Abstract
Cell-extracellular matrix (ECM) is an important property of virtually all cells in multi-cellular organisms. Cell-ECM adhesion research, therefore, has broad impact on biology and medicine. Studies over the past three decades have resulted in tremendous advance in our understanding of the molecular basis and functions of cell-ECM adhesion. Here, I focus on some of the general lessons that we have learned from recent studies on cell-ECM adhesion. In addition, I highlight several topics in this rapidly advancing research area. These topics, which include assembly, disassembly and regulation of cell-ECM adhesion structures, the molecular mechanisms of bi-directional signaling through cell-ECM adhesions, and the tissue and organ pathobiology of cell-ECM adhesion, are pertinent to our understanding of cell-ECM adhesion and signaling.
Key Words: Focal adhesion, integrins, extracellular matrix, cytoskeleton, cell migration
Cell-ECM adhesion is a fundamental process through which cells interact and communicate with the environment. Cell-ECM adhesion is essential for organogenesis during embryonic development. In adult, it is vital for maintenance of tissue integrity and organ functions. Alterations of cell-ECM adhesion hence are frequently associated with human diseases. Because of the broad significance of cell-ECM adhesion in biology and pathology, understanding how cell-ECM adhesion is mediated and regulated and determining how cell-ECM adhesion influences cell behavior have been the subjects of numerous studies. In particular, studies over the past three decades have led to major breakthroughs in our understanding of cell-ECM adhesion. Many of the key discoveries, including identification of integrins as major transmembrane receptors for ECM proteins, demonstration of integrins as bi-directional (outside-in and inside-out) transmembrane signaling machines, identification of talin, focal adhesion kinase (FAK), integrin-linked kinase (ILK) and other cytoplasmic and membrane-associated proteins as key regulators and effectors of integrins, and delineation of multiple downstream signaling pathways that relay signals from cell surface integrins to diverse cytoplasmic and nuclear effectors, have been reviewed in refs. 1–12. In this brief article, I will focus on some of the general features of cell-ECM adhesion and discuss from my personal perspective several key questions that remain to be answered in future studies.
Subcellular Structures that Mediate Cell-ECM Adhesion
The subcellular structures that mediate cell-ECM adhesion are quite heterogeneous, often varying in size, shape, distribution, dynamics, and to certain extent, molecular constituents. The morphological “plasticity” of cell-ECM adhesion perhaps reflects the needs of cells to sense, adapt and response to a variety of extracellular environments. In addition, cell type (e.g., differentiation status, oncogenic transformation, etc.) often exerts marked influence on the structure of cell-ECM adhesions. Based on morphological and molecular criteria, several different types of model cell-ECM adhesion structures including focal adhesions, focal complexes, fibrillar adhesions, podosomes and three-dimensional matrix adhesions have been described.4,13 Despite the heterogeneity ECM adhesion structures do share certain common features. For example, they all connect cell surface integrins to the actin cytoskeleton. One of the earliest and perhaps best-characterized cell-ECM adhesion structures is focal adhesion, which represents strong ECM adhesion structures that are often found in adherent cells (e.g., fibroblasts) cultured on rigid ECM substrata. Although cultured mammalian cells have been widely used as a model system for analyses of focal adhesions, cell-ECM adhesions resembling focal adhesions have been found in vivo, in both invertebrate (e.g., body wall muscle attachment structures in C. elegans) and vertebrate organisms.4
Studies on focal adhesion and focal adhesion-like structures have greatly contributed to our understanding of molecular constituents and organization of cell-ECM adhesions. Indeed, localization to focal adhesions is often considered a good indication for the functionality of a protein in cell-ECM adhesion. To date, more than sixty focal adhesion proteins have been identified in vertebrates and a somewhat smaller number of proteins have been found in focal adhesion-like structures in invertebrates. Based on biochemical activities, these proteins can be classified into two major groups, namely catalytic proteins and adaptor proteins. The catalytic proteins can be further divided into several sub-groups depending on their substrate specificity, which include kinases (e.g., protein tyrosine kinases such as FAK and Src, protein serine/threonine kinases such as ILK and p21-activated kinase (PAK), and phosphoinositide 3-kinase), phosphatases (e.g., protein tyrosine phosphatase-PEST, protein tyrosine phosphatase 1B and PTEN), proteases (e.g., calpain), and other enzymes (e.g., phospholipase C-γ). The adaptor proteins are primarily involved in mediating multiple protein interactions and hence they invariably contain domains mediating protein interactions. One well-characterized protein-binding motif found in focal adhesion adaptor proteins is LIM, a cysteine-rich consensus sequence of approximately 55 amino acids that fold into two zinc fingers. The LIM-containing focal adhesion protein sub-group includes PINCH-1/2, which contain five (the most among all known LIM proteins) LIM domains, four-and-a-half LIM domain protein 2/3, paxillin/Hic-5 (four LIM domains), zyxin and migfilin (three LIM domains), cysteine-rich protein 1 (two LIM domains), and LIM and SH3 protein (LASP) (one LIM domain). Although the primary sequences and the folding of different LIM domains exhibit considerable similarities, they appear to be able to interact with a large number of structurally diverse targets over a wide range of affinities. Thus, the LIM domains provide a versatile system for protein interactions at cell-ECM adhesions. Other well-characterized protein binding motifs found in focal adhesion adaptor proteins include four point one ezrin radixin and moesin (FERM), calponin homology (CH), PSD95/DlgA/ZO-1 homology (PDZ) domain, and Src homology (SH) 2 and 3 domains. Focal adhesion proteins frequently contain more than one type of protein-binding motifs, which provides another mechanism for mediating diverse protein interactions.
Although this classification is useful, it is not absolute. Many focal adhesion proteins contain both catalytic and protein-binding domains. Thus, these proteins can play not only a catalytic role but also an adaptor role at focal adhesions. Indeed, recent studies suggest that ILK, together with its binding partners PINCH and parvins, provide an important physical connection between integrins and the actin cytoskeleton.5,12,14
It is anticipated that new components of focal adhesions will continue to be identified. However, major efforts in the next decade will be shifted to determining how individual components are wired to form a network that connects integrins to the actin cytoskeleton at molecular and atomic levels and how signals are transduced through focal adhesions. Because of the large number of the proteins that are involved, defining the interactions that compose the “integrin-actin” network will be a daunting, yet essential, task.
Although our knowledge about the “integrin-actin” network is still in its infancy, several interesting features have emerged. First, most if not all components of the “integrin-actin” network are multi-domain proteins, which allow the formation of an extensive interaction web. Second, although the “integrin-actin” network comprises a large number of proteins and numerous interactions, a relatively small number of proteins appear to serve as network hubs mediating a large number of interactions. This so called “scale-free” wiring of the “integrin-actin” network probably reflects evolutionary growth of the network.14 Functionally, it facilitates intra-molecular or inter-domain signal transduction and coordination for key network components. Third, the “integrin-actin” network is particularly rich in post-translational modifications (e.g., phosphorylation). The post-translational modifications are often coupled to cell-ECM adhesion and mechanical signals, allowing conversion between chemical and mechanical signals. Fourth, the functionality of the network requires interactions with affinities over a wide range (KD ranging from >10-3 M to <10-7 M). While high affinity (particularly those with low off rates) interactions confer network stability, low affinity interactions are crucial for dynamic regulation of signal transduction. Studies at genetic, molecular and atomic levels in the next several years should shed more light on the wiring of focal adhesions and other types of cell-ECM adhesion structures.
Assembly and Disassembly of Cell-ECM Adhesions
The molecular and atomic interaction maps derived from genetic, biochemical and structural studies provide valuable “snap shots” of cell-ECM adhesion structures. An area of research that is equally valuable to our understanding of cell-ECM adhesion is to determine the dynamics of these structures and how these structures are assembled and disassembled. Recent studies using total internal reflection fluorescence microscopy, fluorescent speckle microscopy and spatio-temporal image correlation spectroscopy have revealed that the interactions between focal adhesion components and actin filaments are dynamic and the correlation of movements between different classes of focal adhesion proteins and actin filaments varies considerably.15,16
It is generally believed that the assembly of cell-ECM adhesion structures is initiated by interactions of cell surface integrins with multivalent adhesive ECM proteins (e.g., fibronectin), which result in integrin clustering and formation of nascent cell-ECM adhesion structures (e.g., focal complexes). Focal complexes can mature into more stable, larger cell-ECM adhesion structures such as focal adhesions, which can in turn drive the formation of fibrillar adhesions.4,17 Conversely, focal adhesions can be disassembled in response to certain extracellular stimuli (e.g., certain “matricellular” proteins such as thrombospondin, tenascin, and SPARC).18
During assembly of cell-ECM adhesions, certain components are recruited to cell-ECM adhesions in a sequential fashion, whereas other components, notably PINCH, ILK and α-parvin, are first assembled into a multi-component protein complex and then recruited simultaneously (as a pre-formed complex) to cell-ECM adhesion sites.19 Thus, assembly of cell-ECM adhesions can be regulated locally (i.e., at cell-ECM adhesion sites) as well as remotely (e.g., during the synthesis or formation of intermediate protein complexes).
How is the assembly of cell-ECM adhesion structures regulated? Rac, a member of the Rho GTPase family, plays a pivotal role in promoting the formation of nascent focal complexes.20,21 Integrin-mediated cell-ECM adhesion activates Rac, which directly binds and activates PAK and other effectors including phosphatidylinositol-4-phosphate 5-kinase, Nap125, PIR121 and IRSp53, resulting in increased membrane protrusions and actin polymerization. Although the precise mechanism by which Rac promotes focal complex formation remains to be determined, increased actin polymerization and branching likely serve as a major driving force in the induction of nascent focal complexes at cell-ECM contacts.
Maturation of focal adhesions is controlled by both tension force and local actin polymerization.4,22 Rho, through downstream effectors Rho kinase (ROCK) and mammalian homolog of diaphanous (mDia), stimulates both contractility and actin polymerization, thereby promoting maturation of focal adhesions.21–23 While small GTPases of the Rho family clearly can function as upstream regulators of cell-ECM adhesions, the localization and activities of Rho small GTPases can also be regulated by signals elicited by cell-ECM adhesion and growth factors.21,24 Defining the cross-talks and coordination between cell-ECM adhesion, Rho/Rac/Cdc42, and growth factor signaling pathways in the assembly of cell-ECM adhesions is an important area of current and future research.
Protein phosphorylation plays important roles in regulation of focal adhesion formation and turnover.25 Several components of focal adhesions (e.g., FAK, paxillin, p130Cas, etc.) are phosphorylated at specific tyrosine residues in response to integrin-mediated cell-ECM adhesion. In general, tyrosine phosphorylation can influence focal adhesion turnover through two mechanisms. First, it regulates catalytic activities of certain key focal adhesion proteins. For example, phosphorylation of Tyr-576 and Tyr-577 within FAK kinase activation loop enhances FAK activity.26 Second, tyrosine phosphorylation provides docking sites for phospho-tyrosine recognition domains (e.g., SH2) and thereby promoting formation of protein complexes that control focal adhesion dynamics. For example, using antibodies that recognize Y31- or Y118-phosphorylated paxillin, and non-phosphorylatable and phosphomimetic mutants of paxillin, Zaidel-Bar et al have shown that phosphorylation of paxillin at Y31 and Y118 promotes the formation of nascent focal complex and turnover of mature focal adhesions, probably through enhanced recruitment of FAK to the tyrosyl-phosphorylated paxillin.27
Signaling through Cell-ECM Adhesions
In addition to mediating physical attachment of cells to ECM, cell-ECM adhesion provides an important means through which cells sense and response to extracellular environment. The ability of integrins to mediate bi-directional (inside-out and outside-in) transmembrane signaling lies at its interactions with both ECM and intracellular proteins and coupling of the extracellular and intracellular interactions. The extracellular ligand binding activities of integrins influence many cellular processes including cell adhesion, migration and extracellular matrix assembly. Integrin ligand binding activity can be regulated via inside-out signaling. A key step in this process is the binding of talin FERM F3 subdomain to β integrin cytoplasmic domains, which induces separation of the α and β integrin cytoplasmic domains and consequently conformational changes in integrin extracellular domains favoring ligand binding (i.e., integrin activation) (reviewed in refs. 11, 28–31). Studies in the next several years will likely yield more information on upstream mechanism that controls the binding of talin to β integrin cytoplasmic domains, factors that cooperate with talin in the binding and activation of integrins, and the structural details of how signals are propagated from the cytoplasmic domains to the extracellular domains of integrins.
Integrin mediated cell-ECM adhesion exerts profound effects on cells, including cell shape change, proliferation, differentiation and survival. Integrins influence cell behavior through regulation of many different intracellular signaling intermediates (e.g., Akt/PKB, MAP kinases and small GTPases). In general, cell-ECM adhesion promotes integrin clustering and recruitment of integrin-associated proteins to integrin-rich adhesion sites. The integrin-associated proteins (e.g., Src, FAK, paxillin and the PINCH-ILK-parvin complexes), in turn, relay signals to specific downstream effectors. Although the precise mechanisms by which signals are transmitted from cell surface integrins to intracellular signaling intermediates are complex and incompletely understood, previous studies have shed light on some of the key events in focal adhesion-mediated signal transduction (Fig. 1).
Figure 1.
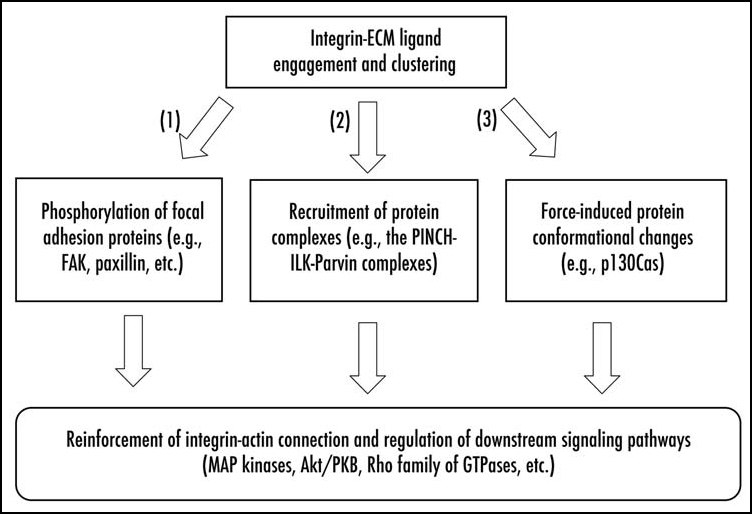
Signaling through cell-ECM adhesions. Integrin-mediated cell-ECM adhesion elicits a plethora of changes at the adhesion sites. Changes that are critical for transduction of signals through cell-ECM adhesions include (1) protein phosphorylation, (2) recruitment of protein complexes, and (3) force-induced protein conformational changes. Proteins depicted in the figure represent examples of these signaling events rather than an inclusive survey. For detailed discussion of integrin-mediated signal transduction, readers are encouraged to read (Refs. 1–12).
One important event in integrin-mediated signaling is cell adhesion-dependent phosphorylation of key focal adhesion proteins such as FAK.9,32–34 Phosphorylation of FAK at Tyr-397 creates a docking site for the SH2 domain of Src family kinases. Binding of Src to FAK phospho-Tyr-397 site releases an autoinhibitory interaction and consequently activates Src. Activated FAK/Src complex in turn phosphorylates components of focal adhesions including FAK, paxillin and p130Cas, resulting in recruitment of additional signaling intermediates (e.g., Grb2) and activation of downstream signaling pathways (e.g., the Ras and MAP kinase signaling pathway).
A second important event in integrin-mediated signaling is recruitment of key protein complexes such as the PINCH-ILK-parvin ternary complexes35 to cell-ECM contacts. The PINCH-ILK-parvin ternary complexes enhance the physical connection between integrins and the actin cytoskeleton.5,12,14 Furthermore, they play important signaling roles.36 For example, ILK, PINCH and α-parvin are required for optimal activation of Akt/PKB in many (albeit not all) cell types. α-parvin facilitates membrane translocation of Akt/PKB, an obligate step in Akt/PKB activation.37 Furthermore, ILK can directly promote phosphorylation of Ser-473, which is crucial for full activation of Akt/PKB.38 Thus, ILK, PINCH and α-parvin appear to play important roles in integrin-mediated regulation of Akt/PKB signaling pathway.
The third important event is the changes of the conformation of certain focal adhesion proteins in response to physical forces exerted on focal adhesions. Mechanotransduction is an essential function of focal adhesions. Conversion of physical signals into chemical signals is critical for many biological and pathological processes including morphogenesis, wound healing, cancer, atherosclerosis and osteoporosis.39,40 In principle, mechanotransduction can be achieved through force-induced protein conformational changes, modifications and/or positional changes.39,40 Recent studies have provided exciting evidence showing that p130Cas, a focal adhesion adaptor protein, serves as an important force sensor in cell-ECM adhesion-dependent mechanotransduction. Using recombinant extendable p130Cas fusion protein containing the substrate domain and antibodies specific for extended p130Cas or tyrosine phosphorylated p130Cas, Sawada et al showed that the conformation and tyrosine phosphorylation of p130Cas are changed in vitro as well as in cells in response to mechanical stretches.41
It is evident from this brief discussion that signals can be transduced through focal adhesions via different mechanisms (e.g., changes in protein phosphorylation, conformation, catalytic activity, interaction and translocation). Furthermore, the effects of cell-ECM adhesion on cell behavior can be influenced by many factors, including the constituents and organization of ECM, the repertoire and activities of cell surface integrins, the wiring of the “integrin-actin” network and the communication with other cell surface receptors and signaling pathways. Thus, the responses of different cell types to ECM adhesion can vary. While studies at the molecular and sub-cellular levels in cultured cells are extremely valuable for elucidating the basic mechanisms of cell-ECM adhesion and signaling, it is important to also investigate the roles of cell-ECM adhesion proteins at the organ and whole organism level.
Cell-ECM Adhesion and Signaling in Embryonic Development and Diseases
Reverse and forward genetics provide powerful approaches to investigate the roles of cell-ECM adhesion proteins in embryonic development and diseases. Many focal adhesion proteins, including β1 integrin,42,43 α4 integrin,44 α545 integrin, talin,46 paxillin,47 vinculin,48 FAK,49 ILK,50 PINCH-151,52 and α-parvin,12 are essential for vertebrates (e.g., mouse) embryonic development. Loss of these proteins often alters cell-ECM adhesion, cytoskeletal organization, polarity, migration and/or survival during embryogenesis. Orthologues of these proteins have been identified in invertebrates such as C. elegans and Drosophila. In many cases, the orthologues are also required for embryonic development in invertebrate organisms. Remarkably, some of these invertebrate orthologues exhibit considerable functional similarities with their mammalian counterparts. For example, invertebrate (e.g., C. elegans and Drosophila) orthologues of PINCH, ILK and α-parvin, like their mammalian counterparts, form a ternary complex linking integrins to the actin cytoskeleton.53–57
Some cell-ECM adhesion proteins are dispensable for mammalian embryonic development but they are critical for tissue and organ functions in postnatal or adult. These proteins often are highly expressed in certain specific cell types and consequently loss of these proteins results in adhesion defects in these cell types. For example, mutations that eliminate or reduce the expression or activity of platelet αIIbβ3 integrin cause Glanzmann thrombasthenia, whereas mutations in β2 integrins, which are exclusively expressed by leukocytes, result in the leukocyte adhesion deficiency-1 disorder (reviewed in ref. 58). Kindler syndrome is a rare genetic disorder that is characterized by skin blister formation, cytoskeleton alteration and atrophy in epidermis and mucosal membranes of the digestive and urinary tracts and other tissues. Recently, it has been found that null or truncation mutations in kindlin-1, which encodes a FERM-domain containing focal adhesion protein, are responsible for Kindler syndrome.59,60
Loss of certain individual cell-ECM adhesion proteins from animals (e.g., mouse) results in no obvious defects in embryonic development and adult. This does not necessarily mean, however, these proteins do not contribute to the embryogenesis or tissue and organ functions. In many cases, these proteins are co-expressed with functionally redundant proteins and therefore defects are not observed unless functional redundant proteins are removed. For example, Nck-1 and Nck-2 are widely expressed in mammalian cells. No defects were detected in mice deficient for either Nck-1 or Nck-2. However, elimination of both Nck-1 and Nck-2 results in embryonic lethality and defects in cell migration and actin cytoskeletal organization.61
Focal adhesion proteins that are essential for embryonic development are often required for the functionality of at least certain tissues and organs in post-natal and adult. For example, ILK, which is required for early embryonic development, is also critical for the functionality of several tissues and organs (e.g., heart, kidney, etc).62–65 Future studies using cell type-specific conditional knockout techniques will provide detailed information on the functions of cell-ECM adhesion proteins in different tissues and organs.
While cell-ECM adhesion proteins are often indispensable for embryonic development and/or normal tissue and organ function, numerous studies have shown that elevated levels of cell-ECM adhesion proteins could also be detrimental. Increased expression or activities of focal adhesion proteins have been associated with many human diseases. For example, increased levels and/or activities of Src, FAK and ILK have been associated with malignant diseases in a variety of tissues and organs (reviewed in refs. 32–34, 36). The oncogenic focal adhesion proteins contribute to multiple facets of cancer, including increased cell proliferation, resistance to apoptosis, elevated cell motility and invasion, and promotion of angiogenesis. Elucidation of the mechanisms, whereby focal adhesion proteins function in the pathological processes represents a major challenge as well as an exciting opportunity in the years to come.
Acknowledgements
This work was supported by NIH grants GM65188 and DK54639 to Chuanyue Wu. I apologize that I was unable to reference many publications in this brief article due to space constraints.
Abbreviations
- CH
calponin homology
- ECM
extracellular matrix
- FERM
four point one ezrin radixin and moesin
- FAK
focal adhesion kinase
- ILK
integrin linked kinase
- mDia
mammalian homolog of diaphanous
- PAK
p21 activated kinase
- PDZ
PSD95/DlgA/ZO 1 homology
- SH
Src homology
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/cellahdesion/abstract.php?id=4081
References
- 1.Schwartz MA, Schaller MD, Ginsberg MH. Integrins: Emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 2.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 3.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 4.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix-cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 5.Wu C, Dedhar S. Integrin linked kinase (ILK) and its interactors: A new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. J Cell Biol. 2001;155:505–510. doi: 10.1083/jcb.200108077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 7.Juliano RL. Signal transduction by cell adhesion receptors and the cytoskeleton: Functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol. 2002;42:283–323. doi: 10.1146/annurev.pharmtox.42.090401.151133. [DOI] [PubMed] [Google Scholar]
- 8.Miranti CK, Brugge JS. Sensing the environment: A historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4:E83–E90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- 9.Parsons JT. Focal adhesion kinase: The first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 10.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: Integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 11.Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol. 2005;17:509–516. doi: 10.1016/j.ceb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Legate KR, Montanez E, Kudlacek O, Fassler R. ILK, PINCH and parvin: The tIPP of integrin signalling. Nat Rev Mol Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- 13.Larsen M, Artym VV, Green JA, Yamada KM. The matrix reorganized: Extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol. 2006;18:463. doi: 10.1016/j.ceb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Wu C. PINCH, N(i)ck and the ILK: Network wiring at cell-matrix adhesions. Trends Cell Biol. 2005;15:460–466. doi: 10.1016/j.tcb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Brown CM, Hebert B, Kolin DL, Zareno J, Whitmore L, Horwitz AR, Wiseman PW. Probing the integrin-actin linkage using high-resolution protein velocity mapping. J Cell Sci. 2006;119:5204–5214. doi: 10.1242/jcs.03321. [DOI] [PubMed] [Google Scholar]
- 16.Hu K, Ji L, Applegate KT, Danuser G, Waterman-Storer CM. Differential transmission of actin motion within focal adhesions. Science. 2007;315:111–115. doi: 10.1126/science.1135085. [DOI] [PubMed] [Google Scholar]
- 17.Zamir E, Katz M, Posen Y, Erez N, Yamada KM, Katz BZ, Lin S, Lin DC, Bershadsky A, Kam Z, Geiger B. Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat Cell Biol. 2000;2:191–196. doi: 10.1038/35008607. [DOI] [PubMed] [Google Scholar]
- 18.Murphy-Ullrich JE. The de-adhesive activity of matricellular proteins: Is intermediate cell adhesion an adaptive state? J Clin Invest. 2001;107:785–790. doi: 10.1172/JCI12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Chen K, Tu Y, Velyvis A, Yang Y, Qin J, Wu C. Assembly of the PINCH-ILK-CH-ILKBP complex precedes and is essential for localization of each component to cell-matrix adhesion sites. J Cell Sci. 2002;115:4777–4786. doi: 10.1242/jcs.00166. [DOI] [PubMed] [Google Scholar]
- 20.Geiger B, Bershadsky A. Assembly and mechanosensory function of focal contacts. Curr Opin Cell Biol. 2001;13:584–592. doi: 10.1016/s0955-0674(00)00255-6. [DOI] [PubMed] [Google Scholar]
- 21.Burridge K, Wennerberg K. Rho and rac take center stage. Cell. 2004;116:167. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 22.Bershadsky AD, Ballestrem C, Carramusa L, Zilberman Y, Gilquin B, Khochbin S, Alexandrova AY, Verkhovsky AB, Shemesh T, Kozlov MM. Assembly and mechanosensory function of focal adhesions: Experiments and models. Eur J Cell Biol. 2006;85:165. doi: 10.1016/j.ejcb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- 24.Bar-Sagi D, Hall A. Ras and rho GTPases: A family reunion. Cell. 2000;103:227. doi: 10.1016/s0092-8674(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 25.Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 26.Ruest PJ, Roy S, Shi E, Mernaugh RL, Hanks SK. Phosphospecific antibodies reveal focal adhesion kinase activation loop phosphorylation in nascent and mature focal adhesions and requirement for the autophosphorylation site. Cell Growth Differ. 2000;11:41–48. [PubMed] [Google Scholar]
- 27.Zaidel-Bar R, Milo R, Kam Z, Geiger B. A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J Cell Sci. 2007;120:137–148. doi: 10.1242/jcs.03314. [DOI] [PubMed] [Google Scholar]
- 28.Critchley DR. Genetic, biochemical and structural approaches to talin function. Biochem Soc Trans. 2005;33:1308–1312. doi: 10.1042/BST0331308. [DOI] [PubMed] [Google Scholar]
- 29.Qin J, Vinogradova O, Plow EF. Integrin bidirectional signaling: A molecular view. PLoS Biol. 2004;2:e169. doi: 10.1371/journal.pbio.0020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell ID, Ginsberg MH. The talin-tail interaction places integrin activation on FERM ground. Trends Biochem Sci. 2004;29:429–435. doi: 10.1016/j.tibs.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Nayal A, Webb DJ, Horwitz AF. Talin: An emerging focal point of adhesion dynamics. Curr Opin Cell Biol. 2004;16:94–98. doi: 10.1016/j.ceb.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Schlaepfer DD, Mitra SK, Ilic D. Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim Biophys Acta. 2004;1692:77–102. doi: 10.1016/j.bbamcr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Playford MP, Schaller MD. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23:7928–7946. doi: 10.1038/sj.onc.1208080. [DOI] [PubMed] [Google Scholar]
- 34.Cohen LA, Guan JL. Mechanisms of focal adhesion kinase regulation. Curr Cancer Drug Targets. 2005;5:629–643. doi: 10.2174/156800905774932798. [DOI] [PubMed] [Google Scholar]
- 35.Wu C. The PINCH-ILK-parvin complexes: Assembly, functions and regulation. Biochim Biophy Acta (BBA) - Mol Cell Res. 2004;1692:55–62. doi: 10.1016/j.bbamcr.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Hannigan G, Troussard A, Dedhar S. Integrin-linked kinase: A cancer therapeutic target unique among its ILK. Nature Rev Cancer. 2005;5:51–63. doi: 10.1038/nrc1524. [DOI] [PubMed] [Google Scholar]
- 37.Fukuda T, Guo L, Shi X, Wu C. CH-ILKBP regulates cell survival by facilitating the membrane translocation of protein kinase B/Akt. J Cell Biol. 2003;160:1001–1008. doi: 10.1083/jcb.200212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci USA. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell. 2006;10:11. doi: 10.1016/j.devcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Bershadsky A, Kozlov M, Geiger B. Adhesion-mediated mechanosensitivity: A time to experiment, and a time to theorize. Curr Opin Cell Biol. 2006;18:472. doi: 10.1016/j.ceb.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fassler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- 43.Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- 44.Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- 45.Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- 46.Monkley SJ, Zhou XH, Kinston SJ, Giblett SM, Hemmings L, Priddle H, Brown JE, Pritchard CA, Critchley DR, Fassler R. Disruption of the talin gene arrests mouse development at the gastrulation stage. Dev Dynamics. 2000;219:560–574. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1079>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 47.Hagel M, George EL, Kim A, Tamimi R, Opitz SL, Turner CE, Imamoto A, Thomas SM. The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol Cell Biol. 2002;22:901–915. doi: 10.1128/MCB.22.3.901-915.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu W, Baribault H, Adamson ED. Vinculin knockout results in heart and brain defects during embryonic development. Development. 1998;125:327–337. doi: 10.1242/dev.125.2.327. [DOI] [PubMed] [Google Scholar]
- 49.Furuta Y, Ilic D, Kanazawa S, Takeda N, Yamamoto T, Aizawa S. Mesodermal defect in late phase of gastrulation by a targeted mutation of focal adhesion kinase, FAK. Oncogene. 1995;11:1989–1995. [PubMed] [Google Scholar]
- 50.Sakai T, Li S, Docheva D, Grashoff C, Sakai K, Kostka G, Braun A, Pfeifer A, Yurchenco PD, Fassler R. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev. 2003;17:926–940. doi: 10.1101/gad.255603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang X, Zhou Q, Li X, Sun Y, Lu M, Dalton N, Ross J, Jr, Chen J. PINCH1 plays an essential role in early murine embryonic development but is dispensable in ventricular cardiomyocytes. Mol Cell Biol. 2005;25:3056–3062. doi: 10.1128/MCB.25.8.3056-3062.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li S, Bordoy R, Stanchi F, Moser M, Braun A, Kudlacek O, Wewer UM, Yurchenco PD, Fassler R. PINCH1 regulates cell-matrix and cell-cell adhesions, cell polarity and cell survival during the peri-implantation stage. J Cell Sci. 2005;118:2913–2921. doi: 10.1242/jcs.02422. [DOI] [PubMed] [Google Scholar]
- 53.Zervas CG, Gregory SL, Brown NH. Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J Cell Biol. 2001;152:1007–1018. doi: 10.1083/jcb.152.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mackinnon AC, Qadota H, Norman KR, Moerman DG, Williams BD. C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr Biol. 2002;12:787–797. doi: 10.1016/s0960-9822(02)00810-2. [DOI] [PubMed] [Google Scholar]
- 55.Lin X, Qadota H, Moerman DG, Williams BD. C. elegans PAT-6/Actopaxin plays a critical role in the assembly of integrin adhesion complexes in vivo. Curr Biol. 2003;13:922–932. doi: 10.1016/s0960-9822(03)00372-5. [DOI] [PubMed] [Google Scholar]
- 56.Clark KA, McGrail M, Beckerle MC. Analysis of PINCH function in Drosophila demonstrates its requirement in integrin-dependent cellular processes. Development. 2003;130:2611–2621. doi: 10.1242/dev.00492. [DOI] [PubMed] [Google Scholar]
- 57.Kadrmas JL, Smith MA, Clark KA, Pronovost SM, Muster N, Yates JRR, Beckerle MC. The integrin effector PINCH regulates JNK activity and epithelial migration in concert with Ras suppressor 1. J Cell Biol. 2004;167:1019–1024. doi: 10.1083/jcb.200408090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hogg N, Bates PA. Genetic analysis of integrin function in man: LAD-1 and other syndromes. Matrix Biol. 2000;19:211–222. doi: 10.1016/s0945-053x(00)00066-4. [DOI] [PubMed] [Google Scholar]
- 59.Jobard F, Bouadjar B, Caux F, Hadj-Rabia S, Has C, Matsuda F, Weissenbach J, Lathrop M, Prud'homme JF, Fischer J. Identification of mutations in a new gene encoding a FERM family protein with a pleckstrin homology domain in Kindler syndrome. Hum Mol Genet. 2003;12:925–935. doi: 10.1093/hmg/ddg097. [DOI] [PubMed] [Google Scholar]
- 60.Siegel DH, Ashton GH, Penagos HG, Lee JV, Feiler HS, Wilhelmsen KC, South AP, Smith FJ, Prescott AR, Wessagowit V, Oyama N, Akiyama M, Al Aboud D, Al Aboud K, Al Githami A, Al Hawsawi K, Al Ismaily A, Al-Suwaid R, Atherton DJ, Caputo R, Fine JD, Frieden IJ, Fuchs E, Haber RM, et al. Loss of kindlin-1, a human homolog of the Caenorhabditis elegans actin-extracellular-matrix linker protein UNC-112, causes kindler syndrome. Am J Hum Genet. 2003;73:174–187. doi: 10.1086/376609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bladt F, Aippersbach E, Gelkop S, Strasser GA, Nash P, Tafuri A, Gertler FB, Pawson T. The murine Nck SH2/SH3 adaptors are important for the development of mesoderm-derived embryonic structures and for regulating the cellular actin network. Mol Cell Biol. 2003;23:4586–4597. doi: 10.1128/MCB.23.13.4586-4597.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.White DE, Coutu P, Shi YF, Tardif JC, Nattel S, St Arnaud R, Dedhar S, Muller WJ. Targeted ablation of ILK from the murine heart results in dilated cardiomyopathy and spontaneous heart failure. Genes Dev. 2006;20:2355–2360. doi: 10.1101/gad.1458906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bendig G, Grimmler M, Huttner IG, Wessels G, Dahme T, Just S, Trano N, Katus HA, Fishman MC, Rottbauer W. Integrin-linked kinase, a novel component of the cardiac mechanical stretch sensor, controls contractility in the zebrafish heart. Genes Dev. 2006;20:2361–2372. doi: 10.1101/gad.1448306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.El-Aouni C, Herbach N, Blattner SM, Henger A, Rastaldi MP, Jarad G, Miner JH, Moeller MJ, St-Arnaud R, Dedhar S, Holzman LB, Wanke R, Kretzler M. Podocyte-specific deletion of integrin-linked kinase results in severe glomerular basement membrane alterations and progressive glomerulosclerosis. J Am Soc Nephrol. 2006 doi: 10.1681/ASN.2005090921. [DOI] [PubMed] [Google Scholar]
- 65.Dai C, Stolz DB, Bastacky SI, St-Arnaud R, Wu C, Dedhar S, Liu Y. Essential role of integrin-linked kinase in podocyte biology: Bridging the integrin and slit diaphragm signaling. J Am Soc Nephrol. 2006;17:2164–2175. doi: 10.1681/ASN.2006010033. [DOI] [PubMed] [Google Scholar]


