Abstract
Directional cell migration is essential for almost all organisms during embryonic development, in adult life and contributes to pathological conditions. This is particularly critical during embryogenesis where it is essential that cells end up in their correct, precise locations in order to build a normal embryo. Many cells have solved this problem by following a gradient of a chemoattractant usually secreted by their target tissues. Our recent research has found an alternative, complimentary, mechanism where intracellular signals are able to generate cell polarity and directional migration in absence of any external chemoattactant. We used neural crest cells to study cell migration in vivo, by performing live imagining of the neural crest cell migrating during embryo development. We show that the Planar Cell Polarity (PCP) or non-canonical Wnt signaling pathway interacts with the proteoglycan syndecan-4 to control the direction in which cell protrusions are generated, and in consequence, the direction of migration. By analyzing the activity of the small GTPases using in vivo FRET imaging we showed that PCP signaling activates RhoA, while syndecan-4 inhibits Rac, both at the back of the neural crest cell. Here we discuss a model where these signals are integrated to generate directional migration in vivo.
Key words: directional migration, cell migration, syndecan-4, PCP, non-canonical Wnt, neural crest, RhoA, Rac
The ability of cells to move in a directed manner is a fundamental requirement for life. In multi-cellular organisms, this requirement begins in the embryo, where morphogenetic processes are dependent on the correct movement of large numbers of cells. In the adult too, cell migration plays a vital role in many systems including the immune system and wound healing. Cell migration defects can contribute to the pathology of many diseases including vascular diseases such as atherosclerosis, and chronic inflammatory diseases like asthma and multiple sclerosis. Likewise, metastasis in cancer is characterized by mis-regulation of the normal cell migration machinery and results in cells that are normally static becoming aggressively motile and invasive.
Cell migration requires cell polarization and the formation of protrusions at one end of the cell. Polarization results in a different molecular ensemble at the front of the cell compared to that at the back. Cell protrusion formation at the front of the cell requires reorganization of the actin and microtubule cytoskeleton to produce a protrusion either in the form of a broad sheet-like lamellipodium or spiky filopodium. Small GTPases are well known modulators of these processes (reviewed in ref. 1).
Several mechanism has been proposed as involved in directional migration during embryo development, such as chemotaxis (migration toward an soluble chemoattractant),2 haptotaxis (migration toward a substrate-bound chemoattractant),3 population pressure (migration from a region of high towards a region of low cell density)4 and contact inhibition of locomotion (change in the direction of migration as a consequence of cell-cell contact),5 being chemotaxis the most widely accepted and studied.
The correct orientation of the cell and its protrusion is the keystone of directional migration and, in the case of chemotaxis, it is supposed to be controlled by the action of external chemical cues (chemoattractants) that are produced by or near to the target tissue.6 One of the best examples for chemoattraction in vivo is the migration of the progenitor germ cells, which are attracted by the chemokine SDF-1.2 It has been shown in vitro and in vivo, that upon receiving a chemotactic signal, the cell becomes polarized in the direction of migration. Nevertheless, it is known that cells cultured in vitro can became polarized and exhibit directional migration in absence of extrinsic chemoattractants.7 Pankov et al. showed that persistent directional migration in vitro can be achieved solely by modulating the activity of the small GTPase, Rac: high levels of Rac promotes the formation of peripheral lamella during random migration, while slightly lower levels of Rac suppress peripheral lamella and favour the formation of a polarized cell with lamella just at the leading edge.7 Is it possible that a similar mechanism of directional migration could occur in vivo?
The migration of Neural Crest (NC) cells has been used as a model to study directional cell migration in vivo.8–10 The neural crest is an embryonic population of cells that are specified at the border between the neural plate and the epidermis.11 Upon induction neural crest cells undergo an epithelial to mesenchymal transition,12 detach from the neural tube and migrate following defined pathways that eventually allow them to colonize almost the entire embryo.13 Finally, after reaching their destination NC cells differentiate to form many different cell types including neurons, glia, cartilage, skeleton and pigment cells.14 The migration of the NC cells is critical for the proper differentiation of their derivatives and there are several human syndromes associated with failures in this process.
The migration of NC cells is a highly ordered process; individual NC cells migrate with high persistence towards the direction of their targets,8 but until now it was not known how this directionality is controlled. A number of molecules have been identified as key players in neural crest migration, such as Ephrins, Semaphorins, Slit/Robo, etc. (reviewed in ref. 13). However most of these molecules work as inhibitory signals, which are required to restrict the migration of NC cells from prohibited areas. Although chemoattraction has been one of the proposed mechanisms to explain this directional migration, no chemoattractant has thus far been found in the NC.
It has been known for many years that NC cells can migrate in vitro with a high directionality even in the absence of external signals.15 Therefore, our work has been focused on understanding how NC directionality is controlled. Recently, we have unveiled some of the molecules that control this directional migration in vitro. More importantly, we have been able to show that the same molecular machinery controls directional migration in vivo.9,10
One of the key factors that controls directional migration of NC cells is the Planar Cell Polarity (PCP) or non-canonical Wnt signaling pathway.9,10,16 PCP signaling was first described in Drosophila, where a number of mutations were identified that disrupt the formation of bristles and hairs on the adult cuticle.17 In the Drosophila wing, epithelial cells are highly polarized, with a single hair outgrowth forming at the distal end of each cell. Mutations in PCP genes cause loss in cell polarity in this tissue with hairs forming in a disorganized pattern.18 In vertebrates, PCP signaling also regulates cell polarity during a number of different developmental processes including neural tube closure, cochlear hair orientation and ciliogenesis.19
We have shown that the PCP pathway is essential for correct neural crest migration in Xenopus. Injection of dominant negative forms of the intracellular PCP component Dishevelled (Dsh), which inhibit the PCP pathway but not canonical Wnt signaling, block the migration of cranial neural crest cells in vivo.9 Recently this role has also been extended to zebrafish where directional migration of neural crest is severely disrupted in the PCP mutant trilobite (strabismus) and in embryos injected with a dominant negative form of Dsh or a morpholino against wnt5a,10 with no effect in neural crest cell motility.9,10 Two factors, pescadillo and syndecan-4 that have recently been proposed as modulators of the PCP signaling,20,21 are also required for NC migration.10,21 Taken together, these data point to an essential role for PCP signaling in neural crest migration.
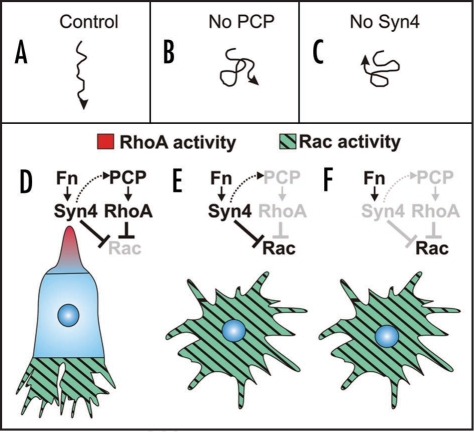
What is the cellular and molecular mechanism by which PCP signaling controls migration of NC cells? In order to investigate this question we analyzed the direction of neural crest migration and cell polarity in vitro and in vivo after interfering with two elements of the PCP signaling pathway: syndecan-4 and Dsh. One of the key finding of our work was that the inhibition of NC migration through syndecan-4 depletion does not affect the velocity of cell migration, but significantly reduces the directional migration of the cells in vivo (Fig. 1A and B). Consequently, when the orientation of cell protrusions was analyzed we found that syndecan-4 depletion does not affect the formation of cell protrusions, but the direction in which the cell protrusions are generated during migration. More precisely, normal cells extend their lamellipodia at the front of the cell (Fig. 1D), while cells where syndecan-4 is inhibited generate protrusion in all directions (Fig. 1E). A similar analysis was performed for embryos expressing a mutated form of Dsh that works as a dominant negative of PCP signaling and an equivalent effect on directional migration and the orientations of cell protrusions was observed (Fig. 1C and F).
Figure 1.
Directional migration of neural crest cells. (A and B) Example of track of a single cell migrating in vivo. (A) Control cell showing persistent directional migration. (B) Cell in which the PCP signaling has been inhibited, showing absence of directional migration. (C) Cell in which syndecan-4 has been inhibited, showing no persistent migration. (D–F) Analysis of cell polarity and model of directional migration. Fn: fibronectin; Syn4: syndecan-4. (D) Control cell. Activation of Fn/Syn4 and PCP/RhoA lead to inhibition of Rac at the back of the cell, with the consequence polarization and directional migration. (E) Inhibition of PCP signaling leads to absence of RhoA activity, and in consequence an increase of Rac activity at the back of the cell. It seems that the inhibition of Rac activity by Syn4 is not sufficient to keep low levels of Rac at the back of the cells. High levels of Rac at the back produce a loss in cell polarity and in directional migration. (F) Inhibition of Syn4 generates high levels of Rac activity by a double mechanism: absence of direct inhibition of Rac and absence of RhoA which is dependent on PCP signaling. High levels of Rac at the back produce a loss of cell polarity and directional migration.
As cell protrusions are known to be controlled by small GTPases and as PCP and syndecan-4 signaling regulates the activities of small GTPases,18,22 we analyzed the activity of cdc42, RhoA and Rac after interfering with Dsh and syndecan-4. We choose to perform FRET analysis of these molecules as it is a technique that allows the visualization of their localized activity. More interestingly we succeeded in performing FRET analysis in cells migrating in vivo for the first time. Our results show that syndecan-4 inhibits Rac activity, while Dsh signaling promotes RhoA activity. In addition, we show that RhoA inhibits Rac in neural crest cells.10 The regulation of Rac by syndecan-4 is similar to that seen in other cells types in vitro.23,24
The model that emerges from these results to explain directional migration of NC cells in vivo is as follows (Fig. 1D). After delamination NC cells come into contact with fibronectin in the extracellular matrix, which is known to provide the main substrate for neural crest cells during their migration.25,26 The interaction of fibronectin with syndecan-4 leads to two major changes in the cell: activation of PCP signaling and inhibition of Rac activity. The activated PCP signaling becomes localized at the back of the cell. From here, PCP contributes to the inhibition of Rac at the back of the cell, through the activation of RhoA. The coordinated activities of syndecan-4 and PCP signaling lead to polarised Rac activity across the cell, with Rac enriched at the leading edge, where it promotes the polymerization of actin and formation of lamellipodia, resulting in directional migration (Fig. 1D). Inhibition of PCP signaling produces high levels of Rac all over the cell as Rac, an inhibitor of RhoA in many cell types including neural crest cells, is absent (Fig. 1E). This generates cell protrusions in all directions with the consequent loss of cell polarity. If syndecan-4 is absent, the levels of Rac activity are also high all over the cell as the inhibition of Rac by syndecan-4 is absent (Fig. 1F), which also leads to a loss of cell polarity.
Although detailed study of the localized activity of small GTPases has not been performed for other migratory cells in vivo, it is likely that the machinery will be similar to the one described here for NC cells. For example, it is well established in Xenopus, zebrafish and chick embryos that the migration of mesodermal cells during gastrulation requires PCP signaling.27–29 It has also been shown that gastrulation in Xenopus20 and in zebrafish (unpublished observations) requires the activity of syndecan-4. Thus, it is expected that cell polarity established during the migration of mesodermal cells will be dependent on small GTPases controlled by non-canonical Wnt signaling and syndecan-4.
This novel integrated view of PCP, syndecan-4 and small GTPase activity during directional cell migration in vivo is an important advance in our knowledge of cell migration. Nevertheless, how the PCP signaling becomes activated only at the back of the cell, is a key question that needs to be answered. Future studies will be necessary to solve this and other crucial problems.
Acknowledgements
The work in R.M. laboratory is supported by grants from MRC and BBSRC. H.M. and C.C.-F. are MRC and Boehringer Ingelheim Fonds Ph.D. scholarship holders respectively.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/6747
References
- 1.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 2.Raz E. Germ cells: sex and repression in mice. Curr Biol. 2005;15:600–603. doi: 10.1016/j.cub.2005.07.043. [DOI] [PubMed] [Google Scholar]
- 3.Cattaruzza S, Perris R. Proteoglycan control of cell movement during wound healing and cancer spreading. Matrix Biology. 2005;24:400–417. doi: 10.1016/j.matbio.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Barlow AJ, Wallace AS, Thapar N, Burns AJ. Critical numbers of neural crest cells are required in the pathways from the neural tube to the foregut to ensure complete enteric nervous system formation. Development. 2008;135:1681–1691. doi: 10.1242/dev.017418. [DOI] [PubMed] [Google Scholar]
- 5.Erickson CA. Control of neural crest cell dispersion in the trunk of the avian embryo. Dev Biol. 1985;111:138–157. doi: 10.1016/0012-1606(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 6.Van Haastert PJ, Devreotes PN. Chemotaxis: signalling the way forward. Nat Rev Mol Cell Biol. 2004;5:626–634. doi: 10.1038/nrm1435. [DOI] [PubMed] [Google Scholar]
- 7.Pankov R, Endo Y, Even-Ram S, Araki M, Clark K, Cukierman E, Matsumoto K, Yamada KM. A Rac switch regulates random versus directionally persistent cell migration. J Cell Biol. 2005;170:793–802. doi: 10.1083/jcb.200503152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teddy JM, Kulesa PM. In vivo evidence for short- and long-range cell communication in cranial neural crest cells. Development. 2004;131:6141–6151. doi: 10.1242/dev.01534. [DOI] [PubMed] [Google Scholar]
- 9.De Calisto J, Araya C, Marchant L, Riaz CF, Mayor R. Essential role of non-canonical Wnt signalling in neural crest migration. Development. 2005;132:2587–2597. doi: 10.1242/dev.01857. [DOI] [PubMed] [Google Scholar]
- 10.Matthews HK, Marchant L, Carmona-Fontaine C, Kuriyama S, Larrain J, Holt MR, et al. Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development. 2008;135:1771–1780. doi: 10.1242/dev.017350. [DOI] [PubMed] [Google Scholar]
- 11.Steventon B, Carmona-Fontaine C, Mayor R. Genetic network during neural crest induction: from cell specification to cell survival. Semin Cell Dev Biol. 2005;16:647–654. doi: 10.1016/j.semcdb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 13.Kuriyama S, Mayor R. Molecular analysis of neural crest migration. Philos Trans R Soc Lond, Ser B: Biol Sci. 2008;363:1349–1362. doi: 10.1098/rstb.2007.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Douarin N, Kalcheim C. The Neural Crest. Cambridge University Press; 1999. [Google Scholar]
- 15.Davis EM, Trinkaus JP. Significance of cell-to cell contacts for the directional movement of neural crest cells within a hydrated collagen lattice. J Embryol Exp Morphol. 1981;63:29–51. [PubMed] [Google Scholar]
- 16.Garriock RJ, Krieg PA. Wnt11-R signaling regulates a calcium sensitive EMT event essential for dorsal fin development of Xenopus. Dev Biol. 2007;304:127–140. doi: 10.1016/j.ydbio.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gubb D, Garcia-Bellido A. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J Embryol Exp Morphol. 1982;68:37–57. [PubMed] [Google Scholar]
- 18.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- 20.Munoz R, Moreno M, Oliva C, Orbenes C, Larrain J. Syndecan-4 regulates non-canonical Wnt signalling and is essential for convergent and extension movements in Xenopus embryos. Nat Cell Biol. 2006;8:492–500. doi: 10.1038/ncb1399. [DOI] [PubMed] [Google Scholar]
- 21.Gessert S, Maurus D, Rossner A, Kuhl M. Pescadillo is required for Xenopus laevis eye development and neural crest migration. Dev Biol. 2007;310:99–112. doi: 10.1016/j.ydbio.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 22.Couchman JR. Syndecans: proteoglycan regulators of cell-surface microdomains? Nat Rev Mol Cell Biol. 2003;4:926–937. doi: 10.1038/nrm1257. [DOI] [PubMed] [Google Scholar]
- 23.Bass MD, Roach KA, Morgan MR, Mostafavi-Pour Z, Schoen T, Muramatsu T, et al. Syndecan-4-dependent Rac1 regulation determines directional migration in response to the extracellular matrix. J Cell Biol. 2007;177:527–538. doi: 10.1083/jcb.200610076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saoncella S, Calautti E, Neveu W, Goetinck PF. Syndecan-4 regulates ATF-2 transcriptional activity in a Rac1-dependent manner. J Biol Chem. 2004;279:47172–47176. doi: 10.1074/jbc.C400299200. [DOI] [PubMed] [Google Scholar]
- 25.Alfandari D, Cousin H, Gaultier A, Hoffstrom BG, DeSimone DW. Integrin alpha5beta1 supports the migration of Xenopus cranial neural crest on fibronectin. Dev Biol. 2003;260:449–464. doi: 10.1016/s0012-1606(03)00277-x. [DOI] [PubMed] [Google Scholar]
- 26.Newgreen D, Thiery JP. Fibronectin in early avian embryos: synthesis and distribution along the migration pathways of neural crest cells. Cell Tissue Res. 1980;211:269–291. doi: 10.1007/BF00236449. [DOI] [PubMed] [Google Scholar]
- 27.Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–4436. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- 28.Montero JA, Heisenberg CP. Gastrulation dynamics: cells move into focus. Trends Cell Biol. 2004;14:620–627. doi: 10.1016/j.tcb.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Hardy KM, Garriock RJ, Yatskievych TA, D'Agostino SL, Antin PB, Krieg PA. Non-canonical Wnt signaling through Wnt5a/b and a novel Wnt11 gene, Wnt11b, regulates cell migration during avian gastrulation. Dev Biol. 2008;320:391–401. doi: 10.1016/j.ydbio.2008.05.546. [DOI] [PMC free article] [PubMed] [Google Scholar]