Abstract
Fetal cells migrate into the mother during pregnancy. Fetomaternal transfer probably occurs in all pregnancies and in humans the fetal cells can persist for decades. Microchimeric fetal cells are found in various maternal tissues and organs including blood, bone marrow, skin and liver. In mice, fetal cells have also been found in the brain. The fetal cells also appear to target sites of injury. Fetomaternal microchimerism may have important implications for the immune status of women, influencing autoimmunity and tolerance to transplants. Further understanding of the ability of fetal cells to cross both the placental and blood-brain barriers, to migrate into diverse tissues, and to differentiate into multiple cell types may also advance strategies for intravenous transplantation of stem cells for cytotherapeutic repair. Here we discuss hypotheses for how fetal cells cross the placental and blood-brain barriers and the persistence and distribution of fetal cells in the mother.
Key Words: fetomaternal microchimerism, stem cells, progenitor cells, placental barrier, blood-brain barrier, adhesion, migration
Microchimerism is the presence of a small population of genetically distinct and separately derived cells within an individual. This commonly occurs following transfusion or transplantation.1–3 Microchimerism can also occur between mother and fetus. Small numbers of cells traffic across the placenta during pregnancy. This exchange occurs both from the fetus to the mother (fetomaternal)4–7 and from the mother to the fetus.8–10 Similar exchange may also occur between monochorionic twins in utero.11–13 There is increasing evidence that fetomaternal microchimerism persists lifelong in many child-bearing women.7,14 The significance of fetomaternal microchimerism remains unclear. It could be that fetomaternal microchimerism is an epiphenomenon of pregnancy. Alternatively, it could be a mechanism by which the fetus ensures maternal fitness in order to enhance its own chances of survival. In either case, the occurrence of pregnancy-acquired microchimerism in women may have implications for graft survival and autoimmunity. More detailed understanding of the biology of microchimeric fetal cells may also advance progress towards cytotherapeutic repair via intravenous transplantation of stem or progenitor cells.
Trophoblasts were the first zygote-derived cell type found to cross into the mother. In 1893, Schmorl reported the appearance of trophoblasts in the maternal pulmonary vasculature.15 Later, trophoblasts were also observed in the maternal circulation.16–20 Subsequently various other fetal cell types derived from fetal blood were also found in the maternal circulation.21,22 These fetal cell types included lymphocytes,23 erythroblasts or nucleated red blood cells,24,25 haematopoietic progenitors7,26,27 and putative mesenchymal progenitors.14,28 While it has been suggested that small numbers of fetal cells traffic across the placenta in every human pregnancy,29–31 trophoblast release does not appear to occur in all pregnancies.32 Likewise, in mice, fetal cells have also been reported in maternal blood.33,34 In the mouse, fetomaternal transfer also appears to occur during all pregnancies.35
Anatomy of the Placenta
Human and rodent placentation is hemochorial, the fetomaternal interaction between the two blood circulations involving direct physical interaction between maternal blood and the chorionic trophoblasts.36 The fetal and maternal blood circulates in channels lined by these zygote-derived cells within the placental region known as the labyrinth in mice or the fetal placenta in humans (Fig. 1). In the human, the channels through which the fetal blood flows, the chorionic villi, form trees with numerous branches and sub-branches terminating in villous blunt-endings. The maternal blood flows in the relatively open intervillous space. In contrast in the mouse, the maternal blood flows through a labyrinthine network of interconnected cavities or lacunae.36 A layer of trophoblast cells forms the interface between the maternal blood and the fetal tissues. It is these trophoblast cells that form the placental barrier between maternal and fetal circulation. In the human, this interface consists of a syncytium of syncytiotrophoblasts directly contacting the maternal blood (Fig. 2B). In the first trimester, there is also a layer of replicating mononuclear cytotrophoblasts beneath the syncytiotrophoblasts. In contrast, in mice there are three layers of trophoblasts. The outer layer consists of mononuclear cytotrophoblasts while the middle and inner layers are syncytiotrophoblastic.36 Between the trophoblasts and the fetal blood there are a trophoblastic basement membrane, in some but not all interfaces a core of extracellular matrix and/or pericytes, an endothelial basement membrane, and fetal capillary endothelial cells36 (Fig. 2B). Fetal blood enters and leaves the fetal placenta/labyrinth via the umbilical cord, whereas maternal blood enters and leaves the fetal placenta/labyrinth via the utero-placental circulation.
Figure 1.
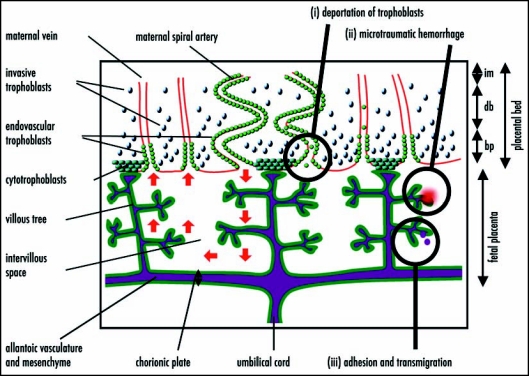
A simplified diagrammatic representation of the structure of the human placenta (adapted from Georgiades et al.36) and hypothesized mechanisms of fetomaternal cell traffic. From the end of the first trimester, maternal blood flows into the fetal placenta via the maternal spiral arteries, through the intervillous space bathing the branches of the villous trees and out through the maternal veins (red arrows on left-hand side). The fetal blood enters via the umbilical cord and circulates to the fetal capillaries in the villous trees. A layer of zygote-derived trophoblasts, in humans a syncytium of syncytiotrophoblasts, on the surface of the villous trees (dark green) forms the barrier between the fetal tissues and the maternal blood. Zygote-derived trophoblasts also progressively invade the placental bed and line the maternal vasculature. By the third trimester the maternal spiral arteries are lined through to the (im), while the maternal veins are lined to the border between the decidua basalis (db) and basal plate (bp). In the mouse, the analogue of the fetal placenta is labyrinthine and the trophoblastic invasion of the maternal blood vessels does not extend beyond the junctional zone analogous to the basal plate. Hypothesized mechanisms of fetomaternal cell traffic include (i) deportation of trophoblasts lining the maternal vessels and intervillous space; (ii) microtraumatic hemorrhage; and (iii) cell adhesion and transmigration across the placental barrier.
Figure 2.
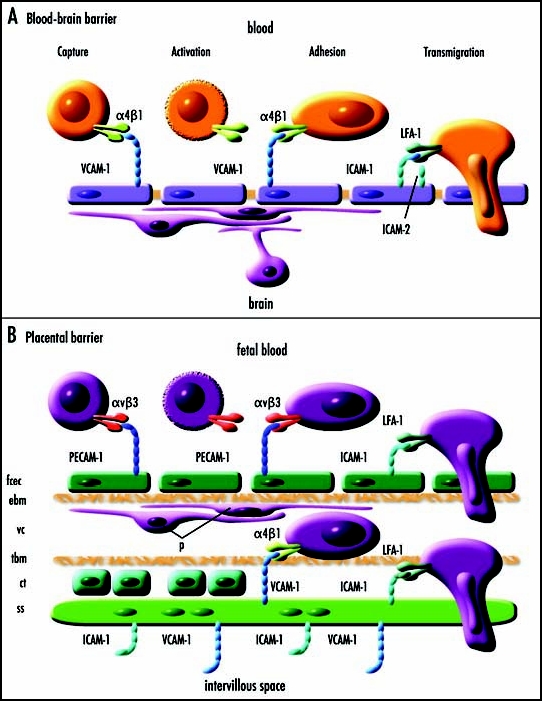
Simplified diagrammatic representations of blood-brain and placental barriers and hypothesized molecular mechanisms of cell adhesion and transmigration. (A) A simplified diagrammatic representation of multistep lymphocyte recognition and capture from blood at the blood brain barrier (adapted from Engelhardt48). Cells expressing α4β1 are captured by VCAM-1 expressed by endothelial cells. There is a rapid activation phase (seconds) that may involve lymphoid chemokines CCL19/ELC and CCL21/SLC. There is a prolonged adhesion phase (hours) followed by slow transmigration (hours) dependent upon binding of LFA-1 to ICAM-1 and/or ICAM-2 on the endothelial cells. It is hypothesized that a similar molecular mechanism may explain fetal cell migration across the blood-brain barrier and the placental barrier. (B) A simplified diagrammatic representation of the human placental barrier showing a hypothetical mechanism of fetal cell capture, adhesion and transmigration. The placental barrier comprises of fetal capillary endothelial cells (fcec), an endothelial basement membrane (ebm), the villous core (vc) which at some interfaces contains pericytes (p) and extracellular matrix, a trophoblastic basement membrane (tbm), in the first trimester a layer of proliferative cytotrophoblasts (ct), and a multinucleated syncytium of syncytiotrophoblasts (ss). In the mouse, the trophoblastic layers differ in that there are two syncytiotrophoblastic layers and the cytotrophoblastic layer is outermost facing the intervillous interface. It is hypothesized that fetal cells may adhere and transmigrate across the placental barrier in a similar manner to that by which lymphocytes cross the blood-brain barrier.
The zone bordering the maternal surface of the fetal placenta/labyrinth is known as the basal plate in humans and the junctional zone or spongiotrophoblast zone in mice. This region is not perfused by fetal blood but is crossed by maternal blood channels lined by zygote-derived trophoblast cells through which the maternal blood flows in and out of the fetal placenta/labyrinth.36 This zone in turn is bordered by the maternal uterine tissue on the maternal side. The maternal uterine tissue becomes progressively invaded by zygote-derived trophoblast cells. In particular, these cells line the maternal blood vessels in the maternal uterine tissue. The maternal uterine tissues of this region, known as the placental bed in humans, can be divided into the decidua basalis adjacent to the basal plate/junctional zone and the myometrium on the maternal side. In humans, trophoblast invasion extends to the inner third of the myometrium but in mice, trophoblast invasion is shallow and is limited to the decidua basalis.36,37 Even within the decidua basalis, maternal arteries and veins remain lined by maternal endothelium rather than trophoblasts in the mouse.38,39 While in the human the trophoblasts stimulate arterial remodeling in the mouse uterine natural killer cells are more important.39–41
The cells of the placenta itself comprise both zygote-derived and maternal cells. In mice, the zygote-derived cells include trophoblasts derived from the polar trophectoderm of the outer cell mass; fetal blood vessels and mesenchyme derived from the allantoic mesenchyme, which in turn is derived from the primitive ectoderm of the inner cell mass; and fetal blood cells of mesodermal lineage. Meanwhile, the maternal cells of the mouse placenta include uterine cells and cells coming from the maternal blood.36 It is generally assumed that the origin of human placental cells is similar to those in the mouse, although not lineage studies have been performed on human placentae.36 However, there is debate over whether the human allantoic vasculature, through which the fetal blood passes, is of trophectodermal or epiblast/hypoblast origin.36,42
The similarities in the anatomy of placentation and placental blood flow in mice and humans36,39 and the role of analogous genes in mouse and human placentation43 make mouse placentation a good model for many aspects of human placentation. However, there are important anatomical differences,36,39 in particular the difference between the villous nature of the human fetal placenta and the labyrinthine nature of the analogous mouse labyrinth and the greater role of invasion by zygote-derived trophoblasts in the maternal circulation in the human placenta.
Cell Traffic Across the Placenta
The mechanism by which cells are exchanged across the placental barrier is unclear. Possible explanations include deportation of trophoblasts, microtraumatic rupture of the placental blood channels or that specific cell types are capable of adhesion to the trophoblasts of the walls of the fetal blood channels and migration through the placental barrier created by the trophoblasts (Fig. 1i–1iii). Intervillous thrombi containing mixed maternal and fetal cells occur in the fetal placenta/labyrinth.44,45 Histological defects in the continuity of the trophoblasts lining the vasculature of the placenta are also reported.46,47 Together these observations suggest the possibility that fetomaternal hemorrhage within the fetal placenta/labyrinth may allow exchange of cells between the fetal and maternal circulation. Microtraumatic dislodgment of trophoblasts from the trophoblast-lined blood channels through which the maternal blood passes may also explain why trophoblasts appear in maternal circulation. The microtraumatic hypothesis of cell exchange does not appear consistent with the hypothesis that fetomaternal microchimerism may be of adaptive value to the fetus but fits well with the hypothesis that fetomaternal microchimerism is an epiphenomenon of pregnancy with potential pathological consequences.
An alternative hypothesis is that cells cross the placental barrier by mechanisms akin to the active adhesion and transmigration that occurs across high endothelial venule (HEV) endothelium in peripheral lymph nodes and at the blood-brain barrier (BBB).48 Intriguingly, in the mouse at least some of the fetal cells that enter the mother are also capable of crossing the blood brain barrier into the brain.35,49
At the BBB and HEV, lymphocyte migration across the endothelial membrane involves a multistep process of recognition and recruitment from the blood involving tethering/rolling or capture, activation, adhesion and finally transmigration (Fig. 2A). In both HEV endothelium and BBB, the final stage of transmigration involves binding of LFA-1 expressed by the lymphocytes to ICAM-1 in HEV endothelium and to ICAM-1 and/or ICAM-2 at the BBB.48,50,51 In the HEV endothelium, the ICAM-1 also appears to be involved in the adhesion preceding transmigration, whereas at the blood brain barrier VCAM-1 is involved in lymphocyte capture and adhesion. Fetal cells crossing the placental barrier must transmigrate both the fetal capillary endothelial cell layer and the trophoblast cell layers (Fig. 2B).
The fetal capillary endothelial cell layer expresses a number of cell adhesion molecules including PECAM-1 and ICAM-1.52,53 While, there is VCAM-1 expression in umbilical cord endothelium there appears to be no evidence for VCAM-1 expression on fetal capillary endothelium in normal placenta at term.52,53 As PECAM-1 plays a role in integrin-mediated neutrophil adhesion and endothelial transmigration,54–56 including migration of CD34+ positive cells57 such as the fetal cells in maternal blood,7 we hypothesize that it is also a candidate for contribution to fetal cell transmigration across the fetal capillary endothelium (Fig. 2B). The functional ligand for PECAM-1 in transmigration is unknown, but it is possible that it is an αvβ3 integrin.58 It is possible that multiple fetal cell types cross the placental barrier by different mechanisms.
Once the fetal cells have crossed the fetal capillary endothelium, they must next cross the trophoblast layer. Trophoblasts express ICAM-1 in vitro and in vivo59–61 and monocytes bind to ICAM-1 expressed by trophoblasts in an LFA-1-dependent manner.60 Similarly, the migration of Toxoplasma gondii across epithelial barriers, including the placental barrier comprised of trophoblast cells, involves interaction of the parasite adhesion molecule, MIC2, with the intercellular adhesion molecule 1 (ICAM-1).62 Together these studies suggest that the molecular apparatus for maternofetal transmigration may be present at the placental barrier. Although there is evidence for greater in vivo expression of ICAM-1 on the apical surface of the villous syncytiotrophoblasts exposed to the maternal blood,60 ICAM-1 is also present throughout the stroma of the chorionic villi,60,61 although it has not been clearly established that it is expressed on the basal surface of the trophoblasts facing the villous core. Trophoblasts also express VCAM-1.63–65 Thus the molecular apparatus for fetomaternal transmigration of fetal cells expressing LFA-1 may also be present at the trophoblast cell layer. Once the fetal cells have crossed the fetal capillary endothelial cell layer, we hypothesize that they cross the trophoblast cell layer again in a manner similar to that in which lymphocytes cross the BBB (Fig. 2B).
We hope that this speculative hypothesis regarding the mechanisms of fetomaternal cell traffic may stimulate further research and that future studies will determine whether active fetomaternal adhesion and transmigration occurs and elucidate the molecular mechanisms involved.
Timing of Onset of Fetomaternal Traffic
In mice, fetal cells generally first appear in the mother in the second week of pregnancy35 (see also Fig. 3). Numbers of fetal cells are present in maternal blood by GD10 to GD12 days (gestational days, the day of vaginal plug detection being designated GD0) in pregnancies from syngenic and allogenic crosses; however the cells do not appear in blood in until GD13 to GD16 in pregnancies from outbred crosses.66 The appearance of fetal cells in maternal blood at GD10 to GD12 in syngenic and allogenic crosses is consistent with the establishment of uteroplacental circulation. Maternal blood first appears in the labyrinth between GD9 and GD10 and extensive fetal capillary formation occurs by GD12.39,67 This coincides with the onset of fetal circulation on the completion of organogenesis at GD9 to GD10.36 In humans, fetal DNA has been detected in maternal blood as early as four weeks and five days after conception and both fetal cells and DNA are consistently detected from seven weeks.68,69 Thus in humans, the first appearance of fetal cells in maternal blood occurs slightly before the completion of fetal organogenesis, the onset of fetal circulation to the placenta, and the appearance of maternal blood within the fetal placenta. Plugs of invading trophoblast cells, which block the tips of the uteroplacental spiral arteries, are progressively dislocated after 10–12 weeks70 and blood only becomes evident in the intervillous space of the fetal placenta after ten weeks gestation.71 Effective arterial circulation of the placenta is not established until around the twelfth week of gestation39,72,73 when the human embryo has largely completed the organogenesis stage.36 In the mouse, the timing of the appearance of fetal cells in maternal blood is consistent with the hypothesis that fetomaternal exchange occurs between fetal and maternal blood at the placental barrier in the fetal placenta/labyrinth. In the fetal placenta/labyrinth, the maternal blood comes into direct contact with the zygote-derived trophoblast and it has been proposed these may also be deported into the maternal circulation.66 The fetal placenta/labyrinth is also very rich in fetal hematopoietic stem cells74–76 and it has been suggested that these cells might able to migrate into the maternal blood.66 The earlier appearance of fetal cells in maternal blood in humans may suggest more active migration of certain fetal cells. Potentially there may be multiple cell types and phases of migration involved. More detailed investigation of the time course of the appearance of maternal blood in the placenta and the appearance of fetal cells in maternal blood in humans may be informative.
Figure 3.
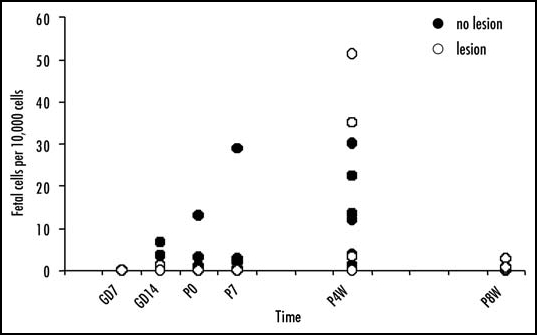
Time course of fetal cell engraftment and persistence in the mouse brain. Adult female mice received intraventricular injection of the excitotoxic NMDA to produce a diffuse brain lesion or were untreated. The mice were crossed with adult male enhanced green fluorescent protein (EGFP) transgenic Green Mice. Fetomaternal microchimerism in the brain was assayed at various time points: gestational days (GD) 7 and 14, the day of parturition (P0), and at seven days (P7), four weeks (P4W) and eight weeks (P8W) post partum (n = 3–8 per group at each time point). The number of fetal cells relative to total cells present in a brain block centered about the site of the injection was quantified by real-time PCR for the EGFP gene in genomic DNA. Procedures were as previously described.49 There are great individual differences, however, in those mothers in which fetal cells were detected in the brain, the number of fetal cells detected in the brain increases by four weeks post partum and declines again by eight weeks post partum. Overall, in those mothers in which fetal cells persist at four weeks and eight weeks post partum, there are greater numbers of fetal cells in the lesioned brains.
The reason for the delay in the appearance of fetal cells in maternal blood in outbred mouse crosses is at present unknown. Outbred crosses were also observed to result in delayed and reduced trophoblast invasion of the decidua basalis.66 It may be that the appearance of fetal cells in maternal blood on outbred crosses is due to a more aggressive immune response; alternatively the delay may be due to a delay in the maturation of the placenta and maternal circulation to the labyrinth. It is hoped that further studies may elucidate the issue.
Intriguingly in syngenic pregnancies, fetal cells were detected in mouse lungs and to a lesser extent spleen and kidney in the first week of gestation before they robustly appear in detectable numbers in maternal circulation.35,66 One explanation might be that, consistent with the appearance of trophoblasts in maternal lungs in humans,15 these cells are trophoblasts. Thus one might hypothesis that the earliest phase of fetomaternal microchimerism involves deportation of zygote-derived trophoblasts as they invade the decidua basalis to line the maternal blood vasculature. In particular, the fate of the trophoblasts that plug the ends of the maternal arteries of the uteroplacental circulation may be to become dislodged into maternal circulation as maternal blood flow begins to break through into the fetal placenta/labyrinth. Trophoblasts being large are rapidly cleared from maternal blood as they become lodged in the microvasculature of the lung and to a lesser extent other organs. While the studies discussed here have made important contributions to establishing the time course of fetomaternal traffic, the question of whether different zygote-derived cell types show different time courses of traffic has not been investigated in depth. It is hoped that future studies will address this important issue.
Frequency and Persistence of Fetomaternal Microchimerism
Fetomaternal microchimerism appears to occur with great frequency following human pregnancy. It has been suggested that fetomaternal traffic occurs in all pregancies.14 Moreover fetal cells are reported to persist in the mother for decades. Male cells have been found in maternal blood even decades after pregnancy,7,77 including in one case in which the women was last pregnant with a male child 27 years earlier.7 Fetal cells also may persist for even longer after engrafting maternal bone marrow14 and perhaps other organs. By engrafting into niches such as the bone marrow, fetal cells may also be able to proliferate and reinfiltrate blood or other tissues later. There is strong evidence that fetal cells with the characteristics of mesenchymal cells do engraft the bone marrow. Male DNA was detected in 48% of CD34-enriched apheresis products from nonpregnant female marrow donors.1 Male cells were also detected in all bone marrow samples from women who had previously been pregnant with males, including one woman who was last pregnant with a son 51 years earlier.14
The absence of Y chromosome markers in samples from women who had never born sons in some studies14 strongly supports the argument that the male cells observed originate from the fetus. However, it is important to note that there are crucial caveats in the use of the Y chromosome alone as a marker for fetomaternal microchimerism that may have led to over estimation of the incidence and persistence of fetomaternal microchimerism in humans. Male cells have been found in the blood of women without sons.78,79 Male cells may occur in the blood of as many as 8–10% of healthy women without sons and no known history of abortion.79 It has been speculated that the male cells arise from unrecognized spontaneous abortions, vanished male twins, an older brother transferred by the maternal circulation, or sexual intercourse. However, a history of unrecognized spontaneous abortions or sexual intercourse cannot explain all cases of the presence of male cells in females as another study detected the presence of the Y chromosome in normal liver from seven of eleven female fetuses and five of six female children.80 Such microchimerism may be best explained, by fetofetal transfer from an undetected vanishing male twin or maternofetal transfer of male cells harbored by the mother. Estimates of the frequency of vanishing twins range from 3.7–100% of pregnancies81 however not all twins share connected placenta vasculature, especially at the early stages of development at which many twins disappear. Maternofetal transfer to the mother may also have occurred if the mother's mother had a history of blood transfusion, transplantation or previous pregnancy with a male fetus. It is difficult to estimate how frequently male cells in females could arise as a result of fetofetal or maternofetal transfer. Although one might expect such events to be rare, the incidence may be high enough to have biased estimates of the incidence of fetomaternal microchimerism in humans. While the possibility that the Y chromosome could also enter the mother via microchimerism as a consequence of previous blood transfusion or transplantation has been considered in most studies, the possibility that male cells detected in the mother may have arrived via fetofetal or maternofetal transfer to the mother in utero has not be systematically excluded. Conclusive proof of fetomaternal microchimerism in humans would require the use of other paternal markers that differentiate between the father of the fetus and the father of the mother. One scenario might be to investigate cases where the mother and the mother's father share a genetic mutation or polymorphism not carried by the father of the fetus. In such cases, evidence of genetic markers derived from the father of the fetus in the mother could provide more conclusive evidence of fetomaternal microchimerism in humans. If the genetic mutation or polymorphism caused disease the presence of fetal cells in the diseased tissue could also offer evidence of the potential of fetomaternal tissue repair.
In contrast, to the suggestion that fetal cells are retained for decades after nearly every human pregnancy,7,14 the retention of fetal cells in mice appears more sporadic and rarely persists for more than a few weeks post partum. The use of mice bearing unique genetic markers such as, the cytogenetic marker chromosome, T626,33 and more recently transgenic mice bearing genetic markers such as enhanced green fluorescent protein (EGPF)35,49,66 has conclusively demonstrated fetomaternal microchimerism. The number of mice in which fetal cells can be detected in maternal blood and the number of fetal cells in maternal blood declines towards the end of gestation, at least in syngenic and allogenic crosses.66 Beyond the first week postpartum, fetal cells are rarely detected in maternal blood;35,66 although they have been found in some mice at 21 days post partum following allogenic crosses and at 42 days post partum, but not 21 days post partum, following outbred crosses.66 Likewise, in maternal bone marrow, spleen, liver, heart, lung and kidney fetal cells do not appear to be retained by maternal mice beyond the first week post partum.35 Even within the first week post partum, the retention of fetal cells is sporadic and highly variable between individuals.35 Our own observations suggest that there might be greater retention of fetal cells within the brain as although fetal cell numbers are low, cells persist to 4 weeks post partum49 (see also Fig. 3). However, by 6–8 weeks post partum, the number of fetal cells has fallen below the limits of detection in blood and all organs studied, including uninjured brain66 (see also Fig. 3). Although the numbers of fetal cells present were very low, fetal cells did persist at eight weeks post partum in some of the lesioned maternal brains (Fig. 3). Together, these data suggest the possibility that, although fetal cells are cleared from the blood and some organs within a few weeks postpartum in mothers of syngenic and allogenic crosses, some fetal cells may remain harbored longer-term in certain niches. In contrast, fetal cells have been detected in the blood of some mice at 42 days post partum following outbred crosses.66 Additionally, there is limited evidence that in some, but not all mice, repeated pregnancies may lead to greater retention of fetal cells,35,49 which may suggest that in some mothers there is longer-term retention of fetal cells. However, the duration of fetal cell retention in those few mice in which fetal cells do persist has not been systematically investigated. The reasons for the large individual differences in the numbers of fetal cells retained and the duration of retention are not known.
During pregnancy the mother develops immune tolerance to the fetus but after pregnancy this suppression of the maternal immune response to the fetus is lifted.82 It is conceivable that, although fetomaternal cell traffic probably occurs in every pregnancy, persistence of microchimeric fetal cells after pregnancy depends upon the immunocompatibility between the mother and fetus. This might explain why fetomaternal microchimerism does not persist in all mothers. The greater preservation of fetal cells in the brain than the blood would be consistent with an immune rejection hypothesis, the brain being an immune privileged site.83 However, it is difficult to reconcile the hypothesis that immune rejection explains the great inter-individual variability and low rate of fetal cell persistence in syngenically crossed mice66 as there is less immune rejection on transplantation between syngenic mice. Although some differences between the mother and fetus may be an advantage as it has been noted that, despite reducing placental expression of major histocompatibility complex (MHC) genes, major histocompatibility complex expression is often reestablished in the most invasive trophoblast cells and may contribute to an immunoprotective effect on the fetus.84
In conclusion, although it has not been studied systematically and there are obvious methodological differences between the mouse and human studies, there appears to greater likelihood of long-term retention of microchimeric fetal cells in humans than in mice. This difference in the retention of fetal cells may be consistent with the hypothesis that fetomaternal microchimerism has developed as a mechanism by which the fetus ensures maternal fitness. As mice wean their offspring by 3–4 weeks postpartum, there would be no need for the fetal cells to continue to survive. In contrast, human mothers nurse their offspring for many months and thereafter continue to nurture their offspring for many decades so there may be an adaptive advantage to fetal cell persistence. Alternatively, if fetal cells have adverse effects on the mother, it may be that rodents have developed greater maternal resistance to fetal cell infiltration as they have far more offspring over a far shorter life span.
Intriguingly, there may in fact be greater retention of fetal cells in outbred mice than in syngenic or allogenic crosses.66 That humans, who are generally outbred, retain fetal cells may be further evidence against the immunocompatibility hypothesis for fetomaternal microchimeric persistence. It is hoped that future studies may investigate the determinants of fetal cell retention. The immunological hypothesis would predict that immunosuppression from late pregnancy and through the post-partum period would increase fetomaternal microchimerism. Another hypothesis might be that hormonal changes coinciding with the later stages of pregnancy and the post partum period lead to rejection of fetal cells. This hypothesis would predict greater fetomaternal microchimerism in mother who did not complete the normal hormonal sequela of delivery and peri- and post-partum hormonal changes. In humans, there is indeed evidence that spontaneous and induced abortions increase the frequency and level of male microchimerism,79,85 but this may equally be explained by trauma associated with abortion leading to greater fetomaternal exchange.
Distribution of Microchimeric Fetal CellS
The microchimeric fetal cells in the mother appear to be of multilineage potential. Y chromosome bearing cells have been identified in numerous tissues, including skin, liver, kidney and bone marrow, in healthy women and in women with autoimmune diseases86–92 and other none immune diseases such as hepatitis C93 and cervical cancer.94 There is now a large literature on fetomaternal microchimerism, especially in autoimmune disease, and overall there appears to be evidence of increased fetal cell presence in diseased tissues than healthy tissues.27,95 It is debatable whether microchimerism plays a role in triggering autoimmune disease,86–89,91 perhaps by stimulating graft-host disease or host-graft disease,96 or whether fetal cells home in on diseased tissue and contribute to tissue repair.27,96 In systematic lupus erythematosus, for example, it appears that microchimeric fetal cells are more likely to be found in severe cases than in mild cases97 suggesting that the fetal cells are not causing the disease but rather are targeting the diseased maternal tissue once the damage reaches a threshold level.27 Similarly, in an animal model of excitotoxic brain injury we found greater numbers of fetal cells in the injured brain region.49 Fetal cells may also persist longer at sites of injury than in uninjured tissue (Fig. 3). This suggests the possibility that fetal cells may target to specific tissues and contribute to tissue repair or function.
There are various manners in which fetal cells might come to target damaged tissue. Sometimes the mechanism by which the zygote-derived cells are sequestered in particular tissues may be mechanical as has been hypothesized for the entrapment of large trophoblast cells in the capillaries of the microvasculature of the lung.15 Likewise, targeting of injured tissues may simply be a mechanical process whereby tissue damage is associated with micro-damage to the blood vessels and cells of all types are more likely to leak out into the damaged tissue. Another hypothesis is that fetal cells invade all maternal tissues but only find a niche conducive to survival in damaged tissues. Alternatively, if this is a process that has evolved to allow the fetus to treat the mother to enhance fetal survival, the fetal cells may actively invade the damaged tissue by a physiological mechanism of adhesion and transmigration across the blood vessel walls followed by active migration through the tissue to sites of damage.
Recently, Khosrotehrani and colleagues98 have used in vivo bioluminescence imaging of fetal cells in which the paternal marker was VEGF receptor 2 promoter controlled luciferase gene expression to demonstrate that fetal cells contribute to neoangiogenesis. This in vivo bioimaging approach will be extremely valuable in determining the extent to which fetal cells invade damaged tissues. Tracking genetically modified fetal cells or the behaviour of fetal cells in genetically modified mothers it may be possible to address important questions about the mechanisms by which fetal cells engraft maternal tissues and home in on injured tissue.
Types of Fetal Cells Involved in Fetomaternal Microchimerism
The fetal cell type or types responsible for fetomaternal microchimerism are unknown. Candidates include all cell types in fetal blood and trophoblasts. However, considerable evidence points towards the conclusion that fetal stem or progenitor cells may also be involved. Subsequent pregnancies appear to trigger further proliferation and mobilization to maternal blood of fetal cells acquired during previous pregnancies.34 The very fact that fetal cells can be detected decades after pregnancy7,14,99 is strong evidence that these cells are replicating in the mother. Moreover, women with older sons have a greater number of male cells suggesting proliferation over time.93 Although fetal cells were not detected in all ex-breeder mice those mice that had fetal cells in the brain tended to have higher numbers than in mice that had only delivered one litter suggesting accumulation or proliferation of fetal cells.49 The numbers of fetal cells detected in the maternal brain also showed marked postnatal increase between the last day of gestation and four weeks post partum (Fig. 3). This evidence that fetal cells can proliferate in the mother is fairly persuasive, but the alternative possibility that the fetal cells engraft in one niche and then subsequently remobilize to another niche without increasing in number has not been excluded.
Fetal cells appear indistinguishable from maternal tissues years after pregnancy and can bear epithelial, leukocyte, hematopoietic, hepatocytic, renal or cardiomyocytic markers.27,95,100 That microchimeric fetal cells also appear to be able to differentiate to adopt cellular characteristics of various host organs suggests that they may be stem or progenitor cells. In injured mouse brain, we have found fetal cells expressing various morphologies, localization and immunocytochemically stained protein markers characteristic of various brain cell types including perivascular macrophages, neurons, astrocytes and oligodendrocytes.49 While the evidence for differentiation may appear persuasive, important alternative hypotheses have yet to be excluded. Notably there have yet to be clear-cut examples of functional differentiation of microchimeric fetal cells. For example, it would be important to show that apparent neuronal differentiation does not just involve location, morphology and expression of a few protein markers but instead that this differentiation leads to functional neuronal characteristics such as the capacity to fire action potentials and synaptic connectivity to repair damaged circuitry. Likewise in the case of apparent oligodendrocytic differentiation, morphology and protein expression should be accompanied by functional wrapping of axons, and recovery of motor function in demyelination models.
At present there is little evidence for or against fusion as a mechanism of the apparent differentiation in microchimeric fetal cells. While a binucleated fetal cell was observed juxtaposed to a blood vessel in the brain in a niche in which other fetal cells adopted a perivascular macrophage-like character,49 it is unclear whether this represents a fusion event, a cell division event, or a multinucleated cell type. Systematic and careful study of fusion events in fetomaternal microchimerism will be important in interpreting whether apparent differentiation of fetal cells is in fact the result of cell fusion. Typically cell fusion in iatrogenic microchimerism following transplantation has been studied by fluorescent in situ hybridization (FISH) for X and Y chromosome markers. The presence of multiple X chromosomes in the cells bearing Y chromosomes has been taken as evidence of fusion. However, the study of cell fusion by this method in fetomaternal microchimerism is complicated. Not only may the Y chromonsome not be a specific marker for fetal cells as discussed above, but the trophoblasts, one of the cell types which contribute to fetomaternal microchimerism, can be multinucleated and due to the mosaic nature of the placenta could naturally carry multiple X chromosomes together with the Y chromosome in cases of vanishing female twins or in the rodent model where most litters contain both male and female offspring. Other strategies will be required to investigate fusion in fetomaternal microchimerism. For example, combining labeling for paternal-specific and maternal specific markers (e.g., crossing male EGFP transgenic mice with DsRed transgenic mice). Alternatively, Cre/lox recombination might be used to detect cell fusion events101 but this approach would require in utero implantation of homozygous embryos, which may alter fetomaternal cell traffic.
If the multilineage differentiation capacity of microchimeric fetal cells does prove to be genuine and functional this suggests that the fetal cells responsible are stem cells. The type of stem cell or stem cells involved is controversial. There is some evidence implicating haematopoietic stem cells. For example, male cells that persist in maternal blood after pregnancy are CD34+/CD38+,7 behave like proliferative haematopoeitc progenitor cells in vitro culture,102 and in haematopoietic tissues, such as the lymph nodes and spleen, the majority of microchimeric male cells express CD45.95 In contrast, there is also evidence suggesting that fetal mesenchymal stem cells (fMSC) are involved. Fetal MSCs have been identified in maternal blood during pregnancy.28,103 Fisk and colleagues appear to favor the interpretation that these cells are fetal mesenchymal stem cells because, at least when found in the bone marrow, male cells in mothers were immunophenotypically mesenchymal.14 However, it has been pointed out that the extent of the multilineage differentiation of microchimeric male cells argues against a strictly mesenchymal lineage.104 Indeed, unless one accepts the still controversial concept of stem cell plasticity and transdifferentiation, neither haematopoietic nor mesenchymal stem cells could explain the full range of differentiation, for example into neural cell types,49 that has been reported. The diversity of cell types into which microchimeric fetal cells can apparently differentiate suggests that, if a single stem or progenitor cell type is involved, it is a very early stem cell type.27,95,100 Bianchi and colleagues have referred to these cells as pregnancy-associated progenitor cells (PAPC) and appear to favor the interpretation that they may be a relatively early stem cell type retaining multilineage potential.27,91,95,100 The alternative possibility that numerous cell types of different lineage enter the mother has not been excluded. Perhaps the involvement of a number of cell types including various types of early stem cells could better explain the diversity of differentiation reported.
Conclusions and Future Prospects
Fetal cells exhibit a remarkable ability to migrate across the placenta into the mother and to integrate with diverse maternal tissues and organs, apparently homing in particularly to sites of damage and disease.49,97 Much remains to be learned about the basic biology of fetomaternal microchimerism. The cell type or types involved have yet to be conclusively characterized. If various cell types are involved, it will be important to understand the time course of the migration of the various cell types and their persistence in the mother. Studies of the process of cellular adhesion and migration that allow the cells to cross the placental barriers, infiltrate tissues and organs, cross the BBB and migrate to sites of damage will be especially informative. Although long-term persistence of fetal cells may be less frequent in the mouse, the mouse appears to offer a useful model for investigating aspects of fetomaternal traffic during pregnancy.
In the longer-term, elucidation of the biology of fetomaternal microchimerism may have important implications for understanding autoimmunity and graft-host interactions. Moreover, knowledge of the cell types and molecular mechanisms that allow for the remarkable migratory and multilineage differentiation capacity of microchimeric fetal cells in the mother may improve strategies for cytotherapeutic repair. Harnessing the capabilities of microchimeric fetal cells may enhance the prospects for minimally invasive intravenous delivery of stem cells.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/abstract.php?id=4082
References
- 1.Adams KM, Nelson JL. Microchimerism: An investigative frontier in autoimmunity and transplantation. JAMA. 2004;291:1127–1131. doi: 10.1001/jama.291.9.1127. [DOI] [PubMed] [Google Scholar]
- 2.Reed W, Lee TH, Norris PJ, Utter GH, Busch MP. Transfusion-associated microchimerism: A new complication of blood transfusions in severely injured patients. Semin Hematol. 2007;44:24–31. doi: 10.1053/j.seminhematol.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Starzl TE. Chimerism and tolerance in transplantation. Proc Natl Acad Sci USA. 2004;101(Suppl 2):14607–14614. doi: 10.1073/pnas.0404829101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iverson GM, Bianchi DW, Cann HM, Herzenberg LA. Detection and isolation of fetal cells from maternal blood using the flourescence-activated cell sorter (FACS) Prenat Diagn. 1981;1:61–73. doi: 10.1002/pd.1970010111. [DOI] [PubMed] [Google Scholar]
- 5.Herzenberg LA, Bianchi DW, Schroder J, Cann HM, Iverson GM. Fetal cells in the blood of pregnant women: Detection and enrichment by fluorescence-activated cell sorting. Proc Natl Acad Sci USA. 1979;76:1453–1455. doi: 10.1073/pnas.76.3.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bianchi DW, Shuber AP, DeMaria MA, Fougner AC, Klinger KW. Fetal cells in maternal blood: Determination of purity and yield by quantitative polymerase chain reaction. Am J Obstet Gynecol. 1994;171:922–926. doi: 10.1016/s0002-9378(94)70059-1. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci USA. 1996;93:705–708. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maloney S, Smith A, Furst DE, Myerson D, Rupert K, Evans PC, Nelson JL. Microchimerism of maternal origin persists into adult life. J Clin Invest. 1999;104:41–47. doi: 10.1172/JCI6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srivatsa B, Srivatsa S, Johnson KL, Bianchi DW. Maternal cell microchimerism in newborn tissues. J Pediatr. 2003;142:31–35. doi: 10.1067/mpd.2003.mpd0327. [DOI] [PubMed] [Google Scholar]
- 10.Nelson JL, Gillespie KM, Lambert NC, Stevens AM, Loubiere LS, Rutledge JC, Leisenring WM, Erickson TD, Yan Z, Mullarkey ME, Boespflug ND, Bingley PJ, Gale EA. Maternal microchimerism in peripheral blood in type 1 diabetes and pancreatic islet beta cell microchimerism. Proc Natl Acad Sci USA. 2007;104:1637–1642. doi: 10.1073/pnas.0606169104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Dijk BA, Boomsma DI, de Man AJ. Blood group chimerism in human multiple births is not rare. Am J Med Genet. 1996;61:264–268. doi: 10.1002/(SICI)1096-8628(19960122)61:3<264::AID-AJMG11>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 12.Shalev SA, Shalev E, Pras E, Shneor Y, Gazit E, Yaron Y, Loewenthal R. Evidence for blood chimerism in dizygotic spontaneous twin pregnancy discordant for Down syndrome. Prenat Diagn. 2006;26:782–784. doi: 10.1002/pd.1503. [DOI] [PubMed] [Google Scholar]
- 13.Walker SP, Meagher S, White SM. Confined blood chimerism in monochorionic dizygous (MCDZ) twins. Prenat Diagn. 2007 doi: 10.1002/pd.1670. [DOI] [PubMed] [Google Scholar]
- 14.O'Donoghue K, Chan J, de la FJ, Kennea N, Sandison A, Anderson JR, Roberts IA, Fisk NM. Microchimerism in female bone marrow and bone decades after fetal mesenchymal stem-cell trafficking in pregnancy. Lancet. 2004;364:179–182. doi: 10.1016/S0140-6736(04)16631-2. [DOI] [PubMed] [Google Scholar]
- 15.Lapaire O, Holzgreve W, Oosterwijk JC, Brinkhaus R, Bianchi DW. Georg schmorl on trophoblasts in the maternal circulation. Placenta. 2007;28:1–5. doi: 10.1016/j.placenta.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Douglas GW, Thomas L, Carr M, Cullen NM, Morris R. Trophoblasts in circualting blood during pregnancy. Am J Obstet Gynecol. 1959;78:960–973. doi: 10.1016/s0002-9378(16)36649-2. [DOI] [PubMed] [Google Scholar]
- 17.Mueller UW, Hawes CS, Wright AE, Petropoulos A, DeBoni E, Firgaira FA, Morley AA, Turner DR, Jones WR. Isolation of fetal trophoblast cells from peripheral blood of pregnant women. Lancet. 1990;336:197–200. doi: 10.1016/0140-6736(90)91731-o. [DOI] [PubMed] [Google Scholar]
- 18.Chua S, Wilkins T, Sargent I, Redman C. Trophoblast deportation in pre-eclamptic pregnancy. Br J Obstet Gynaecol. 1991;98:973–979. doi: 10.1111/j.1471-0528.1991.tb15334.x. [DOI] [PubMed] [Google Scholar]
- 19.Hawes CS, Suskin HA, Petropoulos A, Latham SE, Mueller UW. A morphologic study of trophoblast isolated from peripheral blood of pregnant women. Am J Obstet Gynecol. 1994;170:1297–1300. doi: 10.1016/s0002-9378(94)70144-x. [DOI] [PubMed] [Google Scholar]
- 20.Hawes CS, Suskin HA, Kalionis B, Mueller UW, Casey G, Hall J, Rudzki Z. Detection of paternally inherited mutations for beta-thalassemia in trophoblast isolated from peripheral maternal blood. Ann N Y Acad Sci. 1994;731:181–185. doi: 10.1111/j.1749-6632.1994.tb55767.x. [DOI] [PubMed] [Google Scholar]
- 21.Alston RL. Demonstration of foetal cells in maternal blood. J Med Lab Technol. 1969;26:224–226. [PubMed] [Google Scholar]
- 22.Amo H, Kajino G. Demonstration of foetal cells by chromosome analysis of maternal blood. Nagoya J Med Sci. 1973;36:11–16. [PubMed] [Google Scholar]
- 23.Walknowska J, Conte FA, Grumbach MM. Practical and theoretical implications of fetal-maternal lymphocyte transfer. Lancet. 1969;1:1119–1122. doi: 10.1016/s0140-6736(69)91642-0. [DOI] [PubMed] [Google Scholar]
- 24.Bianchi DW, Flint AF, Pizzimenti MF, Knoll JH, Latt SA. Isolation of fetal DNA from nucleated erythrocytes in maternal blood. Proc Natl Acad Sci USA. 1990;87:3279–3283. doi: 10.1073/pnas.87.9.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganshirt D, Garritsen H, Miny P, Holzgreve W. Fetal cells in maternal circulation throughout gestation. Lancet. 1994;343:1038–1039. doi: 10.1016/s0140-6736(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 26.Gaillard MC, Ouvre E, Liegeois A, Lewin D. The concentration of fetal cells in maternal haematopoietic organs during pregnancy: An experimental study in mice. J Gynecol Obstet Biol Reprod (Paris) 1978;7:1043–1050. [PubMed] [Google Scholar]
- 27.Khosrotehrani K, Bianchi DW. Multi-lineage potential of fetal cells in maternal tissue: A legacy in reverse. J Cell Sci. 2005;118:1559–1563. doi: 10.1242/jcs.02332. [DOI] [PubMed] [Google Scholar]
- 28.O'Donoghue K, Choolani M, Chan J, de la FJ, Kumar S, Campagnoli C, Bennett PR, Roberts IA, Fisk NM. Identification of fetal mesenchymal stem cells in maternal blood: Implications for non-invasive prenatal diagnosis. Mol Hum Reprod. 2003;9:497–502. doi: 10.1093/molehr/gag063. [DOI] [PubMed] [Google Scholar]
- 29.Ariga H, Ohto H, Busch MP, Imamura S, Watson R, Reed W, Lee TH. Kinetics of fetal cellular and cell-free DNA in the maternal circulation during and after pregnancy: Implications for non-invasive prenatal diagnosis. Transfusion. 2001;41:1524–1530. doi: 10.1046/j.1537-2995.2001.41121524.x. [DOI] [PubMed] [Google Scholar]
- 30.Krabchi K, Gros-Louis F, Yan J, Bronsard M, Masse J, Forest JC, Drouin R. Quantification of all fetal nucleated cells in maternal blood between the 18th and 22nd weeks of pregnancy using molecular cytogenetic techniques. Clin Genet. 2001;60:145–150. doi: 10.1034/j.1399-0004.2001.600209.x. [DOI] [PubMed] [Google Scholar]
- 31.Jackson L. Fetal cells and DNA in maternal blood. Prenat Diagn. 2003;23:837–846. doi: 10.1002/pd.705. [DOI] [PubMed] [Google Scholar]
- 32.Sargent IL, Johansen M, Chua S, Redman CW. Clinical experience: Isolating trophoblasts from maternal blood. Ann N Y Acad Sci. 1994;731:154–161. doi: 10.1111/j.1749-6632.1994.tb55762.x. [DOI] [PubMed] [Google Scholar]
- 33.Liegeois A, Escourrou J, Ouvre E, Charreire J. Microchimerism: A stable state of low-ratio proliferation of allogeneic bone marrow. Transplant Proc. 1977;9:273–276. [PubMed] [Google Scholar]
- 34.Liegeois A, Gaillard MC, Ouvre E, Lewin D. Microchimerism in pregnant mice. Transplant Proc. 1981;13:1250–1252. [PubMed] [Google Scholar]
- 35.Khosrotehrani K, Johnson KL, Guegan S, Stroh H, Bianchi DW. Natural history of fetal cell microchimerism during and following murine pregnancy. J Reprod Immunol. 2005;66:1–12. doi: 10.1016/j.jri.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta. 2002;23:3–19. doi: 10.1053/plac.2001.0738. [DOI] [PubMed] [Google Scholar]
- 37.Brosens I, Robertson WB, Dixon HG. The physiological response of the vessels of the placental bed to normal pregnancy. J Pathol Bacteriol. 1967;93:569–579. doi: 10.1002/path.1700930218. [DOI] [PubMed] [Google Scholar]
- 38.Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, Cross JC. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol. 2002;250:358–373. doi: 10.1016/s0012-1606(02)90773-6. [DOI] [PubMed] [Google Scholar]
- 39.Carter AM. Animal models of human placentation - A review. Placenta. 2006 doi: 10.1016/j.placenta.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Monk JM, Leonard S, McBey BA, Croy BA. Induction of murine spiral artery modification by recombinant human interferon-gamma. Placenta. 2005;26:835–838. doi: 10.1016/j.placenta.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 42.Bianchi DW, Wilkins-Haug LE, Enders AC, Hay ED. Origin of extraembryonic mesoderm in experimental animals: Relevance to chorionic mosaicism in humans. Am J Med Genet. 1993;46:542–550. doi: 10.1002/ajmg.1320460517. [DOI] [PubMed] [Google Scholar]
- 43.Rossant J, Cross JC. Placental development: Lessons from mouse mutants. Nat Rev Genet. 2001;2:538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- 44.Devi B, Jennison RF, Langley FA. Significance of placental pathology in transplacental haemorrhage. J Clin Pathol. 1968;21:322–331. doi: 10.1136/jcp.21.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Batcup G, Tovey LA, Longster G. Fetomaternal blood group incompatibility studies in placental intervillous thrombosis. Placenta. 1983;4:449–453. (Spec No) [PubMed] [Google Scholar]
- 46.Schroder J. Transplacental passage of blood cells. J Med Genet. 1975;12:230–242. [PMC free article] [PubMed] [Google Scholar]
- 47.Jauniaux E, Hustin J. Histological examination of first trimester spontaneous abortions: The impact of materno-embryonic interface features. Histopathology. 1992;21:409–414. doi: 10.1111/j.1365-2559.1992.tb00424.x. [DOI] [PubMed] [Google Scholar]
- 48.Engelhardt B. Molecular mechanisms involved in T cell migration across the blood-brain barrier. J Neural Transm. 2006;113:477–485. doi: 10.1007/s00702-005-0409-y. [DOI] [PubMed] [Google Scholar]
- 49.Tan XW, Liao H, Sun L, Okabe M, Xiao ZC, Dawe GS. Fetal microchimerism in the maternal mouse brain: A novel population of fetal progenitor or stem cells able to cross the blood-brain barrier? Stem Cells. 2005;23:1443–1452. doi: 10.1634/stemcells.2004-0169. [DOI] [PubMed] [Google Scholar]
- 50.Greenwood J, Etienne-Manneville S, Adamson P, Couraud PO. Lymphocyte migration into the central nervous system: Implication of ICAM-1 signalling at the blood-brain barrier. Vascul Pharmacol. 2002;38:315–322. doi: 10.1016/s1537-1891(02)00199-4. [DOI] [PubMed] [Google Scholar]
- 51.Lyck R, Reiss Y, Gerwin N, Greenwood J, Adamson P, Engelhardt B. T-cell interaction with ICAM-1/ICAM-2 double-deficient brain endothelium in vitro: The cytoplasmic tail of endothelial ICAM-1 is necessary for transendothelial migration of T cells. Blood. 2003;102:3675–3683. doi: 10.1182/blood-2003-02-0358. [DOI] [PubMed] [Google Scholar]
- 52.Lyall F, Young A, Boswell F, Kingdom JC, Greer IA. Placental expression of vascular endothelial growth factor in placentae from pregnancies complicated by pre-eclampsia and intrauterine growth restriction does not support placental hypoxia at delivery. Placenta. 1997;18:269–276. doi: 10.1016/s0143-4004(97)80061-6. [DOI] [PubMed] [Google Scholar]
- 53.Dye JF, Jablenska R, Donnelly JL, Lawrence L, Leach L, Clark P, Firth JA. Phenotype of the endothelium in the human term placenta. Placenta. 2001;22:32–43. doi: 10.1053/plac.2000.0579. [DOI] [PubMed] [Google Scholar]
- 54.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Newman PJ. The biology of PECAM-1. J Clin Invest. 1997;99:3–8. doi: 10.1172/JCI119129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jackson DE. The unfolding tale of PECAM-1. FEBS Lett. 2003;540:7–14. doi: 10.1016/s0014-5793(03)00224-2. [DOI] [PubMed] [Google Scholar]
- 57.Yong KL, Watts M, Shaun TN, Sullivan A, Ings S, Linch DC. Transmigration of CD34+ cells across specialized and nonspecialized endothelium requires prior activation by growth factors and is mediated by PECAM-1 (CD31) Blood. 1998;91:1196–1205. [PubMed] [Google Scholar]
- 58.Piali L, Hammel P, Uherek C, Bachmann F, Gisler RH, Dunon D, Imhof BA. CD31/PECAM-1 is a ligand for alpha v beta 3 integrin involved in adhesion of leukocytes to endothelium. J Cell Biol. 1995;130:451–460. doi: 10.1083/jcb.130.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Labarrere CA, Faulk WP. Intercellular adhesion molecule-1 (ICAM-1) and HLA-DR antigens are expressed on endovascular cytotrophoblasts in abnormal pregnancies. Am J Reprod Immunol. 1995;33:47–53. doi: 10.1111/j.1600-0897.1995.tb01137.x. [DOI] [PubMed] [Google Scholar]
- 60.Xiao J, Garcia-Lloret M, Winkler-Lowen B, Miller R, Simpson K, Guilbert LJ. ICAM-1-mediated adhesion of peripheral blood monocytes to the maternal surface of placental syncytiotrophoblasts: Implications for placental villitis. Am J Pathol. 1997;150:1845–1860. [PMC free article] [PubMed] [Google Scholar]
- 61.Labarrere CA, Ortiz MA, Sosa MJ, Campana GL, Wernicke M, Baldridge LA, Terry C, DiCarlo HL. Syncytiotrophoblast intercellular adhesion molecule-1 expression in placental villitis of unknown cause. Am J Obstet Gynecol. 2005;193:483–488. doi: 10.1016/j.ajog.2004.12.090. [DOI] [PubMed] [Google Scholar]
- 62.Barragan A, Brossier F, Sibley LD. Transepithelial migration of Toxoplasma gondii involves an interaction of intercellular adhesion molecule 1 (ICAM-1) with the parasite adhesin MIC2. Cell Microbiol. 2005;7:561–568. doi: 10.1111/j.1462-5822.2005.00486.x. [DOI] [PubMed] [Google Scholar]
- 63.Rajashekhar G, Loganath A, Roy AC, Wong YC. Expression and secretion of the vascular cell adhesion molecule-1 in human placenta and its decrease in fetal growth restriction. J Soc Gynecol Investig. 2003;10:352–360. doi: 10.1016/s1071-5576(03)00121-7. [DOI] [PubMed] [Google Scholar]
- 64.Cartwright JE, Balarajah G. Trophoblast interactions with endothelial cells are increased by interleukin-1beta and tumour necrosis factor alpha and involve vascular cell adhesion molecule-1 and alpha4beta1. Exp Cell Res. 2005;304:328–336. doi: 10.1016/j.yexcr.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 65.Rajashekhar G, Loganath A, Roy AC, Chong SS, Wong YC. Hypoxia up-regulated angiogenin and down-regulated vascular cell adhesion molecule-1 expression and secretion in human placental trophoblasts. J Soc Gynecol Investig. 2005;12:310–319. doi: 10.1016/j.jsgi.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 66.Vernochet C, Caucheteux SM, Kanellopoulos-Langevin C. Bi-directional cell trafficking between mother and fetus in mouse placenta. Placenta. 2006 doi: 10.1016/j.placenta.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 67.Muntener M, Hsu YC. Development of trophoblast and placenta of the mouse: A reinvestigation with regard to the in vitro culture of mouse trophoblast and placenta. Acta Anat (Basel) 1977;98:241–252. [PubMed] [Google Scholar]
- 68.Thomas MR, Tutschek B, Frost A, Rodeck CH, Yazdani N, Craft I, Williamson R. The time of appearance and disappearance of fetal DNA from the maternal circulation. Prenat Diagn. 1995;15:641–646. doi: 10.1002/pd.1970150709. [DOI] [PubMed] [Google Scholar]
- 69.Pertl B, Bianchi DW. First trimester prenatal diagnosis: Fetal cells in the maternal circulation. Semin Perinatol. 1999;23:393–402. doi: 10.1016/s0146-0005(99)80005-6. [DOI] [PubMed] [Google Scholar]
- 70.Burton GJ, Jauniaux E, Watson AL. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: The Boyd collection revisited. Am J Obstet Gynecol. 1999;181:718–724. doi: 10.1016/s0002-9378(99)70518-1. [DOI] [PubMed] [Google Scholar]
- 71.Hustin J, Schaaps JP. Echographic and anatomic studies of the maternotrophoblastic border during the first trimester of pregnancy. Am J Obstet Gynecol. 1987;157:162–168. doi: 10.1016/s0002-9378(87)80371-x. [DOI] [PubMed] [Google Scholar]
- 72.Jauniaux E, Jurkovic D, Campbell S. In vivo investigation of the placental circulations by Doppler echography. Placenta. 1995;16:323–331. doi: 10.1016/0143-4004(95)90089-6. [DOI] [PubMed] [Google Scholar]
- 73.Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress: A possible factor in human early pregnancy failure. Am J Pathol. 2000;157:2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Avarez-Silva M, Belo-Diabangouaya P, Salaun J, Dieterlen-Lievre F. Mouse placenta is a major hematopoietic organ. Development. 2003;130:5437–5444. doi: 10.1242/dev.00755. [DOI] [PubMed] [Google Scholar]
- 75.Gekas C, eterlen-Lievre F, Orkin SH, Mikkola HK. The placenta is a niche for hematopoietic stem cells. Dev Cell. 2005;8:365–375. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 76.Ottersbach K, Dzierzak E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev Cell. 2005;8:377–387. doi: 10.1016/j.devcel.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 77.Guetta E, Gordon D, Simchen MJ, Goldman B, Barkai G. Hematopoietic progenitor cells as targets for non-invasive prenatal diagnosis: Detection of fetal CD34+ cells and assessment of post-delivery persistence in the maternal circulation. Blood Cells Mol Dis. 2003;30:13–21. doi: 10.1016/s1079-9796(03)00008-1. [DOI] [PubMed] [Google Scholar]
- 78.Lambert NC, Pang JM, Yan Z, Erickson TD, Stevens AM, Furst DE, Nelson JL. Male microchimerism in women with systemic sclerosis and healthy women who have never given birth to a son. Ann Rheum Dis. 2005;64:845–848. doi: 10.1136/ard.2004.029314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yan Z, Lambert NC, Guthrie KA, Porter AJ, Loubiere LS, Madeleine MM, Stevens AM, Hermes HM, Nelson JL. Male microchimerism in women without sons: Quantitative assessment and correlation with pregnancy history. Am J Med. 2005;118:899–906. doi: 10.1016/j.amjmed.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 80.Guettier C, Sebagh M, Buard J, Feneux D, Ortin-Serrano M, Gigou M, Tricottet V, Reynes M, Samuel D, Feray C. Male cell microchimerism in normal and diseased female livers from fetal life to adulthood. Hepatology. 2005;42:35–43. doi: 10.1002/hep.20761. [DOI] [PubMed] [Google Scholar]
- 81.Landy HJ, Keith LG. The vanishing twin: A review. Hum Reprod Update. 1998;4:177–183. doi: 10.1093/humupd/4.2.177. [DOI] [PubMed] [Google Scholar]
- 82.Veenstra van Nieuwenhoven AL, Heineman MJ, Faas MM. The immunology of successful pregnancy. Hum Reprod Update. 2003;9:347–357. doi: 10.1093/humupd/dmg026. [DOI] [PubMed] [Google Scholar]
- 83.Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: Hiding in plain sight. Immunol Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bainbridge DR. Evolution of mammalian pregnancy in the presence of the maternal immune system. Rev Reprod. 2000;5:67–74. doi: 10.1530/ror.0.0050067. [DOI] [PubMed] [Google Scholar]
- 85.Khosrotehrani K, Johnson KL, Lau J, Dupuy A, Cha DH, Bianchi DW. The influence of fetal loss on the presence of fetal cell microchimerism: A systematic review. Arthritis Rheum. 2003;48:3237–3241. doi: 10.1002/art.11324. [DOI] [PubMed] [Google Scholar]
- 86.Bianchi DW. Fetal cells in the mother: From genetic diagnosis to diseases associated with fetal cell microchimerism. Eur J Obstet Gynecol Reprod Biol. 2000;92:103–108. doi: 10.1016/s0301-2115(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 87.Bianchi DW. Fetomaternal cell trafficking: A new cause of disease? Am J Med Genet. 2000;91:22–28. doi: 10.1002/(sici)1096-8628(20000306)91:1<22::aid-ajmg4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 88.Nelson JL. Microchimerism: Incidental byproduct of pregnancy or active participant in human health? Trends Mol Med. 2002;8:109–113. doi: 10.1016/s1471-4914(01)02269-9. [DOI] [PubMed] [Google Scholar]
- 89.Nelson JL. Microchimerism and human autoimmune diseases. Lupus. 2002;11:651–654. doi: 10.1191/0961203302lu271oa. [DOI] [PubMed] [Google Scholar]
- 90.Nelson JL. Microchimerism in human health and disease. Autoimmunity. 2003;36:5–9. doi: 10.1080/0891693031000067304. [DOI] [PubMed] [Google Scholar]
- 91.Bianchi DW. Fetomaternal cell traffic, pregnancy-associated progenitor cells, and autoimmune disease. Best Pract Res Clin Obstet Gynaecol. 2004;18:959–975. doi: 10.1016/j.bpobgyn.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 92.Johnson KL, Bianchi DW. Fetal cells in maternal tissue following pregnancy: What are the consequences? Hum Reprod Update. 2004;10:497–502. doi: 10.1093/humupd/dmh040. [DOI] [PubMed] [Google Scholar]
- 93.Johnson KL, Samura O, Nelson JL, McDonnell M, Bianchi DW. Significant fetal cell microchimerism in a nontransfused woman with hepatitis C: Evidence of long-term survival and expansion. Hepatology. 2002;36:1295–1297. doi: 10.1053/jhep.2002.35622. [DOI] [PubMed] [Google Scholar]
- 94.Cha D, Khosrotehrani K, Kim Y, Stroh H, Bianchi DW, Johnson KL. Cervical cancer and microchimerism. Obstet Gynecol. 2003;102:774–781. doi: 10.1016/s0029-7844(03)00615-x. [DOI] [PubMed] [Google Scholar]
- 95.Khosrotehrani K, Johnson KL, Cha DH, Salomon RN, Bianchi DW. Transfer of fetal cells with multilineage potential to maternal tissue. JAMA. 2004;292:75–80. doi: 10.1001/jama.292.1.75. [DOI] [PubMed] [Google Scholar]
- 96.Kremer Hovinga I, Koopmans M, de HE, Bruijn JA, Bajema IM. Chimerism in systemic lupus erythematosus-three hypotheses. Rheumatology (Oxford) 2007;46:200–208. doi: 10.1093/rheumatology/kel379. [DOI] [PubMed] [Google Scholar]
- 97.Mosca M, Curcio M, Lapi S, Valentini G, D'Angelo S, Rizzo G, Bombardieri S. Correlations of Y chromosome microchimerism with disease activity in patients with SLE: Analysis of preliminary data. Ann Rheum Dis. 2003;62:651–654. doi: 10.1136/ard.62.7.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huu SN, Oster M, Uzan S, Chareyre F, Aractingi S, Khosrotehrani K. Maternal neoangiogenesis during pregnancy partly derives from fetal endothelial progenitor cells. Proc Natl Acad Sci USA. 2007;104:1871–1876. doi: 10.1073/pnas.0606490104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Evans PC, Lambert N, Maloney S, Furst DE, Moore JM, Nelson JL. Long-term fetal microchimerism in peripheral blood mononuclear cell subsets in healthy women and women with scleroderma. Blood. 1999;93:2033–2037. [PubMed] [Google Scholar]
- 100.Huu SN, Dubernard G, Aractingi S, Khosrotehrani K. Feto-maternal cell trafficking: A transfer of pregnancy associated progenitor cells. Stem Cell Rev. 2006;2:111–116. doi: 10.1007/s12015-006-0017-8. [DOI] [PubMed] [Google Scholar]
- 101.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 102.Osada H, Doi S, Fukushima T, Nakauchi H, Seki K, Sekiya S. Detection of fetal HPCs in maternal circulation after delivery. Transfusion. 2001;41:499–503. doi: 10.1046/j.1537-2995.2001.41040499.x. [DOI] [PubMed] [Google Scholar]
- 103.Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 104.Rossi G. Nature of stem cell involved in fetomaternal microchimerism. Lancet. 2004;364:1936. doi: 10.1016/S0140-6736(04)17469-2. [DOI] [PubMed] [Google Scholar]


