacillin-tazobactam (n = 3) | 30/398 | 15/416 | 2.14 (1.17-3.89) | 0.01 | |
| Cefepime vs imipenem or meropenem (n = 6) | 73/808 | 62/802 | 1.20 (0.88-1.63) | 0.24 | |
| Cefepime vs ceftriaxone or cefotaxime (n = 9) | 39/719 | 27/614 | 1.24 (0.77-1.99) | 0.38 | |
| All-cause mortality by comparator infection | Total (n = 41) | 309/3931 | 220/3457 | 1.26 (1.08-1.49) | 0.005 |
| Neutropenic fever (n = 19) | 130/2043 | 87/1928 | 1.42 (1.09-1.84) | 0.009 | |
| Pneumonia (n = 10) | 110/953 | 84/767 | 1.15 (1.89-1.48) | 0.29 | |
| Urinary tract/gynecological infections (n = 2) | 0/308 | 0/222 | NE | NA | |
| Other infections (n = 10) | 69/627 | 49/540 | 1.20 (0.85-1.69) | 0.3 | |
| Clinical failure by comparator infection | Total (n = 55) | 1715/4656 | 1628/4215 | 0.98 (0.93-1.03) | 0.37 |
| Neutropenic fever (n = 20) | 1258/2258 | 1178/2152 | 1.00 (0.96-1.05) | 0.88 | |
| Community-acquired pneumonia (n = 15) | 107/829 | 105/658 | 0.78 (0.61-1.00) | 0.05 | |
| Hospital-acquired pneumonia (n = 3) | 132/294 | 142/292 | 0.92 (0.77-1.09) | 0.34 | |
| Urinary tract/gynecological infections (n = 5) | 41/397 | 22/276 | 1.12 (0.69-1.81) | 0.66 | |
| Meningitis (n = 2) | 34/146 | 37/152 | 0.96 (0.64-1.44) | 0.85 | |
| Other infections (n = 10) | 143/732 | 144/685 | 0.97 (0.79-1.19) | 0.77 |
CI: Confidence interval; NA: Not applicable; NE: Not estimable; RR: Relative risk.
Data from [97].
Empirical treatment of febrile neutropenia
Despite its potent antimicrobial efficacy, there is ongoing concern regarding the increased mortality among patients with febrile neutropenia who are empirically treated with cefepime compared with other β-lactams. In a meta-analysis performed by Paul et al., cefepime was assessed in 17 trials, of which it was compared with ceftazidime in eight trials, imipenem in four, piperacillin/tazobactam in three and meropenem in two [98]. This analysis showed that all-cause mortality was significantly higher with cefepime compared with other β-lactams (RR = 1.44; 95% confidence interval [CI]: 1.06-1.94; p = 0.02). Four studies recruited only children and mortality was higher with cefepime when compared with ceftazidime (RR = 2.28; 95% CI: 0.53-9.79; p = 0.02) and equal compared with meropenem. The ‘effect estimate’ was higher among studies that used less than the recommended dose for cefepime in febrile neutropenia, but did not reach statistical significance (RR = 2.01; 95% CI: 0.87-5.08). Significant differences were not detected between cefepime and comparators with regard to all secondary efficacy outcomes (i.e., treatment failure, microbiological failure, infection-related mortality, any antibiotic modification, glycopeptide addition, antifungal drug addition, any superinfection, bacterial superinfection and any adverse event) [98]. Similar findings were reported in a more recent meta-analysis by Yahav et al. involving a total of 19 studies (Table 7) [97].
It should be noted that, although it is the point of meta-analyses to combine results of trials so that significance can be achieved, only two out of 19 trials demonstrated statistically significant higher mortality with the use of cefepime [99,100]. One of the trials is a prospective, double-blind, randomized trial of cefepime versus ceftazidime for the treatment of 276 neutropenic cancer patients with fever [100]. Treatment success, clearance of bacteremia and adverse effects were similar in both groups. There was a higher overall mortality within 30 days of discontinuation of the drugs in the cefepime group (15% [22 out of 143] vs 8% [10 out of 133]). However, the deaths were not attributed to administration of the drug. Mortality rates within 2 weeks of stopping the drug were equivalent (8 vs 6%) and only three deaths in each group were due to progression of the initial infection; the rest were deemed to be superinfections.
Overall, we would also emphasize that the authors included studies in which another antibiotic in addition to cefepime was part of the therapy (e.g., glycopeptides and aminoglycosides) and a third of the trials were performed more than 10 years ago, when the spread of multidrug-resistant organisms (e.g., ESBL producers) was still limited and our ability to collect clinical and epidemiological information was less sophisticated.
Meningitis
In patients with community-acquired K. pneumoniae meningitis who were treated with cefepime, there was a lower mortality rate when compared with ceftazidime [101]. Cefepime (2 g every 8 h) plus ciprofloxacin (400 mg every 8 h) was able to eradicate a postoperative meningitis due to E. cloacae [102]. A 6-week course of intravenous cefepime (2 g every 8 h) plus gentamicin (200 mg every 8 h) was able to cure a 56-year-old woman affected by endocarditis and meningitis due to P. aeruginosa [103]. A total of 90 pediatric patients were prospectively randomized to receive cefepime (n = 43) or cefotaxime (n = 47) for therapy of bacterial meningitis. Overall, the following pathogens were detected: H. influenzae type b (61%), N. meningitidis (18%) and S. pneumoniae (13%). Notably, MIC values for cefepime and cefotaxime were constantly no higher than 0.06 mg/l for all bacteria isolated from CSF. In total, six (7%) patients died (two treated with cefepime and four treated with cefotaxime). Clinical response and hospital stay were similar for the two treatment regimens. Concentrations of cefepime in CSF varied from 55- to 95-times greater than the maximal MIC required by the causative pathogens. Audiologic and/or neurologic sequelae were found in 16% of the cefepime-treated and 15% of the cefotaxime-treated patients [49].
Urinary tract & gynecological infections
Results comparable to those obtained with ceftazidime are observed with the use of cefepime in complicated UTI [92,104]. A total of 300 episodes of pyelonephritis were observed in pediatric patients enrolled in a randomized, open label, multicenter trial where cefepime was compared with ceftazidime (both administered parenterally at 50 mg/kg every 8 h). Bacteriologic eradication was achieved in 96 and 94% of cefepime and ceftazidime patients and was maintained after 4-6 weeks in 86 and in 83% of cases, respectively. A satisfactory clinical response occurred in 98 and 96% of cefepime and ceftazidime patients, respectively. Cefepime and ceftazidime were equally safe [105].
Patients with acute gynecological infections were randomized to receive cefepime (2 g every 12 h) or cefotaxime (2 g every 8 h), both intramuscularly or by a 30-min intravenous administration for approximately 4-5 days. Clinical response was satisfactory in 85% of cefepime recipients and 83% of cefotaxime recipients. All pathogens were eradicated in 81% of cefepime-treated and in 86% of cefotaxime-treated patients [106].
Abdominal infections
In a clinical trial, cefepime (2 g every 12 h intravenously) plus metronidazole (500 mg every 6 h) was compared with imipenem-cilastatin (500 mg every 6 h) for the treatment of complicated intra-abdominal infections. Patients treated with cefepime plus metronidazole were cured (88%) more frequently than those receiving imipenem-cilastatin (76%). Pathogens were eradicated in significantly more patients treated with combined cefepime and metronidazole (89%) than with imipenem-cilastatin (76%) (p = 0.01) [107].
A double-blind, randomized study compared cefepime plus metronidazole (2 g intravenously every 12 h and 500 mg intravenously every 8 h) with gentamicin plus clindamycin (1.5 mg/kg intra venously and 0.9 g intravenously every 8 h), administered in surgically treated patients with appendicitis. The efficacy of cefepime plus metronidazole was equivalent to that of clindamycin plus gentamicin [108]. An open, prospective, randomized trial compared cefepime (2 g every 12 h) with gentamicin plus mezlocillin (1.5 mg/kg every 8 h and 3 g every 4 h) in patients affected by acute cholecystitis/cholangitis. All patients treated with gentamicin plus mezlocillin were cured, as were 97.5% of the patients treated with cefepime [109].
In a recent multicenter, open-labeled, randomized study, the prophylactic efficacy of cefepime and ceftriaxone was compared in patients undergoing biliary tract surgery. A total of 209 patients were randomized to receive a preoperative infusion of 2 g of cefepime or ceftriaxone, both in a single intravenous administration. Antimicrobial prophylaxis was successful in 98.9% of patients in the cefepime group and 97.7% in the ceftriaxone group [110].
Osteomyelitis
In one clinical study, 23 patients with osteomyelitis, mainly due to S. aureus, were treated with cefepime 2 g intravenously every 12 h. Of these, 20 were cured, but treatment failed in three [111]. In a prospective, randomized, open-label trial, the efficacy of cefepime in the treatment of osteomyelitis caused by Gram-negative bacilli was evaluated. Cefepime was administered intravenously or intramuscularly (2 g every 8 h or 12 h). Overall, 73.3% of patients were cured [112].
Safety & tolerability
The results of a meta-analysis published in early 2007 indicated a potential increased mortality of 26% in patients treated with cefepime compared with patients treated with other β-lactam drugs [97]. The US FDA requested additional data from the manufacturer and, at this point, has not reached a definitive conclusion as to whether the observed increased mortality is due to cefepime. In the interim, the continued use of cefepime is conditioned by information from that meta-analysis but also from the previously published body of clinical experience with the use of cefepime.
The meta-analysis by Yahav et al. reviewed the efficacy and safety of cefepime in 57 clinical trials [97]. The trials assessed different sites of infection treated with different β-lactam antimicrobials (Table 7). The 30-day all-cause mortality was higher with cefepime than other β-lactams. All antibiotic comparators were associated with lower all-cause mortality, with significance shown for piperacillin-tazobactam. Exclusion of studies that compared cefepime with carbapenems did not eliminate the disadvantages observed for cefepime. All-cause mortality was higher for cefepime in all types of infection, with the exception of UTIs. However, as previously discussed, the difference in all-cause mortality was significant only for febrile neutropenia (Table 7) [97]. Overall, significant differences between groups in treatment failure, superinfection and adverse events were not found. Therefore, a clear explanation for the findings, especially given the previous safety record of cefepime is not available (see later).
In general, toxic effects of cefepime appear to be infrequent and similar to those observed with other cephalosporins, such as ceftazidime. In clinical trials using multiple doses of cefepime, patients were treated with the recommended dosages of cefepime (0.5-2 g intravenously every 12 h). Deaths or permanent disabilities related to drug toxicity were not found. The percentage of cefepime-treated patients who discontinued the study drug was very similar at daily doses of 0.5, 1 and 2 g every 12 h (0.8, 1.1 and 2.0%, respectively). The following adverse events were related to cefepime: local reactions (3%), including phlebitis (1.3%), pain or inflammation (0.6%) and rash (1.1%). Colitis (including pseudomembranous colitis), diarrhea, fever, headache, nausea, oral moniliasis, pruritus, urticaria, vaginitis and vomiting were observed in less than 1% of patients. At the higher dose of 2 g every 6-8 h, the incidence of probably related adverse events was higher: rash (4%), diarrhea (3%), nausea (2%), vomiting (1%), pruritus (1%), fever (1%) and headache (1%) [47,60,92,93,113-115].
Neurotoxicity of cefepime has been reported [116-119]. The spectrum of manifestations has been broad, including confusion, hallucinations, agitation, convulsions, tremor, delirium and coma. Convulsions have been reported in 0.2% of patients. The period between the start of cefepime treatment and neurologic manifestations varied between 1 and 10 days, and the majority of neurological symptoms regressed within 2-7 days after stopping the antibiotic infusion. Almost all patients affected have had renal failure and the dose of cefepime was relatively high for the level of renal failure [116-119].
During cefepime administration, there was a decrease in E. coli and bifidobacteria in feces, whereas Bacteroides spp. and Clostridium spp. showed a slight increase. The numbers of fecal bacteria returned to normal 20-48 days after the study was completed [120]. Cephalosporins have long been implicated as an important predisposing cause of C. difficile-associated disease (CDAD). In particular, the extensive use of the extended-spectrum cephalosporins has been well recognized as an important risk factor for CDAD [121-123]. However, clinical data regarding the possible role of cefepime in CDAD are limited. A matched univariate analysis of case and control patients showed a significant difference between the two groups of cases in the use of cefepime during the 4 weeks prior to the detection of C. difficile toxin in the stool (24.1 vs 13.8%, respectively) [124]. This contrasts with data from animal studies. The subcutaneous administration of cefepime to mice did not facilitate in vitro growth and toxin production by C. difficile in cecal contents, unlike antibiotics that probably disrupt the anaerobic microflora (e.g., clindamycin) [125]. Similarly, cefepime may possess a low potential for promoting rectal colonization with resistant Gram-negative rods among pediatric ICU patients [126]. By contrast, the acquisition of rectal colonization of vancomycin-resistant Enterococcus (VRE) among ICU patients revealed that the administration of cefepime may be associated with the frequent acquisition of VRE [127].
The following adverse laboratory changes have been reported: positive Coombs’ test (16.2%), decreased phosphorous (2.8%), increased serum glutamic oxaloacetic transaminase and serum glutamic pyruvic transaminase (∼2.5%), eosinophils (1.7%) and abnormal PT and PTT (∼1.5%). Whereas, increased alkaline phosphatase, blood urea nitrogen, calcium, creatinine, potassium and total bilirubin or decreased calcium, hematocrit, neutrophils, platelets and white blood cells were recorded in fewer than 1% of patients [47,60,92,93,113-115]. Notably, neutropenia resulted in 62% of patients receiving long-term cefepime therapy (17-30 days) for osteomyelitis. Blood counts returned to normal within 1 week of cefepime discontinuation [128].
Conclusion & expert commentary
Cefepime possess higher in vitro activity than other extended-spectrum cephalosporins against common Gram-negative and Gram-positive pathogens. However, cefepime is ineffective against ESBL-producing Enterobacteriaceae, A. baumannii and highly derepressed AmpC-producing P. aeruginosa isolates. The re-evaluation of the existent CLSI Gram-negative susceptibility breakpoints for cefepime is necessary. The EUCAST susceptibility criteria (e.g., MIC for Enterobacteriaceae ≤ 1 mg/l) seem to better integrate in vitro, pharmacodynamic and clinical data.
Pharmacokinetic studies show that cefepime reaches concentrations in the serum and body capable of inhibiting organisms possessing MICs lower than 2 mg/l. By contrast, retrospective analysis and statistical simulations showed that the standard scheduled dosages are not able to achieve satisfactory probabilities of microbiological eradication for Gram-negative organisms showing MICs for cefepime of at least 2 mg/l. Therefore, we recommend that cefepime should not be used to treat infections due to ESBL-producing Enterobacteriaceae and A. baumannii. For P. aeruginosa, the probability of achieving the pharmacodynamic target for isolates with MIC values of 2-8 mg/l could be improved by increasing the dose of cefepime and/or extending the infusion time. Unless this can be achieved, it is our opinion that the use of cefepime monotherapy to treat serious infection due to P. aeruginosa is not an advisable choice.
Since meta-analyses showed a higher mortality among patients receiving cefepime than other β-lactams, we believe that the use of cefepime as monotherapy should be tempered by a careful consideration of risks and benefits (at least in the case of febrile neutropenia). An extensive review of the previous trials involving cefepime is in progress and, in the near future, the FDA will release a final recommendation.
Five-year view
Questions regarding cefepime that remain to be answered in the next few years include the following:
What susceptibility breakpoint for cefepime leads to the best outcome for infections due to Gram-negative organisms?
Is cefepime able to ensure a better treatment outcome than other extended-spectrum cephalosporins during infections caused by AmpC producers (e.g., Enterobacter spp.)?
What dosing regimens of cefepime offer the best clinical outcomes?
Is cefepime treatment in patients with febrile neutropenia really related to a higher mortality rate than other extended-spectrum β-lactams?
Should cefepime be combined with β-lactamase inhibitors to be used against Enterobacteriaceae-coproducing AmpC and ESBL enzymes?
Key issues.
Cefepime Clinical and Laboratory Standards Institute breakpoints of susceptibility against Gram-negative bacteria should be revised.
Cefepime should not be used to treat infections due to extended-spectrum β-lactamase-producing organisms and Acinetobacter baumannii.
Conventional doses of cefepime should not be used to treat infections caused by Pseudomonas aeruginosa.
According to large international studies, the MIC50/90 for cefepime are lower than those of ceftazidime.
Cefepime remains an option against methicillin-susceptible staphylococci and Streptococcus pneumoniae infections.
Acknowledgments
Financial & competing interests disclosure
RA Bonomo has recieved grants from AstraZeneca, Merck, Elan and Basilea Pharmaceutica, Inc. RA Bonomo serves as a consultant to Wyeth Pharmaceuticals, Pfizer and Basilea Pharmaceutica, Ltd. This work was supported in part by AstraZeneca (to A Endimiani and RA Bonomo), the National Institutes of Health (grant RO1-AI063517 to RA Bonomo), the Veterans Affairs Merit Review Program (RA Bonomo), and Geriatric Research Education and Clinical Center (RA Bonomo). F Perez is supported by the Wyeth Fellowship in Antimicrobial Resistance The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Andrea Endimiani, Department of Medicine, Section of Infectious Diseases, Case Western Reserve University, School of Medicine, Cleveland, OH, USA and Research Service, Louis Stokes Cleveland Department of Veterans Affairs Medical Center, 10701 East Boulevard, Cleveland, OH 44106, USA, Tel.: +1 216 791 3800 ext. 4645, Fax: +1 216 231 3482, aendimiani@gmail.com.
Federico Perez, Division of Infectious Diseases and HIV Medicine, University Hospitals Case Medical Center, Cleveland, OH, USA.
Robert A Bonomo, Research Service, Louis Stokes Cleveland Department of Veterans Affairs Medical Center, Cleveland, OH, USA and Departments of Pharmacology and Molecular Biology and Microbiology, Case Western Reserve University, School of Medicine, Cleveland, OH, USA.
References
- 1.Witte W, Cuny C, Klare I, Nubel U, Strommenger B, Werner G. Emergence and spread of antibiotic-resistant Gram-positive bacterial pathogens. Int. J. Med. Microbiol. 2008;298(56):365–377. doi: 10.1016/j.ijmm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Gaynes R, Edwards JR. Overview of nosocomial infections caused by Gram-negative bacilli. Clin. Infect. Dis. 2005;41(6):848–854. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 3.Meroueh SO, Bencze KZ, Hesek D, et al. Three-dimensional structure of the bacterial cell wall peptidoglycan. Proc. Natl Acad. Sci. USA. 2006;103(12):4404–4409. doi: 10.1073/pnas.0510182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunha BA, Gill MV. Cefepime. Med. Clin. North Am. 1995;79(4):721–732. doi: 10.1016/s0025-7125(16)30035-9. [DOI] [PubMed] [Google Scholar]
- 5.Nikaido H, Liu W, Rosenberg EY. Outer membrane permeability and β-lactamase stability of dipolar ionic cephalosporins containing methoxyimino substituents. Antimicrob. Agents Chemother. 1990;34(2):337–342. doi: 10.1128/aac.34.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan WC, Li RC, Ling JM, Cheng AF, Schentag JJ. Markedly different rates and resistance profiles exhibited by seven commonly used and newer β-lactams on the selection of resistant variants of Enterobacter cloacae. J. Antimicrob. Chemother. 1999;43(1):55–60. doi: 10.1093/jac/43.1.55. [DOI] [PubMed] [Google Scholar]
- 7.Konstantinou K, Baddam K, Lanka A, Reddy K, Zervos M. Cefepime versus ceftazidime for treatment of pneumonia. J. Int. Med. Res. 2004;32(1):84–93. doi: 10.1177/147323000403200114. [DOI] [PubMed] [Google Scholar]
- 8.Carsenti-Etesse H, Cavallo JD, Roger PM, et al. Effect of β-lactam antibiotics on the in vitro development of resistance in Pseudomonas aeruginosa. Clin. Microbiol. Infect. 2001;7(3):144–151. doi: 10.1046/j.1469-0691.2001.00225.x. [DOI] [PubMed] [Google Scholar]
- 9.Paterson DL, Bonomo RA. Extended-spectrum β-lactamases: a clinical update. Clin. Microbiol. Rev. 2005;18(4):657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Queenan AM, Bush K. Carbapenemases: the versatile β-lactamases. Clin. Microbiol. Rev. 2007;20(3):440–458. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez F, Endimiani A, Hujer KM, Bonomo RA. The continuing challenge of ESBLs. Curr. Opin. Pharmacol. 2007;7(5):459–469. doi: 10.1016/j.coph.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luzzaro F, Docquier JD, Colinon C, et al. Emergence in Klebsiella pneumoniae and Enterobacter cloacae clinical isolates of the VIM-4 metallo-β-lactamase encoded by a conjugative plasmid. Antimicrob. Agents Chemother. 2004;48(2):648–650. doi: 10.1128/AAC.48.2.648-650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossolini GM, Mantengoli E. Treatment and control of severe infections caused by multiresistant Pseudomonas aeruginosa. Clin. Microbiol. Infect. 2005;11(Suppl 4):17–32. doi: 10.1111/j.1469-0691.2005.01161.x. [DOI] [PubMed] [Google Scholar]
- 14.Juan C, Gutierrez O, Oliver A, Ayestaran JI, Borrell N, Perez JL. Contribution of clonal dissemination and selection of mutants during therapy to Pseudomonas aeruginosa antimicrobial resistance in an intensive care unit setting. Clin. Microbiol. Infect. 2005;11(11):887–892. doi: 10.1111/j.1469-0691.2005.01251.x. [DOI] [PubMed] [Google Scholar]
- 15.Hocquet D, Nordmann P, El Garch F, Cabanne L, Plesiat P. Involvement of the MexXY-OprM efflux system in emergence of cefepime resistance in clinical strains of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2006;50(4):1347–1351. doi: 10.1128/AAC.50.4.1347-1351.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta V. Metallo-β-lactamases in Pseudomonas aeruginosa and Acinetobacter species. Expert Opin. Investig. Drugs. 2008;17(2):131–143. doi: 10.1517/13543784.17.2.131. [DOI] [PubMed] [Google Scholar]
- 17.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2007;51(10):3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bratu S, Landman D, Martin DA, Georgescu C, Quale J. Correlation of antimicrobial resistance with β-lactamases, the OmpA-like porin, and efflux pumps in clinical isolates of Acinetobacter baumannii endemic to New York City. Antimicrob. Agents Chemother. 2008;52(9):2999–3005. doi: 10.1128/AAC.01684-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walther-Rasmussen J, Hoiby N. Class A carbapenemases. J. Antimicrob. Chemother. 2007;60(3):470–482. doi: 10.1093/jac/dkm226. [DOI] [PubMed] [Google Scholar]
- 20.Hujer KM, Hamza NS, Hujer AM, et al. Identification of a new allelic variant of the Acinetobacter baumannii cephalosporinase, ADC-7 β-lactamase: defining a unique family of class C enzymes. Antimicrob. Agents Chemother. 2005;49(7):2941–2948. doi: 10.1128/AAC.49.7.2941-2948.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walther-Rasmussen J, Hoiby N. OXA-type carbapenemases. J. Antimicrob. Chemother. 2006;57(3):373–383. doi: 10.1093/jac/dki482. [DOI] [PubMed] [Google Scholar]
- 22.Hanson ND. AmpC β-lactamases: what do we need to know for the future? J. Antimicrob. Chemother. 2003;52(1):2–4. doi: 10.1093/jac/dkg284. [DOI] [PubMed] [Google Scholar]
- 23.Ramphal R, Ambrose PG. Extended-spectrum β-lactamases and clinical outcomes: current data. Clin. Infect. Dis. 2006;42(Suppl 4):S164–S172. doi: 10.1086/500663. [DOI] [PubMed] [Google Scholar]
- 24.Walsh TR. The emergence and implications of metallo-β-lactamases in Gram-negative bacteria. Clin. Microbiol. Infect. 2005;11(Suppl 6):2–9. doi: 10.1111/j.1469-0691.2005.01264.x. [DOI] [PubMed] [Google Scholar]
- 25.Rice LB, Bellais S, Carias LL, et al. Impact of specific PBP5 mutations on expression of β-lactam resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 2004;48(8):3028–3032. doi: 10.1128/AAC.48.8.3028-3032.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hujer AM, Kania M, Gerken T, et al. Structure-activity relationships of different β-lactam antibiotics against a soluble form of Enterococcus faecium PBP5, a type II bacterial transpeptidase. Antimicrob. Agents Chemother. 2005;49(2):612–618. doi: 10.1128/AAC.49.2.612-618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rice LB. Antimicrobial resistance in Gram-positive bacteria. Am. J. Infect. Control. 2006;34(5 Suppl 1):S11–S19. doi: 10.1016/j.ajic.2006.05.220. [DOI] [PubMed] [Google Scholar]
- 28.Clinical and Laboratory Standard Institute . Performance Standards For Antimicrobial Susceptibility Testing: 17th Informational Supplement. CLSI; Wayne, PA, USA: 2007. [Google Scholar]
- 29.Sanders WE, Jr, Tenney JH, Kessler RE. Efficacy of cefepime in the treatment of infections due to multiply resistant Enterobacter species. Clin. Infect. Dis. 1996;23(3):454–461. doi: 10.1093/clinids/23.3.454. [DOI] [PubMed] [Google Scholar]
- 30.Bhat SV, Peleg AY, Lodise TP, Jr, et al. Failure of current cefepime breakpoints to predict clinical outcomes of bacteremia caused by Gram-negative organisms. Antimicrob. Agents Chemother. 2007;51(12):4390–4395. doi: 10.1128/AAC.01487-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fritsche TR, Sader HS, Jones RN. Antimicrobial activity of ceftobiprole, a novel anti-methicillin-resistant Staphylococcus aureus cephalosporin, tested against contemporary pathogens: results from the SENTRY Antimicrobial Surveillance Program (2005-2006) Diagn. Microbiol. Infect. Dis. 2008;61(1):86–95. doi: 10.1016/j.diagmicrobio.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Bell JM, Turnidge JD, Jones RN. Prevalence of extended-spectrum β-lactamase-producing Enterobacter cloacae in the Asia-Pacific region: results from the SENTRY Antimicrobial Surveillance Program, 1998 to 2001. Antimicrob. Agents Chemother. 2003;47(12):3989–3993. doi: 10.1128/AAC.47.12.3989-3993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirakata Y, Matsuda J, Miyazaki Y, et al. Regional variation in the prevalence of extended-spectrum β-lactamase-producing clinical isolates in the Asia-Pacific region (SENTRY 1998-2002) Diagn. Microbiol. Infect. Dis. 2005;52(4):323–329. doi: 10.1016/j.diagmicrobio.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Goossens H, Grabein B. Prevalence and antimicrobial susceptibility data for extended-spectrum β-lactamase- and AmpC-producing Enterobacteriaceae from the MYSTIC Program in Europe and the United States (1997-2004) Diagn. Microbiol. Infect. Dis. 2005;53(4):257–264. doi: 10.1016/j.diagmicrobio.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Sader HS, Fritsche TR, Jones RN. Potency and spectrum trends for cefepime tested against 65746 clinical bacterial isolates collected in North American medical centers: results from the SENTRY Antimicrobial Surveillance Program (1998-2003) Diagn. Microbiol. Infect. Dis. 2005;52(3):265–273. doi: 10.1016/j.diagmicrobio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Livermore DM, Carter MW, Bagel S, et al. In vitro activities of ertapenem (MK-0826) against recent clinical bacteria collected in Europe and Australia. Antimicrob. Agents Chemother. 2001;45(6):1860–1867. doi: 10.1128/AAC.45.6.1860-1867.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuriyama T, Karasawa T, Nakagawa K, Nakamura S, Yamamoto E. Antimicrobial susceptibility of major pathogens of orofacial odontogenic infections to 11 β-lactam antibiotics. Oral. Microbiol. Immunol. 2002;17(5):285–289. doi: 10.1034/j.1399-302x.2002.170504.x. [DOI] [PubMed] [Google Scholar]
- 38.Loza E, Morosini MI, Canton R, Almaraz F, Reig M, Baquero F. Comparative in vitro activity of ertapenem against aerobic and anaerobic bacteria. Rev. Esp. Quimioter. 2003;16(2):209–215. [PubMed] [Google Scholar]
- 39.Johnson DM, Stilwell MG, Fritsche TR, Jones RN. Emergence of multidrug-resistant Streptococcus pneumoniae: report from the SENTRY Antimicrobial Surveillance Program (1999-2003) Diagn. Microbiol. Infect. Dis. 2006;56(1):69–74. doi: 10.1016/j.diagmicrobio.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Jones RN, Sader HS, Fritsche TR, Pottumarthy S. Comparisons of parenteral broad-spectrum cephalosporins tested against bacterial isolates from pediatric patients: report from the SENTRY Antimicrobial Surveillance Program (1998-2004) Diagn. Microbiol. Infect. Dis. 2007;57(1):109–116. doi: 10.1016/j.diagmicrobio.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 41.Fritsche TR, Sader HS, Jones RN. Comparative activity and spectrum of broad-spectrum β-lactams (cefepime, ceftazidime, ceftriaxone, piperacillin/tazobactam) tested against 12,295 staphylococci and streptococci: report from the SENTRY antimicrobial surveillance program (North America: 2001-2002) Diagn. Microbiol. Infect. Dis. 2003;47(2):435–440. doi: 10.1016/s0732-8893(03)00114-7. [DOI] [PubMed] [Google Scholar]
- 42.Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin. Infect. Dis. 2004;39(9):1267–1284. doi: 10.1086/425368. [DOI] [PubMed] [Google Scholar]
- 43.Blumer JL, Reed MD, Knupp C. Review of the pharmacokinetics of cefepime in children. Pediatr. Infect. Dis. J. 2001;20(3):337–342. doi: 10.1097/00006454-200103000-00032. [DOI] [PubMed] [Google Scholar]
- 44.Lipman J, Wallis SC, Rickard C. Low plasma cefepime levels in critically ill septic patients: pharmacokinetic modeling indicates improved troughs with revised dosing. Antimicrob. Agents Chemother. 1999;43(10):2559–2561. doi: 10.1128/aac.43.10.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sampol E, Jacquet A, Viggiano M, et al. Plasma, urine and skin pharmacokinetics of cefepime in burns patients. J. Antimicrob. Chemother. 2000;46(2):315–317. doi: 10.1093/jac/46.2.315. [DOI] [PubMed] [Google Scholar]
- 46.Barbhaiya RH, Forgue ST, Gleason CR, et al. Pharmacokinetics of cefepime after single and multiple intravenous administrations in healthy subjects. Antimicrob. Agents Chemother. 1992;36(3):552–557. doi: 10.1128/aac.36.3.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barbhaiya RH, Forgue ST, Gleason CR, et al. Safety, tolerance, and pharmacokinetic evaluation of cefepime after administration of single intravenous doses. Antimicrob. Agents Chemother. 1990;34(6):1118–1122. doi: 10.1128/aac.34.6.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rhoney DH, Tam VH, Parker D, Jr, McKinnon PS, Coplin WM. Disposition of cefepime in the central nervous system of patients with external ventricular drains. Pharmacotherapy. 2003;23(3):310–314. doi: 10.1592/phco.23.3.310.32108. [DOI] [PubMed] [Google Scholar]
- 49.Saez-Llorens X, Castano E, Garcia R, et al. Prospective randomized comparison of cefepime and cefotaxime for treatment of bacterial meningitis in infants and children. Antimicrob. Agents Chemother. 1995;39(4):937–940. doi: 10.1128/aac.39.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chadha D, Wise R, Baldwin DR, Andrews JM, Ashby JP, Honeybourne D. Cefepime concentrations in bronchial mucosa and serum following a single 2 gram intravenous dose. J. Antimicrob. Chemother. 1990;25(6):959–963. doi: 10.1093/jac/25.6.959. [DOI] [PubMed] [Google Scholar]
- 51.Boselli E, Breilh D, Duflo F, et al. Steady-state plasma and intrapulmonary concentrations of cefepime administered in continuous infusion in critically ill patients with severe nosocomial pneumonia. Crit. Care Med. 2003;31(8):2102–2106. doi: 10.1097/01.CCM.0000069734.38738.C8. [DOI] [PubMed] [Google Scholar]
- 52.Breilh D, Saux MC, Delaisement C, et al. Pharmacokinetic population study to describe cefepime lung concentrations. Pulm. Pharmacol. Ther. 2001;14(2):69–74. doi: 10.1006/pupt.2000.0269. [DOI] [PubMed] [Google Scholar]
- 53.Aras C, Ozdamar A, Ozturk R, Karacorlu M, Ozkan S. Intravitreal penetration of cefepime after systemic administration to humans. Ophthalmologica. 2002;216(4):261–264. doi: 10.1159/000063838. [DOI] [PubMed] [Google Scholar]
- 54.Okamoto MP, Gill MA, Nakahiro RK, et al. Tissue concentrations of cefepime in acute cholecystitis patients. Ther. Drug Monit. 1992;14(3):220–225. doi: 10.1097/00007691-199206000-00008. [DOI] [PubMed] [Google Scholar]
- 55.Petrikkos G, Kastanakis M, Markogiannakis A, et al. Pharmacokinetics of cefepime in bile and gall bladder tissue after prophylactic administration in patients with extrahepatic biliary diseases. Int. J. Antimicrob. Agents. 2006;27(4):331–334. doi: 10.1016/j.ijantimicag.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 56.Delcenserie R, Dellion-Lozinguez MP, Yzet T, et al. Pancreatic concentrations of cefepime. J. Antimicrob. Chemother. 2001;47(5):711–713. doi: 10.1093/jac/47.5.711. [DOI] [PubMed] [Google Scholar]
- 57.Breilh D, Boselli E, Bel JC, Chassard D, Saux MC, Allaouchiche B. Diffusion of cefepime into cancellous and cortical bone tissue. J. Chemother. 2003;15(2):134–138. doi: 10.1179/joc.2003.15.2.134. [DOI] [PubMed] [Google Scholar]
- 58.Tam VH, McKinnon PS, Akins RL, Drusano GL, Rybak MJ. Pharmacokinetics and pharmacodynamics of cefepime in patients with various degrees of renal function. Antimicrob. Agents Chemother. 2003;47(6):1853–1861. doi: 10.1128/AAC.47.6.1853-1861.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van der Auwera P, Santella PJ. Pharmacokinetics of cefepime: a review. J. Antimicrob. Chemother. 1993;32(Suppl B):103–115. doi: 10.1093/jac/32.suppl_b.103. [DOI] [PubMed] [Google Scholar]
- 60.Barbhaiya RH, Knupp CA, Pittman KA. Effects of age and gender on pharmacokinetics of cefepime. Antimicrob. Agents Chemother. 1992;36(6):1181–1185. doi: 10.1128/aac.36.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cronqvist J, Nilsson-Ehle I, Oqvist B, Norrby SR. Pharmacokinetics of cefepime dihydrochloride arginine in subjects with renal impairment. Antimicrob. Agents Chemother. 1992;36(12):2676–2680. doi: 10.1128/aac.36.12.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elwell RJ, Frye RF, Bailie GR. Pharmacokinetics of intraperitoneal cefepime in automated peritoneal dialysis. Perit. Dial. Int. 2005;25(4):380–386. [PubMed] [Google Scholar]
- 63.Trotman RL, Williamson JC, Shoemaker DM, Salzer WL. Antibiotic dosing in critically ill adult patients receiving continuous renal replacement therapy. Clin. Infect. Dis. 2005;41(8):1159–1166. doi: 10.1086/444500. [DOI] [PubMed] [Google Scholar]
- 64.Malone RS, Fish DN, Abraham E, Teitelbaum I. Pharmacokinetics of cefepime during continuous renal replacement therapy in critically ill patients. Antimicrob. Agents Chemother. 2001;45(11):3148–3155. doi: 10.1128/AAC.45.11.3148-3155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Allaouchiche B, Breilh D, Jaumain H, Gaillard B, Renard S, Saux MC. Pharmacokinetics of cefepime during continuous venovenous hemodiafiltration. Antimicrob. Agents Chemother. 1997;41(11):2424–2427. doi: 10.1128/aac.41.11.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lima-Rogel V, Medina-Rojas EL, Del Carmen Milan-Segovia R, et al. Population pharmacokinetics of cefepime in neonates with severe nosocomial infections. J. Clin. Pharm. Ther. 2008;33(3):295–306. doi: 10.1111/j.1365-2710.2008.00913.x. [DOI] [PubMed] [Google Scholar]
- 67.Huls CE, Prince RA, Seilheimer DK, Bosso JA. Pharmacokinetics of cefepime in cystic fibrosis patients. Antimicrob. Agents Chemother. 1993;37(7):1414–1416. doi: 10.1128/aac.37.7.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Castanheira M, Sader HS, Deshpande LM, Fritsche TR, Jones RN. Antimicrobial activities of tigecycline and other broad-spectrum antimicrobials tested against serine carbapenemase- and metallo-β-lactamase-producing Enterobacteriaceae: report from the SENTRY antimicrobial surveillance program. Antimicrob. Agents Chemother. 2008;52(2):570–573. doi: 10.1128/AAC.01114-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bonapace CR, White RL, Friedrich LV, Norcross ED, Bosso JA. Pharmacokinetics of cefepime in patients with thermal burn injury. Antimicrob. Agents Chemother. 1999;43(12):2848–2854. doi: 10.1128/aac.43.12.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ambrose PG, Bhavnani SM, Jones RN. Pharmacokinetics-pharmacodynamics of cefepime and piperacillin-tazobactam against Escherichia coli and Klebsiella pneumoniae strains producing extended-spectrum β-lactamases: report from the ARREST program. Antimicrob. Agents Chemother. 2003;47(5):1643–1646. doi: 10.1128/AAC.47.5.1643-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Craig WA. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn. Microbiol. Infect. Dis. 1995;22(12):89–96. doi: 10.1016/0732-8893(95)00053-d. [DOI] [PubMed] [Google Scholar]
- 72.Frei CR, Burgess DS. Continuous infusion β-lactams for intensive care unit pulmonary infections. Clin. Microbiol. Infect. 2005;11(5):418–421. doi: 10.1111/j.1469-0691.2005.01106.x. [DOI] [PubMed] [Google Scholar]
- 73.Burgess DS, Frei CR. Comparison of β-lactam regimens for the treatment of Gram-negative pulmonary infections in the intensive care unit based on pharmacokinetics/pharmacodynamics. J. Antimicrob. Chemother. 2005;56(5):893–898. doi: 10.1093/jac/dki335. [DOI] [PubMed] [Google Scholar]
- 74.Tam VH, McKinnon PS, Akins RL, Rybak MJ, Drusano GL. Pharmacodynamics of cefepime in patients with Gram-negative infections. J. Antimicrob. Chemother. 2002;50(3):425–428. doi: 10.1093/jac/dkf130. [DOI] [PubMed] [Google Scholar]
- 75.Tam VH, Louie A, Lomaestro BM, Drusano GL. Integration of population pharmacokinetics, a pharmacodynamic target, and microbiologic surveillance data to generate a rational empiric dosing strategy for cefepime against Pseudomonas aeruginosa. Pharmacotherapy. 2003;23(3):291–295. doi: 10.1592/phco.23.3.291.32110. [DOI] [PubMed] [Google Scholar]
- 76.Lee SY, Kuti JL, Nicolau DP. Cefepime pharmacodynamics in patients with extended spectrum β-lactamase (ESBL) and non-ESBL infections. J. Infect. 2007;54(5):463–468. doi: 10.1016/j.jinf.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 77.Roos JF, Bulitta J, Lipman J, Kirkpatrick CM. Pharmacokinetic-pharmacodynamic rationale for cefepime dosing regimens in intensive care units. J. Antimicrob. Chemother. 2006;58(5):987–993. doi: 10.1093/jac/dkl349. [DOI] [PubMed] [Google Scholar]
- 78.Eagye KJ, Nicolau DP, Lockhart SR, et al. A pharmacodynamic analysis of resistance trends in pathogens from patients with infection in intensive care units in the United States between 1993 and 2004. Ann. Clin. Microbiol. Antimicrob. 2007;6:11. doi: 10.1186/1476-0711-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.DeRyke CA, Kuti JL, Nicolau DP. Pharmacodynamic target attainment of six β-lactams and two fluoroquinolones against Pseudomonas aeruginosa, Acinetobacter baumannii, Escherichia coli, and Klebsiella species collected from United States intensive care units in 2004. Pharmacotherapy. 2007;27(3):333–342. doi: 10.1592/phco.27.3.333. [DOI] [PubMed] [Google Scholar]
- 80.Lodise TP, Jr, Rhoney DH, Tam VH, McKinnon PS, Drusano GL. Pharmacodynamic profiling of cefepime in plasma and cerebrospinal fluid of hospitalized patients with external ventriculostomies. Diagn. Microbiol. Infect. Dis. 2006;54(3):223–230. doi: 10.1016/j.diagmicrobio.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 81.Kotapati S, Kuti JL, Nicolau DP. Pharmacodynamic modeling of β-lactam antibiotics for the empiric treatment of secondary peritonitis: a report from the OPTAMA program. Surg. Infect. (Larchmt) 2005;6(3):297–304. doi: 10.1089/sur.2005.6.297. [DOI] [PubMed] [Google Scholar]
- 82.Sun HK, Kuti JL, Nicolau DP. Pharmacodynamics of antimicrobials for the empirical treatment of nosocomial pneumonia: a report from the OPTAMA Program. Crit. Care Med. 2005;33(10):2222–2227. doi: 10.1097/01.ccm.0000181528.88571.9b. [DOI] [PubMed] [Google Scholar]
- 83.Maglio D, Kuti JL, Nicolau DP. Simulation of antibiotic pharmacodynamic exposure for the empiric treatment of nosocomial bloodstream infections: a report from the OPTAMA program. Clin. Ther. 2005;27(7):1032–1042. doi: 10.1016/j.clinthera.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 84.Masterton RG, Kuti JL, Turner PJ, Nicolau DP. The OPTAMA programme: utilizing MYSTIC (2002) to predict critical pharmacodynamic target attainment against nosocomial pathogens in Europe. J. Antimicrob. Chemother. 2005;55(1):71–77. doi: 10.1093/jac/dkh511. [DOI] [PubMed] [Google Scholar]
- 85.Kuti JL, Nightingale CH, Nicolau DP. Optimizing pharmacodynamic target attainment using the MYSTIC antibiogram: data collected in North America in 2002. Antimicrob. Agents Chemother. 2004;48(7):2464–2470. doi: 10.1128/AAC.48.7.2464-2470.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sader HS, Bhavnani SM, Ambrose PG, Jones RN, Pfaller MA. Re-evaluation of the role of broad-spectrum cephalosporins against staphylococci by applying contemporary in-vitro results and pharmacokinetic-pharmacodynamic principles. J. Chemother. 2007;19(1):38–43. doi: 10.1179/joc.2007.19.1.38. [DOI] [PubMed] [Google Scholar]
- 87.Lodise TP, Jr, Nau R, Kinzig M, Jones RN, Drusano GL, Sorgel F. Comparison of the probability of target attainment between ceftriaxone and cefepime in the cerebrospinal fluid and serum against Streptococcus pneumoniae. Diagn. Microbiol. Infect. Dis. 2007;58(4):445–452. doi: 10.1016/j.diagmicrobio.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 88.Labombardi VJ, Rojtman A, Tran K. Use of cefepime for the treatment of infections caused by extended spectrum β-lactamase-producing Klebsiella pneumoniae and Escherichia coli. Diagn. Microbiol. Infect. Dis. 2006;56(3):313–315. doi: 10.1016/j.diagmicrobio.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 89.Goethaert K, Van Looveren M, Lammens C, et al. High-dose cefepime as an alternative treatment for infections caused by TEM-24 ESBL-producing Enterobacter aerogenes in severely-ill patients. Clin. Microbiol. Infect. 2006;12(1):56–62. doi: 10.1111/j.1469-0691.2005.01290.x. [DOI] [PubMed] [Google Scholar]
- 90.Paterson DL, Ko WC, Von Gottberg A, et al. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum β-lactamases: implications for the clinical microbiology laboratory. J. Clin. Microbiol. 2001;39(6):2206–2212. doi: 10.1128/JCM.39.6.2206-2212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kotapati S, Kuti JL, Nightingale CH, Nicolau DP. Clinical implications of extended spectrum β-lactamase (ESBL) producing Klebsiella species and Escherichia coli on cefepime effectiveness. J. Infect. 2005;51(3):211–217. doi: 10.1016/j.jinf.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 92.Oster S, Edelstein H, Cassano K, McCabe R. Open trial of cefepime (BMY 28142) for infections in hospitalized patients. Antimicrob. Agents Chemother. 1990;34(6):954–957. doi: 10.1128/aac.34.6.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Edelstein H, Chirurgi V, Oster S, et al. A randomized trial of cefepime (BMY-28142) and ceftazidime for the treatment of pneumonia. J. Antimicrob. Chemother. 1991;28(4):569–575. doi: 10.1093/jac/28.4.569. [DOI] [PubMed] [Google Scholar]
- 94.Ambrose PG, Owens RC, Jr, Garvey MJ, Jones RN. Pharmacodynamic considerations in the treatment of moderate to severe pseudomonal infections with cefepime. J. Antimicrob. Chemother. 2002;49(3):445–453. doi: 10.1093/jac/49.3.445. [DOI] [PubMed] [Google Scholar]
- 95.Gouin F, Papazian L, Martin C, et al. A non-comparative study of the efficacy and tolerance of cefepime in combination with amikacin in the treatment of severe infections in patients in intensive care. J. Antimicrob. Chemother. 1993;32(Suppl B):205–214. doi: 10.1093/jac/32.suppl_b.205. [DOI] [PubMed] [Google Scholar]
- 96.Zanetti G, Bally F, Greub G, et al. Cefepime versus imipenem-cilastatin for treatment of nosocomial pneumonia in intensive care unit patients: a multicenter, evaluator-blind, prospective, randomized study. Antimicrob. Agents Chemother. 2003;47(11):3442–3447. doi: 10.1128/AAC.47.11.3442-3447.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yahav D, Paul M, Fraser A, Sarid N, Leibovici L. Efficacy and safety of cefepime: a systematic review and meta-analysis. Lancet Infect. Dis. 2007;7(5):338–348. doi: 10.1016/S1473-3099(07)70109-3. [DOI] [PubMed] [Google Scholar]
- 98.Paul M, Yahav D, Fraser A, Leibovici L. Empirical antibiotic monotherapy for febrile neutropenia: systematic review and meta-analysis of randomized controlled trials. J. Antimicrob. Chemother. 2006;57(2):176–189. doi: 10.1093/jac/dki448. [DOI] [PubMed] [Google Scholar]
- 99.Gomez L, Estrada C, Gomez I, et al. Cefepime plus amikacina versus piperacillin-tazobactam plus amikacin in the treatment of patients with fever and granulacytopenia; Presented at: 41st Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago, IL, USA. 16-19 December 2001. [Google Scholar]
- 100.Chandrasekar PH, Arnow PM. Cefepime versus ceftazidime as empiric therapy for fever in neutropenic patients with cancer. Ann. Pharmacother. 2000;34(9):989–995. doi: 10.1345/aph.10001. [DOI] [PubMed] [Google Scholar]
- 101.Lee PY, Chang WN, Lu CH, et al. Clinical features and in vitro antimicrobial susceptibilities of community-acquired Klebsiella pneumoniae meningitis in Taiwan. J. Antimicrob. Chemother. 2003;51(4):957–962. doi: 10.1093/jac/dkg158. [DOI] [PubMed] [Google Scholar]
- 102.Rousseau JM, Soullie B, Villevielle T, Koeck JL. Efficiency of cefepime in postoperative meningitis attributable to Enterobacter aerogenes. J. Trauma. 2001;50(5):971. doi: 10.1097/00005373-200105000-00041. [DOI] [PubMed] [Google Scholar]
- 103.Venkatesan A, Spalding C, Speedie A, Sinha G, Rumbaugh JA. Pseudomonas aeruginosa infective endocarditis presenting as bacterial meningitis. J. Infect. 2005;51(4):E199–E202. doi: 10.1016/j.jinf.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 104.Gentry LO, Rodriguez-Gomez G. Randomized comparison of cefepime and ceftazidime for treatment of skin, surgical wound, and complicated urinary tract infections in hospitalized subjects. Antimicrob. Agents Chemother. 1991;35(11):2371–2374. doi: 10.1128/aac.35.11.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schaad UB, Eskola J, Kafetzis D, et al. European Society for Paediatric Infectious Diseases (ESPID) Pyelonephritis Study Group Cefepine vs. ceftazidime treatment of pyelonephritis: a European, randomized, controlled study of 300 pediatric cases. Pediatr. Infect. Dis. J. 1998;17(7):639–644. doi: 10.1097/00006454-199807000-00012. [DOI] [PubMed] [Google Scholar]
- 106.Newton ER, Yeomans ER, Pastorek JG, Soper DE, Hemsell DL. Randomized comparative study of cefepime and cefotaxime in the treatment of acute obstetric and gynaecological infections. J. Antimicrob. Chemother. 1993;32(Suppl B):195–204. doi: 10.1093/jac/32.suppl_b.195. [DOI] [PubMed] [Google Scholar]
- 107.Barie PS, Vogel SB, Dellinger EP, et al. Cefepime Intra-Abdominal Infection Study Group A randomized, double-blind clinical trial comparing cefepime plus metronidazole with imipenem-cilastatin in the treatment of complicated intra-abdominal infections. Arch. Surg. 1997;132(12):1294–1302. doi: 10.1001/archsurg.1997.01430360040008. [DOI] [PubMed] [Google Scholar]
- 108.Berne TV, Yellin AE, Appleman MD, Heseltine PN, Gill MA. A clinical comparison of cefepime and metronidazole versus gentamicin and clindamycin in the antibiotic management of surgically treated advanced appendicitis. Surg. Gynecol. Obstet. 1993;177(Suppl):18–22. doi: 10.1016/0020-7292(94)90428-6. [DOI] [PubMed] [Google Scholar]
- 109.Thompson JE, Jr, Bennion RS, Roettger R, Lally KP, Hopkins JA, Wilson SE. Cefepime for infections of the biliary tract. Surg. Gynecol. Obstet. 1993;177(Suppl):30–34. [PubMed] [Google Scholar]
- 110.Del Rio P, Vellone M, Fragapane P, et al. Cefepime for prophylaxis of infections in the surgery of cholelithiasis. Results of a multicentric comparative trial. Acta. Biomed. 2008;79(1):23–27. [PubMed] [Google Scholar]
- 111.Jauregui L, Matzke D, Scott M, Minns P, Hageage G. Cefepime as treatment for osteomyelitis and other severe bacterial infections. J. Antimicrob. Chemother. 1993;32(Suppl B):141–149. doi: 10.1093/jac/32.suppl_b.141. [DOI] [PubMed] [Google Scholar]
- 112.Barberan J, Gomis M, Sanchez B, et al. Cefepime in the treatment of osteomyelitis caused by Gram negative bacilli. Rev. Esp. Quimioter. 2000;13(4):366–373. [PubMed] [Google Scholar]
- 113.Eggimann P, Glauser MP, Aoun M, Meunier F, Calandra T. Cefepime monotherapy for the empirical treatment of fever in granulocytopenic cancer patients. J. Antimicrob. Chemother. 1993;32(Suppl B):151–163. doi: 10.1093/jac/32.suppl_b.151. [DOI] [PubMed] [Google Scholar]
- 114.Mouton Y, Chidiac C, Humbert G, et al. A non-comparative, multicentre study of cefepime in the treatment of serious bacterial infections. J. Antimicrob. Chemother. 1993;32(Suppl B):133–140. doi: 10.1093/jac/32.suppl_b.133. [DOI] [PubMed] [Google Scholar]
- 115.Kieft H, Hoepelman AI, Rozenberg-Arska M, et al. Cefepime compared with ceftazidime as initial therapy for serious bacterial infections and sepsis syndrome. Antimicrob. Agents Chemother. 1994;38(3):415–421. doi: 10.1128/aac.38.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dakdouki GK, Al-Awar GN. Cefepime-induced encephalopathy. Int. J. Infect. Dis. 2004;8(1):59–61. doi: 10.1016/j.ijid.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 117.Lam S, Gomolin IH. Cefepime neurotoxicity: case report, pharmacokinetic considerations, and literature review. Pharmacotherapy. 2006;26(8):1169–1174. doi: 10.1592/phco.26.8.1169. [DOI] [PubMed] [Google Scholar]
- 118.Sonck J, Laureys G, Verbeelen D. The neurotoxicity and safety of treatment with cefepime in patients with renal failure. Nephrol. Dial. Transplant. 2008;23(3):966–970. doi: 10.1093/ndt/gfm713. [DOI] [PubMed] [Google Scholar]
- 119.Bresson J, Paugam-Burtz C, Josserand J, Bardin C, Mantz J, Pease S. Cefepime overdosage with neurotoxicity recovered by high-volume haemofiltration. J. Antimicrob. Chemother. 2008;62(4):849–850. doi: 10.1093/jac/dkn256. [DOI] [PubMed] [Google Scholar]
- 120.Bacher K, Schaeffer M, Lode H, Nord CE, Borner K, Koeppe P. Multiple dose pharmacokinetics, safety, and effects on faecal microflora, of cefepime in healthy volunteers. J. Antimicrob. Chemother. 1992;30(3):365–375. doi: 10.1093/jac/30.3.365. [DOI] [PubMed] [Google Scholar]
- 121.Spencer RC. The role of antimicrobial agents in the aetiology of Clostridium difficile-associated disease. J. Antimicrob. Chemother. 1998;41(Suppl C):21–27. doi: 10.1093/jac/41.suppl_3.21. [DOI] [PubMed] [Google Scholar]
- 122.Pepin J, Valiquette L, Alary ME, et al. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004;171(5):466–472. doi: 10.1503/cmaj.1041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 2005;353(23):2442–2449. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 124.Muto CA, Pokrywka M, Shutt K, et al. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect. Control Hosp. Epidemiol. 2005;26(3):273–280. doi: 10.1086/502539. [DOI] [PubMed] [Google Scholar]
- 125.Pultz NJ, Donskey CJ. Effect of antibiotic treatment on growth of and toxin production by Clostridium difficile in the cecal contents of mice. Antimicrob. Agents Chemother. 2005;49(8):3529–3532. doi: 10.1128/AAC.49.8.3529-3532.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Toltzis P, Dul M, O’Riordan MA, et al. Cefepime use in a pediatric intensive care unit reduces colonization with resistant bacilli. Pediatr. Infect. Dis. J. 2003;22(2):109–114. doi: 10.1097/01.inf.0000050241.65703.2e. [DOI] [PubMed] [Google Scholar]
- 127.Paterson DL, Muto CA, Ndirangu M, et al. Acquisition of rectal colonization by vancomycin-resistant Enterococcus among intensive care unit patients treated with piperacillin-tazobactam versus those receiving cefepime-containing antibiotic regimens. Antimicrob. Agents Chemother. 2008;52(2):465–469. doi: 10.1128/AAC.01316-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wong BB, Ko GJ. Neutropenia in patients receiving long-term cefepime therapy for osteomyelitis. Am. J. Health Syst. Pharm. 2003;60(21):2229–2232. doi: 10.1093/ajhp/60.21.2229. [DOI] [PubMed] [Google Scholar]
- 129.Queenan AM, Shang W, Kania M, Page MG, Bush K. Interactions of ceftobiprole with β-lactamases from molecular classes A to D. Antimicrob. Agents Chemother. 2007;51(9):3089–3095. doi: 10.1128/AAC.00218-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Queenan AM, Foleno B, Gownley C, Wira E, Bush K. Effects of inoculum and β-lactamase activity in AmpC- and extended-spectrum β-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae clinical isolates tested by using NCCLS ESBL methodology. J. Clin. Microbiol. 2004;42(1):269–275. doi: 10.1128/JCM.42.1.269-275.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bradford PA, Cherubin CE, Idemyor V, Rasmussen BA, Bush K. Multiply resistant Klebsiella pneumoniae strains from two Chicago hospitals: identification of the extended-spectrum TEM-12 and TEM-10 ceftazidime-hydrolyzing β-lactamases in a single isolate. Antimicrob. Agents Chemother. 1994;38(4):761–766. doi: 10.1128/aac.38.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Poirel L, Mammeri H, Nordmann P. TEM-121, a novel complex mutant of TEM-type β-lactamase from Enterobacter aerogenes. Antimicrob. Agents Chemother. 2004;48(12):4528–4531. doi: 10.1128/AAC.48.12.4528-4531.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Poirel L, Gniadkowski M, Nordmann P. Biochemical analysis of the ceftazidime-hydrolysing extended-spectrum β-lactamase CTX-M-15 and of its structurally related β-lactamase CTX-M-3. J. Antimicrob. Chemother. 2002;50(6):1031–1034. doi: 10.1093/jac/dkf240. [DOI] [PubMed] [Google Scholar]
- 134.Poirel L, Naas T, Nicolas D, et al. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-β-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob. Agents Chemother. 2000;44(4):891–897. doi: 10.1128/aac.44.4.891-897.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Riccio ML, Franceschini N, Boschi L, et al. Characterization of the metallo-β-lactamase determinant of Acinetobacter baumannii AC-54/97 reveals the existence of bla(IMP) allelic variants carried by gene cassettes of different phylogeny. Antimicrob. Agents Chemother. 2000;44(5):1229–1235. doi: 10.1128/aac.44.5.1229-1235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wachino J, Kurokawa H, Suzuki S, et al. Horizontal transfer of blaCMY-bearing plasmids among clinical Escherichia coli and Klebsiella pneumoniae isolates and emergence of cefepime-hydrolyzing CMY-19. Antimicrob. Agents Chemother. 2006;50(2):534–541. doi: 10.1128/AAC.50.2.534-541.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vakulenko SB, Golemi D, Geryk B, et al. Mutational replacement of Leu-293 in the class C Enterobacter cloacae P99 β-lactamase confers increased MIC of cefepime. Antimicrob. Agents Chemother. 2002;46(6):1966–1970. doi: 10.1128/AAC.46.6.1966-1970.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jones RN, Kirby JT, Rhomberg PR. Comparative activity of meropenem in US medical centers (2007): initiating the 2nd decade of MYSTIC program surveillance. Diagn. Microbiol. Infect. Dis. 2008;61(2):203–213. doi: 10.1016/j.diagmicrobio.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 139.Mathai D, Jones RN, Pfaller MA. Epidemiology and frequency of resistance among pathogens causing urinary tract infections in 1,510 hospitalized patients: a report from the SENTRY Antimicrobial Surveillance Program (North America) Diagn. Microbiol. Infect. Dis. 2001;40(3):129–136. doi: 10.1016/s0732-8893(01)00254-1. [DOI] [PubMed] [Google Scholar]
- 140.Sader HS, Jones RN, Andrade-Baiocchi S, Biedenbach DJ. Four-year evaluation of frequency of occurrence and antimicrobial susceptibility patterns of bacteria from bloodstream infections in Latin American medical centers. Diagn. Microbiol. Infect. Dis. 2002;44(3):273–280. doi: 10.1016/s0732-8893(02)00469-8. [DOI] [PubMed] [Google Scholar]
- 141.Nijssen S, Florijn A, Bonten MJ, Schmitz FJ, Verhoef J, Fluit AC. β-lactam susceptibilities and prevalence of ESBL-producing isolates among more than 5000 European Enterobacteriaceae isolates. Int. J. Antimicrob. Agents. 2004;24(6):585–591. doi: 10.1016/j.ijantimicag.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 142.Turnidge J, Bell J, Biedenbach DJ, Jones RN. Pathogen occurrence and antimicrobial resistance trends among urinary tract infection isolates in the Asia-Western Pacific region: report from the SENTRY Antimicrobial Surveillance Program, 1998-1999. Int. J. Antimicrob. Agents. 2002;20(1):10–17. doi: 10.1016/s0924-8579(02)00050-x. [DOI] [PubMed] [Google Scholar]
- 143.Hoban DJ, Biedenbach DJ, Mutnick AH, Jones RN. Pathogen of occurrence and susceptibility patterns associated with pneumonia in hospitalized patients in North America: results of the SENTRY Antimicrobial Surveillance Study (2000) Diagn. Microbiol. Infect. Dis. 2003;45(4):279–285. doi: 10.1016/s0732-8893(02)00540-0. [DOI] [PubMed] [Google Scholar]
- 144.Gales AC, Sader HH, Jones RN. Respiratory tract pathogens isolated from patients hospitalized with suspected pneumonia in Latin America: frequency of occurrence and antimicrobial susceptibility profile: results from the SENTRY Antimicrobial Surveillance Program (1997-2000) Diagn. Microbiol. Infect. Dis. 2002;44(3):301–311. doi: 10.1016/s0732-8893(02)00499-6. [DOI] [PubMed] [Google Scholar]
- 145.Tognim MC, Andrade SS, Silbert S, Gales AC, Jones RN, Sader HS. Resistance trends of Acinetobacter spp. in Latin America and characterization of international dissemination of multi-drug resistant strains: five-year report of the SENTRY Antimicrobial Surveillance Program. Int. J. Infect. Dis. 2004;8(5):284–291. doi: 10.1016/j.ijid.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 146.Streit JM, Jones RN, Sader HS, Fritsche TR. Assessment of pathogen occurrences and resistance profiles among infected patients in the intensive care unit: report from the SENTRY Antimicrobial Surveillance Program (North America, 2001) Int. J. Antimicrob. Agents. 2004;24(2):111–118. doi: 10.1016/j.ijantimicag.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 147.Traczewski MM, Brown SD. In vitro activity of doripenem against Pseudomonas aeruginosa and Burkholderia cepacia isolates from both cystic fibrosis and non-cystic fibrosis patients. Antimicrob. Agents Chemother. 2006;50(2):819–821. doi: 10.1128/AAC.50.2.819-821.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen Y, Garber E, Zhao Q, et al. In vitro activity of doripenem (S-4661) against multidrug-resistant Gram-negative bacilli isolated from patients with cystic fibrosis. Antimicrob. Agents Chemother. 2005;49(6):2510–2511. doi: 10.1128/AAC.49.6.2510-2511.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mendes C, Oplustil C, Sakagami E, Turner P, Kiffer C. Antimicrobial susceptibility in intensive care units: MYSTIC Program Brazil 2002. Braz. J. Infect. Dis. 2005;9(1):44–51. doi: 10.1590/s1413-86702005000100008. [DOI] [PubMed] [Google Scholar]
- 150.Fritsche TR, Stilwell MG, Jones RN. Antimicrobial activity of doripenem (S-4661): a global surveillance report (2003) Clin. Microbiol. Infect. 2005;11(12):974–984. doi: 10.1111/j.1469-0691.2005.01271.x. [DOI] [PubMed] [Google Scholar]
- 151.Sader HS, Jones RN. Antimicrobial susceptibility of uncommonly isolated non-enteric Gram-negative bacilli. Int. J. Antimicrob. Agents. 2005;25(2):95–109. doi: 10.1016/j.ijantimicag.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 152.Fedler KA, Biedenbach DJ, Jones RN. Assessment of pathogen frequency and resistance patterns among pediatric patient isolates: report from the 2004 SENTRY Antimicrobial Surveillance Program on 3 continents. Diagn. Microbiol. Infect. Dis. 2006;56(4):427–436. doi: 10.1016/j.diagmicrobio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 153.Castanheira M, Gales AC, Pignatari AC, Jones RN, Sader HS. Changing antimicrobial susceptibility patterns among Streptococcus pneumoniae and Haemophilus influenzae from Brazil: report from the SENTRY Antimicrobial Surveillance Program (1998-2004) Microb. Drug Resist. 2006;12(2):91–98. doi: 10.1089/mdr.2006.12.91. [DOI] [PubMed] [Google Scholar]
- 154.Wang B, Xu JS, Wang CX, et al. Antimicrobial susceptibility of Neisseria gonorrhoeae isolated in Jiangsu Province, China, with a focus on fluoroquinolone resistance. J. Med. Microbiol. 2006;55(Pt 9):1251–1255. doi: 10.1099/jmm.0.46401-0. [DOI] [PubMed] [Google Scholar]
- 155.Rennie RP, Jones RN, Mutnick AH. Occurrence and antimicrobial susceptibility patterns of pathogens isolated from skin and soft tissue infections: report from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 2000) Diagn. Microbiol. Infect. Dis. 2003;45(4):287–293. doi: 10.1016/s0732-8893(02)00543-6. [DOI] [PubMed] [Google Scholar]
- 156.Barbhaiya RH, Knupp CA, Tenney J, Martin RR, Weidler DJ, Pittman KA. Safety, tolerance, and pharmacokinetics of cefepime administered intramuscularly to healthy subjects. J. Clin. Pharmacol. 1990;30(10):900–910. doi: 10.1002/j.1552-4604.1990.tb03569.x. [DOI] [PubMed] [Google Scholar]
- 201.EUCAST. European Committee on Antimicrobial Susceptibility Testing www.escmid.org/sites/index_f.aspx?par=2.4.
- 202.EUCAST. European Commettee on Antimicrobial Susceptibility Testing. Expert rules in antimicrobial susceptibility testing 2008 www.escmid.org/files/eucast-barcelona-poster2008.pdf.


