Abstract
The mouse model of pleurisy induced by carrageenan is characterized by a significant enhancement of cell migration due to neutrophils 4 h after pleurisy induction. Forty-eight hours after pleurisy induction, a significant increase in cell migration due to mononuclear cells occurs. Recently, studies in our laboratory have demonstrated that cyclosporine A (CsA) inhibits leukocyte migration in the pleural cavity and lungs in the mouse model of pleurisy induced by carrageenan. In the present work we evaluated whether CsA was able to downregulate CD11a/CD18 adhesion molecule in the lungs, as well as TNFα and IL-1β levels in the fluid leakage of the pleural cavity in this model. Our results showed that CsA significantly decreased CD11a/CD18 in the lungs, as well as TNFα and IL-1β levels in the fluid leakage of the pleural cavity 4 h and 48 h after pleurisy induction. It is our hypothesis that the inhibitory effect elicited by CsA upon these adhesion molecules may be also be attributed to the downregulation of TNFα and IL-1β cytokines.
Key words: cyclosporin A, CD11a/CD18 adhesion molecules, pleurisy, TNFα and IL-1β
Introduction
Cell adhesion is critical for the genesis and maintenance of tissue structure and function. The complex adhesion molecule CD11a/CD18 heterodimer are expressed in neutrophils,1 monocytes,2 macrophages and other cells.3 In fact, these adhesion molecules have an essential role in leukocyte recognition through the endothelium and extracellular matrix at the site of inflammation.4 Furthermore, adhesion molecules control the trafficking of leukocytes to the site of the inflammatory process by means of a firm membrane adhesion between leukocyte and endothelium.1,55 In this context, Wilson et al.6 and also Carlos and Harlan7 had demonstrated that CD11a/CD18 is an important adhesion molecule involved in the leukocyte chemotaxis during the inflammatory process. Further, Takeshita et al.8 had also shown that this heterodimer protein is the most important adhesion molecule involved in the leukocyte influx at the site of the inflammatory reaction.
Moreover, proinflammatory mediators such as cytokines (TNFα and IL-1β) are some of the many other mediators responsible for inducing adhesion molecule and leukocyte chemotaxis.9 These cytokines profoundly affect structural and functional cells, promoting cellular chemotaxis and other responses.10 Further, studies from Fröde et al.11 as well as from Cailhier et al.12 and Mazzon and Cuzzocrea13 had shown that these two cytokines (TNFα and IL-1β) are the prior mediators involved in the inflammatory response, and the leukocytes are known to be both the source and the target of these cytokines in the mouse model of pleurisy induced by carrageenan.
Many studies have been carried out to investigate new drugs with antiinflammatory properties by inhibiting adhesion molecules. One of the most promising antiinflammatory drugs is cyclosporine A (CsA), an immunosuppressive drug that inhibits the production of proinflammatory cytokines.14
Today, CsA is used in allografting, particularly in organ transplantation.15–17 Studies have addressed the antiinflammatory properties of CsA, especially in autoimmune diseases, such as rheumatoid arthritis,18 bronchial asthma,19 Crohn's disease20 and psoriasis.21
In the present study, following the investigation of the antiinflammatory effect of CsA,22,23 we evaluated whether CsA was able to downregulate CD11a/CD18 adhesion molecule in the lungs, as well as TNFα and IL-1β in the fluid leakage of the pleural cavity, using the mouse model of pleurisy induced by carrageenan.
Results
Effect of cyclosporin A on CD11a/CD18 adhesion molecule in the lungs.
At the studied doses (1 mg/kg and 2 mg/kg), CsA significantly inhibited CD11a in the lungs in the first (4 h) (p = 0.0017) and late (48 h) phases (p < 0.0001) of the inflammatory response induced by carrageenan in mice. At the same condition, this drug also decreased CD18 at 4 h (p < 0.0001) and 48 h (p = 0.0017) (Figs. 1 and 2, Table 1).
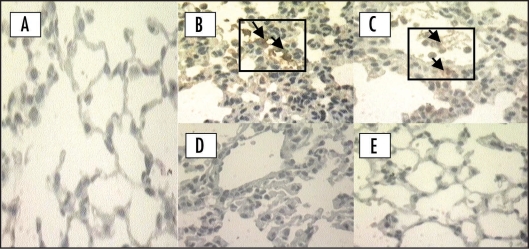
Figure 1.
Immunohistochemical location of CD11a/CD18 adhesion molecule in the lung in the early phase (4 h) of the inflammatory response induced by carrageenan. (A) Negative control: animal treated with sterile saline only; (B) Positive controls of CD11a and (C) CD18: animals treated with carrageenan only. Animals pretreated with cyclosporin A (1 mg/kg, i.p., 0.5 h) upon (D) CD11a and (E) CD18 adhesion molecule. Original magnification (×400). Each figure is representative of four experiments performed on different experimental days. Arrows indicate positive CD11a and CD18.
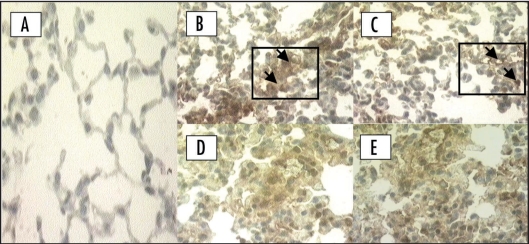
Figure 2.
Immunohistochemical location of CD11a/CD18 adhesion molecule in the lung in the late phase (48 h) of the inflammatory response induced by carrageenan. (A) Negative control: animal treated with sterile saline only; (B) Positive controls of CD11a and (C) CD18: animals treated with carrageenan only. Animals pretreated with cyclosporin A (2 mg/kg, i.p., 1 h) upon (D) CD11a and (E) CD18 adhesion molecule. Original magnification (×400). Each figure is representative of four experiments performed on different experimental days. Arrows indicate the positive CD11a and CD18.
Table 1.
Effect of cyclosporine A upon CD11a/CD18 expression in the lungs in the two phases (4 h and 48 h) of mouse pleurisy induced by carrageenan
| Adhesion molecules | ||||
| 4 h | 48 h | |||
| Groups | CD11a | CD18 | CD11a | CD18 |
| C | 1 | 2 | 4 | 4 |
| CsA (1 or 2 mg/kg) | 0 | 0 | 2 | 3 |
| p value | 0.0017 | <0.0001 | <0.0001 | 0.0017 |
Data are reported as scores on a scale of 0 to 3, with 0 = none, 1 = mild, 2 = moderate and 3 = severe, in accordance with Motohiro et al., (1996); Lossos et al., (2000). Control = C = animals treated only with carrageenan (1%), CsA = animals pretreated with cyclosporin A: 1 mg/kg (4 h) or 2 mg/kg (48 h). Five slides of a pool of lungs were analysed. p = Significance between C and CsA groups.
Effects of cyclosporin A on TNFα and IL-1β levels.
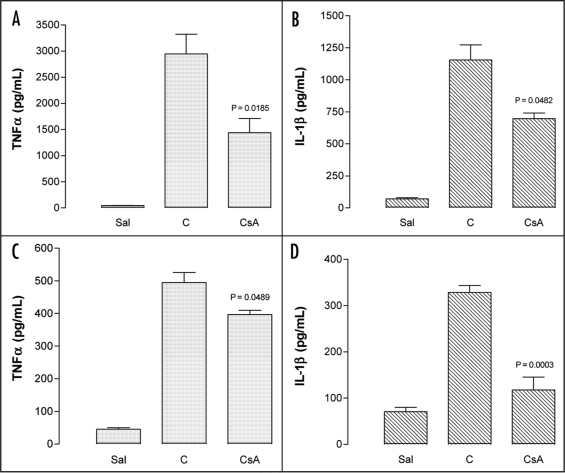
In the first (4 h) phase of inflammation in the pleural cavity induced by carrageenan, acute administration of CsA (1 mg/kg, i.p.) resulted in a significant reduction of TNFα levels (% of inhibition: 85.3 ± 10) (p = 0.0185) (Fig. 3A) and also the IL-1β levels (% of inhibition: 43.3 ± 16) (p = 0.0482) (Fig. 3B) in the fluid leakage of the pleural cavity. In the second phase of this inflammatory process (48 h later), Cyclosporin A (2 mg/kg, i.p.) also significantly inhibited TNFα (% of inhibition: 20 ± 2.6) (p = 0.0489) (Fig. 3C) and IL-1β levels (% of inhibition: 62.4 ± 5.5) (p = 0.0003) (Fig. 3D).
Figure 3.
Effects of cyclosporin A upon inflammation in the murine model of pleurisy induced by carrageenan. (A) TNFα and (B) IL-1β levels in the early (4 h) phase and (C) TNFα and (D) IL-1β levels in the late (48 h) phase. CsA: Effects of cyclosporin A (1 or 2 mg/kg, i.p.) administered 0.5 h or 1 h before carrageenan on the pleurisy induced in animals at 4 h or 48 h, respectively. Sal: Negative control: animals treated with sterile saline only; Cg: Positive control: animals treated with carrageenan only. Each column represents the mean of five animals and the vertical bars the SEM. p < 0.05 mean statistical differences between controls and experimental groups.
It is worth noting that the degree of IL-1β reduction did not differ between the analyzed periods of time. In contrast, TNFα reduction was more significant at 4 h than 48 h.
Discussion
Previous studies in our laboratory had demonstrated that cyclosporine A inhibits neutrophil and mononuclear influx into the pleural cavity. A similar inhibition of influx into the lungs was observed by Dalmarco et al.22,23 in the mouse model of pleurisy induced by carrageenan. In this present work, using the same experimental model, we have demonstrated that CsA significantly inhibits the heterodimer CD11a/CD18 in the lungs. Our results are in accordance with Takeshita et al.8 who has also demonstrated that CD11a/CD18 is the most important protein adhesion molecule involved in the leukocyte migration from blood and other tissue compartments to the pleural cavity and lungs in the mouse model of pleurisy induced by carrageenan.
We also demonstrated that CsA elicited a pronounced inhibitory effect upon proinflammatory cytokines such TNFα and IL-1β, which are potent mediators that trigger chemotaxis of the leukocytes in inflammatory processes.24,25 In this context, previous studies11–13 had also shown that these two cytokines are the prior mediators involved in the inflammatory response as well as the inflammation caused by carrageenan in the mouse model of pleurisy and the leukocytes are targets of TNFα and IL-1β.
In inflammation, leukocyte function is closely linked with cell adhesion and cytokine production that facilitate migration to the inflammatory site. Our findings are in agreement with those of other studies which have demonstrated that CsA inhibits inflammation through a variety of mechanisms including downregulation of CD11a/CD18 adhesion molecule. Charreau et al.26 have demonstrated that CsA inhibits neutrophil adhesion molecules (E-selectin and ICAM-1) in the inflammation induced by either LPS or TNFα in porcine endothelial cells. Likewise, CsA has also been shown to inhibit proinflammatory mediators, leukocyte migration and/or adhesion molecule in some other in-vivo and in-vitro experiments.27–30 In this context, Farivar et al.31 have demonstrated that this modulatory effect of CsA upon leukocyte migration and adhesion molecules occurs via inhibition of nuclear factor transcription (NFkappaB and EGR-1) in rat pulmonary artery endothelial cells. These findings are also in agreement with a great variety of clinical studies which have demonstrated a significant reduction of adhesion molecules either in the endothelial cells or in the leukocytes.32 However, the molecular basis of this effect needs further study.
In fact, CsA's effects upon cytokine production are well known. The main result of the present work was that acute pretreatment with this drug inhibited both TNFα and IL-1β. However, the degree of inhibition was not similar for both cytokines. In this bimodal model of inflammation, with different patterns of cell migration and consequently of proinflammatory mediator expression, the degrees of inhibition of IL-1β levels at both 4 h and 48 h were similar, but not for TNFα. Again, these differences highlight different patterns of the antiinflammatory effects of CsA.
In conclusion, the results of this study demonstrate that CsA can acutely decrease the leukocyte adhesion molecules CD11a/CD18, an effect that is linked to traffic of the cells to the inflammatory site and production of proinflammatory cytokines that are responsible for the intercellular communication and for controlling the inner environment of the cells. Its our hypothesis that the inhibitory effect elicited by CsA upon these adhesion molecules is also attributable to the downregulation of TNFα and IL-1β cytokines.
Material and Methods
Animals.
Swiss mice, weighing 18–25 g, were housed under standardized conditions (room at a constant temperature of 22 ± 2°C with alternating 12 h periods of light and darkness, humidity 50–60%) and were fed on a standard mouse diet with water ad libitum before use. This study was approved by the Committee for Ethics in Animal Research of our university (protocol number: 23080.001032/2001-74).
The following groups of animals were studied: (1) untreated and (2) pretreated with cyclosporin A prior to carrageenan-induced pleurisy (n = 5). In parallel, three animals that had received an injection of either sterile saline (NaCl, 0.9%) via intrapleural (i.pl.) route or cyclosporin A via intraperitoneal (i.p.) route were included in each experimental group.
Experimental protocol.
Pleurisy caused by carrageenan (0.1%, i.p.)33 exhibits a biphasic response (4 h and 48 h). Thus, both interval-points were chosen to analyse the CD11a/CD18 adhesion molecule in the lungs, as well as TNFα and IL-1β levels in the fluid leakage of the pleural cavity.
Doses of cyclosporin A were chosen as previously established.22,23 Briefly, the doses of 1 mg/kg and 2 mg/kg (1 h and 0.5 h before) of cyclosporine (i.p.) were effective in significantly inhibiting neutrophil and mononuclear influxes to the pleural cavity, 4 h and 48 h after pleurisy induction.
Immunohistochemical analysis.
At the established time-intervals (4 h and 48 h) following pleurisy induction, the lungs were removed, washed in phosphate buffered saline (PBS: NaCl 137 mM, KCl 2 mM and phosphate buffer 10 mM, pH 7.6) and placed in 2 ml of buffered formalin (10%) for 48 h. Immunostaining of lung sections was conducted using a Dako Envision System (Dako, Carpinteria, CA, USA) according to the manufacturer's instructions. In brief, endogenous peroxidase activity was blocked with distilled water and the slides were incubated for 1 h with rat antimouse primary antibody against CD11a or CD18 adhesion molecules (Sigma-Aldrich, St. Louis, Missouri, USA) diluted 1:50 in TRIS-HCL buffer (composition: TRIS 13.9 g; TRIS-HCL 60.6 g, NaCl 87.66 g, pH 7.6) at 36–37°C, in a humid chamber. After two washes in automation buffer (PBS) the sections were incubated with IgG/IgM secondary antibodies (Dako, Carpinteria, CA, USA) for 25 min at room temperature in a humid chamber. The slides were then incubated with peroxidase-conjugate (Dako, Carpinteria, CA, USA) for 25 min at room temperature in a humid chamber. Following another wash in automation buffer, sections were stained with the DAB chromogen (diaminobenzidine; Dako, Carpinteria, CA, USA). Counterstaining was performed with hematoxylin and sections were examined in a blinded fashion by a pathologist. At least four slides per group were analyzed.
Quantification of TNFα and IL-1β.
Samples of the pleural fluid leakage obtained from carrageenan-treated animals only and animals pretreated with CsA were collected and immediately prepared for the analysis of cytokine levels. In parallel, negative control specimens from animals injected with sterile saline alone were included. In this protocol, commercially available kits were used with monoclonal antibodies for each cytokine. The cytokine levels were measured by enzyme-linked immunosorbent assay (ELISA), using Bioscience Pharmigen, USA (for TNFα) and Immuno Biological Laboratories Co., Ltd., Japan (for IL-1β) kits according to the manufacturers' instructions. The ranges of the values detected by these assays were: TNFα (5–2000 pg/mL) and IL-1β (100–6400 pg/mL). The intra- and interassay coefficients of variation (CV) for TNFα and IL-1β were: intra CV: TNFα = 7.8 ± 0.9% and IL-1β = 6.2 ± 0.4%; inter CV: TNFα = 9.6 ± 2.1% and IL-1β = 5.1 ± 0.6% with sensitivities of TNFα = 5 pg/ml and IL-1β = 1.67 pg/ml. Using an ELISA plate reader (Organon Teknika, Roseland, NJ, USA), all cytokine concentrations were estimated by means of colorimetric measurement at 450 mn by interpolation from a standard curve.
Drugs and reagents.
The following drugs and reagents were used: cyclosporin A (Sandimmun, Novartis, Basel, Switzerland); lambda carrageenan (grade IV), monoclonal rat antimouse CD11a and CD18 antibodies (Sigma-Aldrich, St. Louis, Missouri, USA); hematoxylin, xylene and Entellan (Merck, Armstard, Germany); diaminobenzidine (DAB) (ACROS-Organics, New Jersey, USA); ethyl ether (Dinamica, São Paulo, Brazil); IgG/IgM secondary antibodies, streptavidin-biotin-peroxidase (Dako, Carpinteria, CA, USA), enzyme-linked immunosorbent assay (ELISA) for quantitative determination of mouse TNFα (BD—Biosciences Pharmingen, San Diego, CA, USA) and mouse IL-1β (IBL-Immuno Biological Laboratories Co., Ltd., Fujioka, Gunma, Japan). Other reagents used were of analytical grade and were obtained from different commercial sources.
Statistical analysis.
The TNFα and IL-1β levels were reported as mean ± SEM. Significant differences between groups were determined by analysis of variance (ANOVA) complemented with Dunnett's and/or Student's t tests. In experiments involving immunohistochemical analysis, the figures shown are representative of at least four experiments performed on different experimental days. Data from the immunohistochemical studies are reported as scores. The scores are on a scale of 0 to 3, with 0 = none, 1 = mild, 2 = moderate and 3 = severe.34,35 Statistical differences between these groups were determined using ANOVA (Kruskal-Wallis) and the Mann Whitney U-test. The differences were considered to be significant at p < 0.05.
Acknowledgements
This study was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil. The authors thank Cláudia Pinto Figueiredo for providing excellent technical support during the study and HEMOSC (Centro de Hemoterapia do Estado de Santa Catarina) for their kind donation of kits used in this study.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/7251
References
- 1.Bijuklic K, Sturn DH, Jennings P, Kountchev J, Pfaller W, Wiedermann CJ, et al. Mechanisms of neutrophil transmigration across renal proximal tubular HK-2 cells. Cell Physiol Biochem. 2006;17:233–244. doi: 10.1159/000094128. [DOI] [PubMed] [Google Scholar]
- 2.Kirchberger S, Vetr H, Majdic O, Stockinger H, Stöckl J. Engagement of ICAM-1 by major group rhinoviruses activates the LFA-1/ICAM-3 cell adhesion pathway in mononuclear phagocytes. Immunobiology. 2006;211:537–547. doi: 10.1016/j.imbio.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Nishibori M, Takahashi HK, Mori S. The regulation of ICAM-1 and LFA-1 interaction by autacoids and statins: a novel strategy for controlling inflammation and immune responses. J Pharmacol Sci. 2003;92:7–12. doi: 10.1254/jphs.92.7. [DOI] [PubMed] [Google Scholar]
- 4.Golias C, Tsoutsi E, Matziridis A, Makridis P, Batistatou A, Charalabopoulos K. Review. Leukocyte and endothelial cell adhesion molecules in inflammation focusing on inflammatory heart disease. In Vivo. 2007;21:757–769. [PubMed] [Google Scholar]
- 5.Schymeinsky J, Mócsai A, Walzog B. Neutrophil activation via beta(2) integrins (CD11/CD18): Molecular mechanisms and clinical implications. Thromb Haemost. 2007;98:262–273. [PubMed] [Google Scholar]
- 6.Wilson RW, Ballantyne CM, Smith CW, Montgomery C, Bradley A, O'Brien WE, et al. Gene targeting yields a CD18-mutant mouse for study of inflammation. J Immunol. 1993;151:1571–1578. [PubMed] [Google Scholar]
- 7.Carlos TM, Harlan JM. Leukocyte-Endothelial Adhesion Molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- 8.Takeshita K, Bacon KB, Gantner F. Critical Role of L-Selectin and Histamine H4 Receptor in Zymosan-Induced Neutrophil Recruitment from the Bone Marrow: Comparison with Carrageenan. J Pharmacol and Exp Ther. 2004;310:272–280. doi: 10.1124/jpet.103.063776. [DOI] [PubMed] [Google Scholar]
- 9.Grzelewska-Rzymowska I, Pietrzkowicz M. Role of tumor necrosis factor-alpha in allergic inflammation and airway hyperresponsiveness. Pol Merkur Lekarski. 2004;16:173–178. [PubMed] [Google Scholar]
- 10.Agarwal SK, Brenner MB. Role of adhesion molecules in synovial inflammation. Curr Opin Rheumatol. 2006;18:268–276. doi: 10.1097/01.bor.0000218948.42730.39. [DOI] [PubMed] [Google Scholar]
- 11.Fröde TS, Souza GE, Calixto JB. The modulatory role played by TNFalpha and IL-1beta in the inflammatory responses induced by carrageenan in the mouse model of pleurisy. Cytokine. 2001;13:162–168. doi: 10.1006/cyto.2000.0816. [DOI] [PubMed] [Google Scholar]
- 12.Cailhier JF, Sawatzky DA, Kipari T, Houlberg K, Walbaum D, Watson S, et al. Resident pleural macrophages are key orchestrators of neutrophil recruitment in pleural inflammation. Am J Respir Crit Care Med. 2005;173:540–547. doi: 10.1164/rccm.200504-538OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazzon E, Cuzzocrea S. Role of TNF-alpha in lung tight junction alteration in mouse model of acute lung inflammation. Resp Res. 2007;8:1–19. doi: 10.1186/1465-9921-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho ML, Ju JH, Kim KW, Moon YM, Lee SY, Min SY, et al. Cyclosporine A inhibits IL-15-induced IL-17 production in CD4+ T cells via downregulation of PI3K/Akt and NFkappaB. Immunol Lett. 2007;108:88–96. doi: 10.1016/j.imlet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Calne R. Cyclosporine as a milestone in immunosuppression. Transplant Proc. 2004;36:13–15. doi: 10.1016/j.transproceed.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 16.Tabbara KF. Pharmacologic strategies in the prevention and treatment of corneal transplant rejection. Int Ophthalmol. 2008;28:223–232.. doi: 10.1007/s10792-007-9100-7. [DOI] [PubMed] [Google Scholar]
- 17.Zijlstra GS, Rijkeboer M, Jan van Drooge D, Sutter M, Jiskoot W, van de Weert M, et al. Characterization of a cyclosporine solid dispersion for inhalation. AAPS J. 2007;9:190–199. doi: 10.1208/aapsj0902021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tlustochowicz M. Cyclosporine combined with another basic drug for the management of rheumatoid arthritis. Ann Acad Med Stetin. 2006;52:23–27. [PubMed] [Google Scholar]
- 19.Niven AS, Argyros G. Alternate treatments in asthma. Chest. 2003;123:1254–1265. doi: 10.1378/chest.123.4.1254. [DOI] [PubMed] [Google Scholar]
- 20.Sohi S, Cohen RD. Management of refractory ulcerative colitis. Curr Treat Options Gastroenterol. 2006;9:234–245. doi: 10.1007/s11938-006-0042-3. [DOI] [PubMed] [Google Scholar]
- 21.Thappa DM, Laxmisha C. Immunomodulators in the treatment of psoriasis. Indian J Dermatol Venereol Leprol. 2004;70:1–9. [PubMed] [Google Scholar]
- 22.Dalmarco EM, Fröde TS, Medeiros YS. Additional evidence of acute anti-inflammatory effects of cyclosporin A in a murine model of pleurisy. Transp Immunol. 2004;12:151–157. doi: 10.1016/j.trim.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Dalmarco EM, Fröde TS. In vivo effects of cyclosporin A on expression of adhesion molecules in tissues in mice. Front Med Biol Eng. 2007;9:111–123. [Google Scholar]
- 24.Chen Y, Xie QM, Yang QH, Chen JQ. Effect of inhaled cyclosporin A on antigen-induced airway inflammation in asthmatic rats. Yao Xue Xue Bao. 2004;39:486–490. [PubMed] [Google Scholar]
- 25.Lampinen M, Carlson M, Håkansson LD, Venge P. Cytokine-regulated accumulation of eosinophils in inflammatory disease. Allergy. 2004;59:793–805. doi: 10.1111/j.1398-9995.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- 26.Charreau B, Coupel S, Goret F, Pourcel C, Soulillou JP. Association of glucocorticoids and cyclosporin A or rapamycin prevents E-selectin and IL-8 expression during LPS- and TNFalpha-mediated endothelial cell activation. Transplantation. 2000;69:945–953. doi: 10.1097/00007890-200003150-00047. [DOI] [PubMed] [Google Scholar]
- 27.Rincón J, Parra G, Quiroz Y, Benatuil L, Rodríguez-Iturbe B. Cyclosporin A reduces expression of adhesion molecules in the kidney of rats with chronic serum sickness. Clin Exp Immunol. 2000;121:391–398. doi: 10.1046/j.1365-2249.2000.01251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magari K, Miyata S, Ohkubo Y, Mutoh S, Goto T. Calcineurin inhibitors exert rapid reduction of inflammatory pain in rat adjuvant-induced arthritis. Br J Pharmacol. 2003;139:927–934. doi: 10.1038/sj.bjp.0705310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabryel B, Labuzek K, Małecki A, Herman ZS. Immunophilin ligands decrease release of proinflammatory cytokines (IL-1beta, TNF-alpha and IL-2 in rat astrocyte cultures exposed to simulated ischemia in vitro. Pol J Pharmacol. 2004;56:129–136. [PubMed] [Google Scholar]
- 30.Soriano-Izquierdo A, Gironella M, Massaguer A, Salas A, Gil F, Piqué JM, Panés J. Effect of cyclosporin A on cell adhesion molecules and leukocyte-endothelial cell interactions in experimental colitis. Inflamm Bowel Dis. 2004;10:789–800. doi: 10.1097/00054725-200411000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Farivar AS, Mackinnon-Patterson BC, Barnes AD, McCourtie AS, Mulligan MS. Cyclosporine modulates the response to hypoxia-reoxygenation in pulmonary artery endothelial cells. Ann Thorac Surg. 2005;79:1010–1060. doi: 10.1016/j.athoracsur.2004.08.078. [DOI] [PubMed] [Google Scholar]
- 32.Umaña A, MPhil AG, Durán MM, Porras L. Lymphocyte subtypes and adhesion molecules in actinic prurigo: observations with cyclosporin A. Intern J Dermatol. 2002;41:139–145. doi: 10.1046/j.1365-4362.2002.01419.x. [DOI] [PubMed] [Google Scholar]
- 33.Saleh TS, Calixto JB, Medeiros YS. Anti-inflammatory effects of theophylline, cromolyn and salbutamol in a murine model of pleurisy. British Journal of Pharmacology. 1996;118:811–819. doi: 10.1111/j.1476-5381.1996.tb15472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Motohiro A, Furukawa K, Yasumoto K, Inokuchi K. Mechanisms nvolved in acute lung edema induced in dogs by oleic acid. Eur Surg Res. 1986;18:50–57. doi: 10.1159/000128505. [DOI] [PubMed] [Google Scholar]
- 35.Lossos IS, Izbicki G, Or R, Goldstein RH, Breuer R. The effects of suramin on bleomycininduced lung injury. J Life Sci. 2000;67:2873–2881. doi: 10.1016/s0024-3205(00)00865-1. [DOI] [PubMed] [Google Scholar]