Abstract
One solution to the shortage of human organs available for transplantation envisions growing new organs in situ. This can be accomplished by transplantation of developing organ anlagen/primordia. Allotransplantation of embryonic day 15 metanephroi into the omentum of adult hosts is followed by differentiation, growth, vascularization and function of the implants. Here we show that survival of rats with all native renal mass removed can be increased by prior metanephros transplantation and ureteroureterostomy. Excretion of urine formed by metanephroi is prerequisite for enhanced survival. This is the first demonstration that life can be extended following de novo renal organogenesis.
Key Words: cell therapy, end-stage renal disease, kidney, metanephros, transplantation
Introduction
The kidney is a structurally complex organ the physiology of which encompasses a myriad of excretory, metabolic, and reabsorptive functions.1–3 The removal of both kidneys from experimental animals or its human equivalent, progression of renal failure to end-stage, is incompatible with life.2 The precise identities of individual or multiple renal functions the absence of which causes death in end-stage renal disease (ESRD) are unknown. However, the fact that life can be prolonged in humans by dialysis, a procedure that results in excretion of solutes from plasma by diffusion into dialysate and removal of fluid down favorable hydrostatic or osmotic gradients, suggests that comparable processes carried out by the kidney are among its essential life-preserving functions.2 Kidney transplantation, an alternative to dialysis for treatment for ESRD, provides a better quality of life for many patients. However, transplantation is limited by lack of organ availability.3,4
One novel alternative to the transplantation of a structurally complex organ such as the kidney is de novo organogenesis as applied through the growing of a new organ in situ from primitive cells.4 Renal organogenesis from human renal embryonic tissue (metanephroi) has been carried out in immunodeficient mice.5,6 Metanephroi from non-human animals also undergo organogenesis post-transplantation across allogeneic,7–14 or concordant9 or highly disparate10 xenogeneic barriers into immune-competent hosts.
Developed human metanephroi exhibit reabsorptive function as reflected by uptake of the infused radioisotope 99technitium-dimercaptosuccinic acid.5 Function of rat metanephroi is reflected by clearance of creatinine and inulin following ureteroureterostomy between transplant and host.7 If incubated with growth factors prior to implantation8,11,13 or post-implantation,13 glomerular filtration rate (inulin clearance) is increased, tubular function can be demonstrated (p-aminohippurate secretion) and metanephroi secrete a concentrated urine.12 The level of inulin clearance achievable by a single transplanted metanephros approaches a level that would be expected to support life in an otherwise anephric rat15 and the level that supports life in patients on dialysis (10% of baseline).2 However, to date prolongation of life in otherwise anephric animals by a transplanted metanephros has not been demonstrated. To ascertain whether life can be prolonged post-metanephros transplantation, we compared survival in rats with or without transplants following the removal of both native kidneys. To our knowledge, ours is the first demonstration that life can be extended following de novo organogenesis of a kidney.
Methods
Animal care followed Institutional Animal Care and Use Committee (IUPAC) standards (approval number A3381-01). Metanephroi were surgically dissected from embryonic day (E)15 Sprague Dawley rat embryos under a dissecting microscope exactly as previously described,7 and placed immediately into ice-cold Dulbecco's modified Eagles Medium: Hams F12 (DMEM:HF12) solution containing recombinant human growth factors and other growth-promoting agents, combinations of which we have been shown enhance function of transplanted metanephroi.8,9,11 For the studies reported here, DMEM:HF12 contained human recombinant fibroblast growth factor 2 (FGF2) (R&D Systems, Minneapolis MN), 0.5 µg/ml; recombinant human hepatocyte growth factor (HGF) (R&D Systems), 0.5 µg/ml; recombinant human insulin-like growth factor II (IGF II) (Bachem, Torrance CA), 10−8 M; recombinant human transforming growth factor alpha (TGFα) (Upstate Biotechnology, Lake Placid New York), 5 µg/ml; 0.1 µg/ml transforming growth factor beta 2 (TGFβ2) (R&D Systems), 0.1 µg/ml; 25-hydroxyvitamin D3 [25(OH)D3] (Sigma Chemicals, St. Louis MO), 1 µg/ml; retinoic acid (RA), 10−6 M (Sigma Chemicals, St. Louis MO); Tamm Horsfall protein (Biomedical Technologies, Stoughton MA), 1 µg/ml; corticotropin releasing hormone (CRH), 1 µg/ml (Sigma Chemicals); and interleukin 10 (IL10) (R&D Systems), 0.1 µg/ml. After 45 minutes in the media, metanephroi were implanted into the omentum of 5–6 week-old adult female Sprague Dawley rats that had one kidney removed at the time of implantation.7–14
We have shown previously that metanephroi can be allotransplanted (Sprague-Dawley to Sprague-Dawley) without host immunosuppression. However, for the present experiments all groups of host rats were administered 50 µg/day i.p. anti-rat TCRαβ (Pharmingen, San Diego CA) beginning 2 days prior to transplantation and daily through 4 weeks post transplantation. A similar ‘short-term’ dosage regimen of anti-rat TCRαβ (optimal dosage level 50 µg/day) was shown to induce unresponsiveness to cardiac allografts in rats.16 Three weeks post-implantation of metanephroi, ureteroureterostomy was performed between the ureter of the developing metanephros and the ureter that remained in the host post-uninephrectomy.7 As before,13 at the time of ureteroureterostomy, metanephroi were bathed for 30 minutes in 100 µl sterile saline containing growth factors. For the present studies, the growth factors were recombinant human IGF I, 10−9 M (Genentech Inc. South San Francisco CA); HGF, 1 µg; and recombinant human vascular endothelial growth factor (VEGF), 6.25 µg (Genentech).
We have shown previously that the size (weight) and function (inulin clearance) of transplanted metanephroi remain constant between 12–32 weeks post-implantation.14 In the present studies, 17 weeks post urteroureterostomy (20 weeks post-implantation) the remaining host kidneys were removed from all rats, and animals were observed subsequently every 12 hours (0.5 days) with death as the endpoint. So as to not impact on survival by inducing volume loss, blood samples were not collected from rats post-operatively (following removal of the remaining native kidney).
A first group of rats in which ureteoureterostomy and nephrectomies were performed as delineated above was designated the Transplant/Excretion (TX-EXCR) group. Urine formed by the transplanted metanephros was excreted into the host bladder from which it passed through the host urethra to the outside. A second group of rats designated the Transplant (TX) group was treated identically to the TX-EXCR group, with the single exception that the ureteroureterostomy was severed at the time the second host kidney was removed. A ligature was tied around the host ureter (distal end of the ureterouretorostomy), precluding any excretion of filtered solutes into the host bladder. However, the ureter originating from the from the transplanted metanephros (proximal end) remained open and urine was observed to flow unimpeded into the host peritoneal cavity. Thus, there was no obstruction of the transplanted metanephros. A third group of rats (Control) underwent laparotomy and uninephrectomy followed by laparotomy and removal of the remaining kidney. However, no metanephros was implanted at the time of the initial laparotomy.
Metanephroi were photographed, fixed, embedded in paraffin, sectioned, and stained with hematoxylin and eosin exactly as in previous studies.7 Student-Newman-Keuls multiple comparison procedure was used to analyze data shown in Figure 3. Differences were considered to be significant if p < 0.05 for 2-tailed analysis.
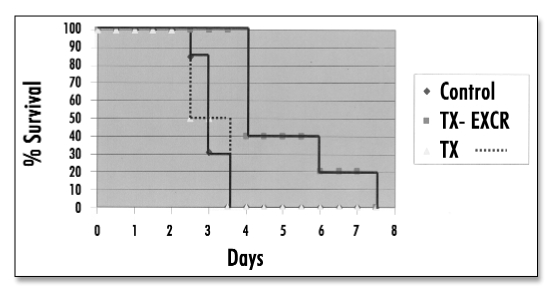
Figure 3.
Survival of control and transplanted rats as a function of time post-removal of all native renal mass.
Results
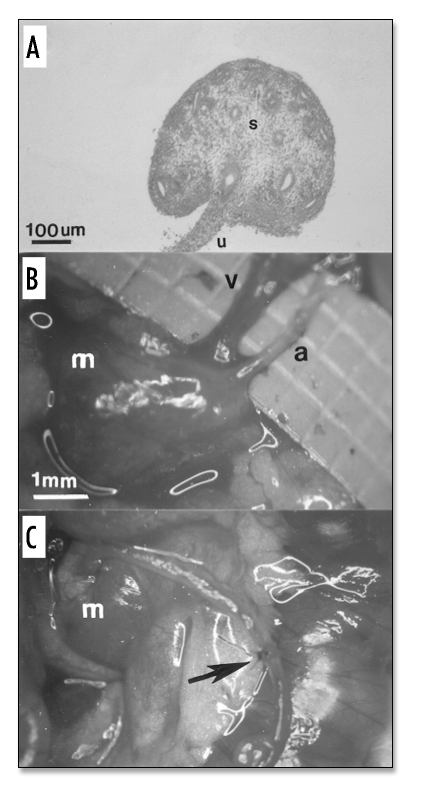
Shown in Figure 1A is a hematoxylin and eosin (H&E)-stained section of an E15 rat metanephros consisting largely of undifferentiated stroma (s). The former ureteric bud is in the process of differentiating into a ureter (u). The metanephros was transplanted into the omentum of a host rat and unilateral host nephrectomy was performed at the same time. At 3 weeks post-transplantation into the omentum of a host rat, the metanephros has enlarged (Fig. 1B and C). An artery (a) and vein (v) connecting the metanephros with the host omentum can be delineated (Fig. 1B). An ureteroureterostomy between host and transplant ureters performed at 3 weeks post metanephros transplantation is shown (arrow, Fig. 1C).
Figure 1.
(Left). (A) Photomicrograph and (B and C) photographs of rat metanephroi. (A) H&E-stained section of an E15 rat metanephros. (s) stroma; (u) ureter. (B and C) Developed metanephroi (m) 3 weeks post transplantation. Artery (a); Vein (v). Arrow delineates ureteroureterostomy. Magnifications are shown for A, B and C (in B).
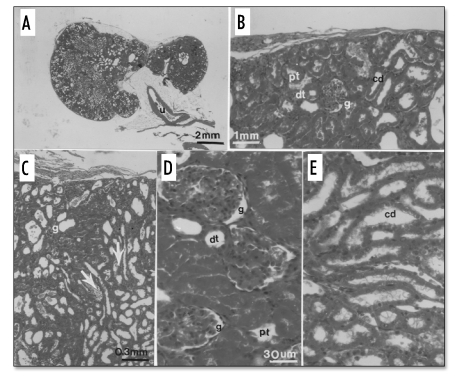
At 20 weeks post-transplantation (17 weeks following ureteroureterostomy), the remaining host kidney was removed. Differentiated structures at 20 weeks post-implantation are shown in Figure 2 that depicts H&E-stained sections of a developed metanephros. The cross sectional diameter of the metanephros shown in Figure 2A (∼1.2 cm) is about one-half the diameter of a normal rat kidney.7 Its ureter (u) is labeled. Figure 2B shows a glomerulus (g) proximal tubule (pt), distal tubule (dt) and collecting duct (cd) in the cortex. A glomerulus (g) and collecting duct (arrow) are labeled in Figure 2C. A glomerulus (g) proximal tubule (pt), and distal tubule (dt) are labeled in Figure 2D. A collecting duct (cd) is shown in Figure 2E.
Figure 2.
(Left). H&E-stained sections of a developed metanephros 20 weeks post-transplantation. (A) The ureter (u) is shown; (B) A glomerulus (g) proximal tubule (pt), distal tubule (dt) and collecting duct (cd) in the cortex; (C). A glomerulus (g) and collecting duct (arrow) are labeled; (D) A glomerulus (g) proximal tubule (pt), and distal tubule (dt) are labeled; (E) A collecting duct (cd) is labeled. Magnifications are shown in A, B, C and D (for D and E).
Survival as a function of time post-removal of all native renal mass (all renal function from the implant) is shown in Figure 3. Control rats (n = 13) lived 67 ± 2.7 hours (range 48–78 hours) post-removal of all native host renal mass. Rats in the TX group (transplanted metanephros, but no externalization of urine) (n = 4) lived 65 ± 6.0 hours (range 55–76 hours), no longer than Controls. Rats in the TX-EXCR group (transplanted metanephros and excretion of urine) (n = 5) lived 125 ± 12 hours (range 108–170 hours), significantly > than Control or TX rats.
Discussion
The metanephric kidneys originate on E12 of rat development when outgrowths of the mesonephric ducts, so-called ureteric buds, invade the intermediate mesoderm (metanephric blastema) located caudal to the mesonephros. Numerous outgrowths arise from the distal end of the ureteric bud which push radially into the surrounding mass of metanephric blastema and give rise to the collecting ducts of the kidneys. The proximal ends of the ureteric bud give rise to the ureter and renal pelvis. The metanephric blastema differentiates into all of the tubular structures of the adult nephron. The collecting system (collecting ducts and ureter) derives from the ureteric bud.17
The differentiated kidney is a remarkably complex organ. Its metabolic, secretory and excretory functions are dependent on the growth differentiation and organization of its precursor cells in three dimensions to comprise interconnected nephron structures.17,18 Taking morphology, location and function into account, Al Awqati and Oliver have estimated that there are at least twenty-six terminally differentiated cell types in the kidney of a newborn mouse that arise from at least four cell types present when renal development begins.18
Isotransplantation19,20 allotransplantation,7–14 and xenotransplantation5,6,9,10 of whole,5,6,7–14 sectioned,19 or collagen-digested, cultured and reconstituted,20 rodent7–9,11–13,19), cloned bovine,20 porcine,6,10 or human5,6 metanephroi has been carried out into sub-renal capsular,5–7 renal parenchymal,19 intraperitoneal5,7–14 and other20 sites as a means to effect renal organogenesis. As part of their development in situ, metanephroi transplanted across a xenogeneic barrier are able to attract a host vasculature from an appropriate bed.6,9 To the extent that their vasculature is host-derived, kidneys that differentiate post-transplantation of metanephroi are less susceptible than donor-vascularized kidneys to hyperacute and acute vascular rejection mediated by circulating antibodies directed against donor endothelial antigens or by incompatibilities between host and xenogeneic vasculatures.4,21 For this reason it is proposed that transplantation of metanephroi across a discordant xenogeneic barrier into humans (pig to human) would be advantageous relative to the transplantation of developed pig kidneys in lieu of human organs the availability of which is rate limiting.3,4,21
Transplanted renal primordia and developed metanephroi have been shown to recapitulate a number of metabolic and excretory functions performed by native kidneys. These include synthesis of erythropoetin and 1,25-dihydroxyvitamin D3,20 and formation of an ultrafiltrate of plasma.5–8,11–13,19,20 However, the preservation of life in anephric hosts post renal organogenesis has never been demonstrated prior to this study.
Our observations do not permit us to conclude that all functions normally subserved by native kidneys1–3 are being carried out by a single transplanted metanephros. In addition the data do not delineate precisely which functions contribute to prolongation of life in otherwise anephric rats. However, the findings that survival is longer in the TX-EXCR group (transplanted metanephros and excretion of urine) than in the Control group, and not different between Control and TX (transplanted metanephros, but no externalization of urine) groups provides strong evidence that excretion of solutes and fluid is essential for enhanced survival, since most if not all non-excretory functions should be preserved in the TX group. The finding that survival is not different between Control and TX groups shows that neither the transplantation of embryonic tissue nor the presence of a developed metanephros, nor growth factor treatment, nor anti-rat TCRαβ treatment per se is sufficient to prolong life.
To be clinically significant, metanephros transplantation will need to prolong life for more than several days in a rat. Indeed, it will need to so in a human. We envision the implementation of metanephros transplantation will be xenotransplantation of pig metanephroi into humans.
In that humans and pigs are of comparable size, and share a similar renal physiology, pigs represent an ideal kidney donor.22
The development of metanephros transplantation as a clinical therapy will take time. To understand that this is the case, one need only contemplate the chronology of events that led to the routine clinical use of hemodialysis and renal allotransplantation. More than half a century elapsed between the first attempts at hemodialysis or renal transplantation in humans and the first demonstrations of clinical utility.23 At the present time we are one hundred years into hemodialysis and renal transplantation, but less than a decade into metanephros transplantation as a means to effect renal organogenesis.23 As was the case in the development of hemodialysis and renal transplantation, we anticipate that the problems inherent in pig to human xenotransplantation of metanephroi can be overcome and renal function of sufficient magnitude to prolong life in humans can be achieved post-xenotransplantation of one or more renal primordia.
Our data establish that life can be extended following renal organogenesis in situ. To our knowledge, ours is the first demonstration of life-preserving function following the in situ development of a kidney (renal organogenesis).
Acknowledgements
S.A.R. and M.R.H. were supported by grant DK63379 from the National Institutes of Health (NIH). S.A.R., M.R.H. and Washington University may receive income based on a license of related technology by Washington University to Intercytex Ltd. and based on equity holdings in Intercytex Ltd.
Footnotes
Previously published online as an Organogenesis E-publication: http://www.landesbioscience.com/journals/organogenesis/abstract.php?id=1009
References
- 1.Rose BD, Rennke HG. Review of Renal Physiology. In: Rose BD, Rennke HG, editors. Renal Pathophysiology- The essentials. Baltimore: Williams and Wilkins; 1994. pp. 1–28. [Google Scholar]
- 2.Rose BD, Rennke HG. Signs and Symptoms of Chronic Renal Failure. In: Rose BD, Rennke HG, editors. Renal Pathophysiology- The essentials. Baltimore: Williams and Wilkins; 1994. pp. 276–300. [Google Scholar]
- 3.Hammerman MR. Tissue engineering the kidney. Kidney Int. 2003;63:1195–1204. doi: 10.1046/j.1523-1755.2003.00890.x. [DOI] [PubMed] [Google Scholar]
- 4.Cascalho M, Platt J. Xenotransplantation and other means of organ replacement. Nat Rev Immunol. 2001;1:154–160. doi: 10.1038/35100578. [DOI] [PubMed] [Google Scholar]
- 5.Dekel B, Amariglio F, Kaminski N, Schwartz A, Goshen E, Arditti FD, et al. Engraftment and differentiation of human metanephroi into functional mature nephrons after transplantation into mice is accompanied by a profile of gene expression similar to normal human kidney. J Am Soc Nephrol. 2002;13:977–990. doi: 10.1681/ASN.V134977. [DOI] [PubMed] [Google Scholar]
- 6.Dekel B, Burakova T, Arditti FD, Reich-Zeliger S, Milstein O, Aviel-Ronen S, et al. Human and porcine early kidney precursors as a new source for transplantation. Nat Med. 2003;9:53–60. doi: 10.1038/nm812. [DOI] [PubMed] [Google Scholar]
- 7.Rogers SA, Lowell JA, Hammerman NA, Hammerman MR. Transplantation of developing metanephroi into adult rats. Kidney Int. 1998;54:27–37. doi: 10.1046/j.1523-1755.1998.00971.x. [DOI] [PubMed] [Google Scholar]
- 8.Rogers SA, Liapis H, Hammerman MR. Transplantation of metanephroi across the major histocompatibility complex in rats. Am J Physiol. 2001;280:R132–R136. doi: 10.1152/ajpregu.2001.280.1.R132. [DOI] [PubMed] [Google Scholar]
- 9.Rogers SA, Hammerman MR. Transplantation of rat metanephroi into mice. Am J Physiol. 2001;280:R1865–R1869. doi: 10.1152/ajpregu.2001.280.6.R1865. [DOI] [PubMed] [Google Scholar]
- 10.Rogers SA, Talcott M, Hammerman MR. Transplantation of pig metanephroi. ASAIO J. 2003;49:48–53. doi: 10.1097/00002480-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Rogers SA, Hammerman MR. Transplantation of metanephroi after preservation in vitro. Am J Physiol. 2001;281:R661–R665. doi: 10.1152/ajpregu.2001.281.2.R661. [DOI] [PubMed] [Google Scholar]
- 12.Hammerman MR. Transplantation of developing kidneys. Transplant Rev. 2002;16:62–71. [Google Scholar]
- 13.Hammerman MR. Transplantation of renal precursor cells: a new therapeutic approach. Pediatr Nephrol. 2000;14:513–517. doi: 10.1007/s004670050805. [DOI] [PubMed] [Google Scholar]
- 14.Rogers SA, Powell-Braxton L, Hammerman MR. Insulin-like growth factor I regulates renal development in rodents. Dev Genet. 1999;24:293–298. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<293::AID-DVG12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 15.Miller SB, Hansen VA, Hammerman MR. Effects of growth hormone and IGF I on renal function in rats with normal and reduced renal mass. Am J Physiol. 1990;259:F747–F751. doi: 10.1152/ajprenal.1990.259.5.F747. [DOI] [PubMed] [Google Scholar]
- 16.Tsuchida M, Hirahara H, Matsumoto Y, Abo T, Eguchi S. Induction of specific unresponsiveness to cardiac allografts by short-term administration of anti-T cell receptor ab antibody. Transplantation. 1994;57:256–262. doi: 10.1097/00007890-199401001-00018. [DOI] [PubMed] [Google Scholar]
- 17.Horster MF, Braun GS, Huber SM. Renal embryonic epithelial induction nephrogenesis and cell differentiation. Physiol Rev. 1999;70:1157–1191. doi: 10.1152/physrev.1999.79.4.1157. [DOI] [PubMed] [Google Scholar]
- 18.Al-Awqati Q, Oliver JA. Stem cells in the kidney. Kidney International. 2002;61:387–395. doi: 10.1046/j.1523-1755.2002.00164.x. [DOI] [PubMed] [Google Scholar]
- 19.Wolf AS, Palmer SJ, Snow ML, Fine LG. Creation of a functioning mammalian chimeric kidney. Kidney International. 1990;38:991–997. doi: 10.1038/ki.1990.303. [DOI] [PubMed] [Google Scholar]
- 20.Lanza RP, Chung HY, Yoo JJ, Wettstein PJ, Blackwell C, Borson N, et al. Generation of histocompatible tissues using nuclear transplantation. Nat Biotechnol. 2001;20:689–696. doi: 10.1038/nbt703. [DOI] [PubMed] [Google Scholar]
- 21.Edge ASB, Gosse ME, Dinsmore J. Xenogeneic cell therapy: current progress and future developments in porcine cell transplantation. Cell Transplant. 1998;7:525–539. doi: 10.1177/096368979800700603. [DOI] [PubMed] [Google Scholar]
- 22.Sachs DH. The pig as a potential xenograft donor. Vet Immunol Immunopathol. 1994;43:185–191. doi: 10.1016/0165-2427(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 23.Hammerman MR. Treatment for end-stage renal disease: an organogenesis/tissue engineering odyssey. Transplant Immunol. 2004;12:211–218. doi: 10.1016/j.trim.2003.12.001. [DOI] [PubMed] [Google Scholar]