Abstract
Glycosylation is one of the most abundant posttranslational modification reactions, and nearly half of all known proteins in eukaryotes are glycosylated. In fact, changes in oligosaccharide structure (glycan) are associated with many physiological and pathological events, including cell adhesion, migration, cell growth, cell differentiation and tumor invasion. Glycosylation reactions are catalyzed by the action of glycosyltransferases, which add sugar chains to various complex carbohydrates such as glycoproteins, glycolipids and proteoglycans. Functional glycomics, which uses sugar remodeling by glycosyltransferases, is a promising tool for the characterization of glycan functions. Here, we will focus on the positive and negative regulation of biological functions of integrins by the remodeling of N-glycans with N-acetylglucosaminyltransferase III (GnT-III) and N-acetylglucosaminyltransferase V (GnT-V), which catalyze branched N-glycan formations, bisecting GlcNAc and β1,6 GlcNAc, respectively. Typically, integrins are modified by GnT-III, which inhibits cell migration and cancer metastasis. In contrast, integrins modified by GnT-V promote cell migration and cancer invasion.
Key words: integrin, E-cadherin, GnT-III, GnT-V, N-glycosylation, glycosyltransferase
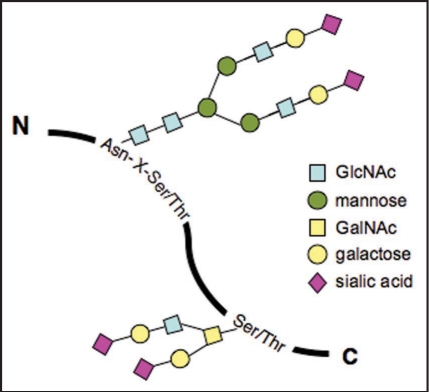
Protein glycosylation encompasses N-glycans, O-glycans and Glycosaminoglycans. N-glycans are linked to asparagine residues of proteins, which is a specific subset residing in the Asn-X-Ser/Thr motif, whereas O-glycans are attached to a subset of serines and threonines (Fig. 1).1 An increasing body of evidence indicates that glycans in glycoproteins are involved in the regulation of cellular functions including cell-cell communication and signal transduction.2,3 In fact, most receptors on the cell surface are N-glycosylated—integrins and epithelial growth factor receptors; and transforming growth factor β receptors. Here, we focus mainly on the modification of N-glycans of integrin α3β1 and α5β1 to address the important roles of N-glycans in cell adhesion and migration.
Figure 1.
Two major types of protein glycosylation. N-glycans are covalently linked to asparagine (Asn) residue of proteins, specifically the Asn-X-Ser/Thr motif. In contrast, O-glycans are attached to a subset of glycosidically linked hydroxyl groups of the amino acids serine (Ser) and threonine (Thr).
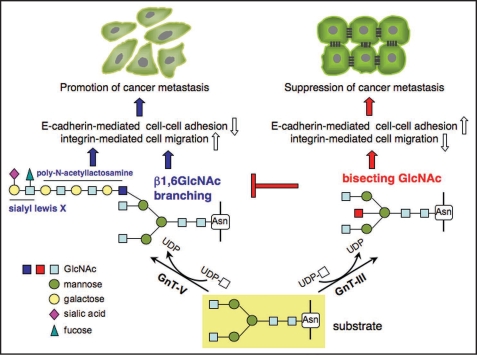
Previous studies indicate that the presence of the appropriate oligosaccharide can modulate integrin activation. When human fibroblasts were cultured in the presence of l-deoxymannojirimycin, an inhibitor of α-mannosidase II, which prevents N-linked oligosaccharide processing, immature α5β1 integrin appeared at the cell surface, and fibronectin (FN)-dependent adhesion was greatly reduced.4 In addition, the treatment of purified integrin α5β1 with N-glycosidase F, which cleaves between the innermost GlcNAc and asparagine residues of N-glycans from N-linked glycoproteins, resulted in the blockage of α5β1 binding to FN and the inherent association of both subunits,5 suggesting that N-glycosylation is essential for functional integrin α5β1. The production of glycoprotein glycans is catalyzed by various glycosyltransferases. N-Acetylglucosaminyltransferase III (GnT-III) transfers N-acetylglucosamine (GlcNAc) from UDP-GlcNAc to a β1, 4 mannose in N-glycans to form a “bisecting” GlcNAc linkage, as shown in Figure 2. Bisecting GlcNAc linkage is found in various hybrid and complex N-glycans. GnT-III is generally regarded as a key glycosyltransferase in N-glycan biosynthetic pathways. Introduction of a bisecting GlcNAc suppresses further processing and elongation of N-glycans catalyzed by N-acetylglucosaminyltransferase V (GnT-V), which is strongly associated with cancer metastasis, since GnT-V cannot utilize the bisected oligosaccharide as a substrate.6–8 It has also been reported that GnT-V activity and β1, 6 branched N-glycan levels are increased in highly metastatic tumor cell lines.9,10 When NIH3T3 cells were transformed with the oncogenic Ras gene, cell spreading on FN was greatly enhanced due to an increase in β1, 6 GlcNAc branched tri- and tetra-antennary oligosaccharides in α5β1 integrins.9 Similarly, the characterization of N-glycans of integrin α3β1 from non-metastatic and metastatic human melanoma cell lines showed that β1, 6 GlcNAc branched structures were expressed at high levels in metastatic cells compared with non-metastatic cells.10 Cancer metastasis was consistently, and significantly, suppressed in GnT-V knockout mice.11
Figure 2.
Glycosylation reactions catalyzed by the action of glycosyltransferase GnT-III and GnT-V. The remodeled N-glycans regulate cell adhesion and migration. Enhanced expression of GnT-V in epithelial cells results in a loss of cell-cell adhesion, increasing integrin-mediated cell migration. In contrast, overexpression of GnT-III strengthens cell-cell interaction and downregulates integrin-mediated cell migration, which may contribute to the suppression of cancer metastasis. The β1,6GlcNAc branching is preferentially modified by polylactosamine and other sugar motifs such as sialyl Lewis X, which also contribute to promotion of cancer metastasis. It is worth mentioning that GnT-III could be proposed as an antagonistic of GnT-V, since GnT-V cannot utilize the bisected oligosaccharide as a substrate.
To explore the possible mechanisms involved in increased β1, six branched N-glycans on cancer cells, Guo et al. found that cell migration toward FN and invasion through the matrigel were both substantially stimulated in cells in which the expression of GnT-V was induced.12 Increased branched sugar chains inhibited the clustering of integrin α5β1 and the organization of F-actin into extended microfilaments in cells plated on FN-coated plates, which supports the hypothesis that the degree of adhesion of cells to their extracellular matrix (ECM) substrate is a critical factor in regulating the rate of cell migration, i.e., migration is maximal under conditions of intermediate levels of cell adhesion.13 Conversely, GnT-V null mouse embryonic fibroblasts (MEF) displayed enhanced cell adhesion to, and spreading on, FN-coated plates with the concomitant inhibition of cell migration. The restoration of GnT-V cDNA in the null MEF reversed these abnormal characteristics, indicating the direct involvement of N-glycosylation events in these phenotypic changes.
In contrast to GnT-V, the overexpression of GnT-III resulted in an inhibition of α5β1 integrin-mediatedcell spreading and migration, and the phosphorylation of the focal adhesion kinase.14 The affinity of the binding of integrin α5β1 to FN was significantly reduced as a result of the introduction of a bisecting GlcNAc to the α5 subunit. In addition, overexpression of GnT-III in highly metastatic melanoma cells reduced β1, six branching in cell-surface N-glycans and increased bisected N-glycans.15 Therefore, GnT-III has been proposed as an antagonistic of GnT-V, thereby contributing to the suppression of cancer metastasis. In fact, the opposing effects of GnT-III and GnT-V have been observed for the same target protein, integrin α3β1.16 GnT-V stimulates α3β1 integrin-mediated cell migration, while overexpression of GnT-III inhibits GnT-V-induced cell migration. The modification of the α3 subunit by GnT-III supersedes modification by GnT-V. As a result, GnT-III inhibits GnT-V-induced cell migration. These results strongly suggest that remodeling of glycosyltransferase-modified N-glycan structures either positively or negatively modulates cell adhesion and migration.
In addition, sialylation on the non-reducing terminus of N-glycans of α5β1 integrin plays an important role in cell adhesion. The increased sialylation of the β1 integrin subunit was correlated with a decreased adhesiveness and metastatic potential.17–19 On the other hand, the enzymatic removal of α2, eight-linked oligosialic acids from the α5 integrin subunit inhibited cell adhesion to FN,20 supporting the observation that the N-glycans of α and β integrin subunits play distinct roles in cell-ECM interactions.21 Collectively, these findings suggest that the interaction of integrin α5β1 with FN is dependent on N-glycosylation and the processing status of N-glycans.
Although alteration of the oligosaccharide portion on integrin α5β1 could affect cis- and trans-interactions caused by GnT-III, ST6GalI and GnT-V, as described above, the molecular mechanism remains unclear. Considering integrin α5β1 contains 26 potential N-linked glycosylation sites (14 in the α subunit and 12 in the β subunit), the determination of those crucial N-glycosylation sites for its biological function is, therefore, quite important for an understanding of the underlying mechanism. We sequentially mutated either one or a combination of asparagine residues in the putative N-glycosylation sites of glutamine residues, and found that N-glycosylation on the β-propeller domain of the α5 subunit (in particular sites number 3–5) is essential for its hetero-dimer formation and its biological functions such as cell spreading and cell migration, as well as for the proper folding of the α5 subunit.22 On the other hand, N-glycans on β1 integrin also play important roles in the regulation of its biological functions23,24 (and our unpublished data). Very recently, we also found that GnT-III specifically modifies one of the important glycosylation sites, which results in functional regulation (unpublished data). We postulate that these important sites may participate in supramolecular complex formation on the cell surface, which controls intracellular signal transduction.
It also is worth noting that N-glycans regulate cell-ECM association as well as cell-cell adhesion. Overexpression of GnT-III slowed E-cadherin turnover, resulting in increased E-cadherin expression on the surface of B16 melanoma cells.25 E-cadherin engagement at cell-cell contacts is known to suppress cell migration, and that effect has been best described in the context of tumorigenesis.26 Conversely, the disruption of E-cadherin-mediated cell adhesion appears to be a central event in the transition from non-invasive to invasive carcinomas. Interestingly, we recently found that E-cadherin-mediated cell-cell interaction upregulated GnT-III expression,27,28 suggesting that regulation of GnT-III and E-cadherin expression may exist as a positive feedback loop. Taken together, the overexpression of GnT-III inhibits cell migration by at least two mechanisms: an enhancement in cell-cell adhesion and a downregulation of cell-ECM adhesion (Fig. 2).
Indeed, glycosylation defects in humans and their links to disease have shown that the mammalian glycome contains a significant amount of biological information.29 The mammalian glycome repertoire is estimated to be between hundreds and thousands of glycan structures and could be larger than its proteome counterpart. Nevertheless, characterization of the biological functions of each glycan could one day make a significant contribution to the diagnosis and treatment of disease.
Acknowledgements
These works were partly supported by Core Research for Evolutional Science and Technology (CREST), the Japan Science and Technology Agency (JST) and the “Academic Frontier” Project for Private Universities from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the core to core program (JSPS). The authors are deeply indebted to the outstanding related papers which have not been cited in the present article due to limited space.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/6748
References
- 1.Schachter H. The joys of HexNAc. The synthesis and function of N- and O-glycan branches. Glycoconj J. 2000;17:465–483. doi: 10.1023/a:1011010206774. [DOI] [PubMed] [Google Scholar]
- 2.Saxon E, Bertozzi CR. Chemical and biological strategies for engineering cell surface glycosylation. Annu Rev Cell Dev Biol. 2001;17:1–23. doi: 10.1146/annurev.cellbio.17.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Taniguchi N, Miyoshi E, Gu J, Honke K, Matsumoto A. Decoding sugar functions by identifying target glycoproteins. Curr Opin Struct Biol. 2006;16:561–566. doi: 10.1016/j.sbi.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Akiyama SK, Yamada SS, Yamada KM. Analysis of the role of glycosylation of the human fibronectin receptor. J Biol Chem. 1989;264:18011–18018. [PubMed] [Google Scholar]
- 5.Zheng M, Fang H, Hakomori S. Functional role of N-glycosylation in alpha5beta1 integrin receptor. De-N-glycosylation induces dissociation or altered association of alpha5 and beta1 subunits and concomitant loss of fibronectin binding activity. J Biol Chem. 1994;269:12325–12331. [PubMed] [Google Scholar]
- 6.Gu J, Nishikawa A, Tsuruoka N, Ohno M, Yamaguchi N, Kangawa K, et al. Purification and characterization of UDP-N-acetylglucosamine: alpha-6-D-mannoside beta1-6N-acetyl-glucosaminyltransferase (N-acetylglucosaminyltransferase V) from a human lung cancer cell line. J Biochem (Tokyo) 1993;113:614–619. doi: 10.1093/oxfordjournals.jbchem.a124091. [DOI] [PubMed] [Google Scholar]
- 7.Schachter H. Biosynthetic controls that determine the branching and microheterogeneity of protein-bound oligosaccharides. Adv Exp Med Biol. 1986;205:53–85. doi: 10.1007/978-1-4684-5209-9_2. [DOI] [PubMed] [Google Scholar]
- 8.Schachter H, Narasimhan S, Gleeson P, Vella G. Control of branching during the biosynthesis of asparagine-linked oligosaccharides. Can J Biochem Cell Biol. 1983;61:1049–1066. doi: 10.1139/o83-134. [DOI] [PubMed] [Google Scholar]
- 9.Asada M, Furukawa K, Segawa K, Endo T, Kobata A. Increased expression of highly branched N-glycans at cell surface is correlated with the malignant phenotypes of mouse tumor cells. Cancer Res. 1997;57:1073–1080. [PubMed] [Google Scholar]
- 10.Pochec E, Litynska A, Amoresano A, Casbarra A. Glycosylation profile of integrin alpha3beta1 changes with melanoma progression. Biochim Biophys Acta. 2003;7:1–3. doi: 10.1016/j.bbamcr.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Granovsky M, Fata J, Pawling J, Muller WJ, Khokha R, Dennis JW. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat Med. 2000;6:306–312. doi: 10.1038/73163. [DOI] [PubMed] [Google Scholar]
- 12.Guo HB, Lee I, Kamar M, Akiyama SK, Pierce M. Aberrant N-glycosylation of beta1 integrin causes reduced alpha5beta1 integrin clustering and stimulates cell migration. Cancer Res. 2002;62:6837–6845. [PubMed] [Google Scholar]
- 13.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 14.Isaji T, Gu J, Nishiuchi R, Zhao Y, Takahashi M, Miyoshi E, et al. Introduction of bisecting GlcNAc into integrin alpha5beta1 reduces ligand binding and downregulates cell adhesion and cell migration. J Biol Chem. 2004;3:3. doi: 10.1074/jbc.M311627200. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimura M, Nishikawa A, Ihara Y, Taniguchi S, Taniguchi N. Suppression of lung metastasis of B16 mouse melanoma by N-acetylglucosaminyltransferase III gene transfection. Proc Natl Acad Sci USA. 1995;92:8754–8758. doi: 10.1073/pnas.92.19.8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Y, Nakagawa T, Itoh S, Inamori K, Isaji T, Kariya Y, et al. N-acetylglucosaminyltransferase III antagonizes the effect of N-acetylglucosaminyltransferase V on alpha3beta1 integrin-mediated cell migration. J Biol Chem. 2006;281:32122–32130. doi: 10.1074/jbc.M607274200. [DOI] [PubMed] [Google Scholar]
- 17.Pretzlaff RK, Xue VW, Rowin ME. Sialidase treatment exposes the beta1-integrin active ligand binding site on HL60 cells and increases binding to fibronectin. Cell Adhes Commun. 2000;7:491–500. doi: 10.3109/15419060009040306. [DOI] [PubMed] [Google Scholar]
- 18.Kawano T, Takasaki S, Tao TW, Kobata A. Altered glycosylation of beta1 integrins associated with reduced adhesiveness to fibronectin and laminin. Int J Cancer. 1993;53:91–96. doi: 10.1002/ijc.2910530118. [DOI] [PubMed] [Google Scholar]
- 19.Dennis J, Waller C, Timpl R, Schirrmacher V. Surface sialic acid reduces attachment of metastatic tumour cells to collagen type IV and fibronectin. Nature. 1982;300:274–276. doi: 10.1038/300274a0. [DOI] [PubMed] [Google Scholar]
- 20.Nadanaka S, Sato C, Kitajima K, Katagiri K, Irie S, Yamagata T. Occurrence of oligosialic acids on integrin alpha5 subunit and their involvement in cell adhesion to fibronectin. J Biol Chem. 2001;276:33657–33664. doi: 10.1074/jbc.M011100200. [DOI] [PubMed] [Google Scholar]
- 21.Chammas R, Veiga SS, Travassos LR, Brentani RR. Functionally distinct roles for glycosylation of alpha and beta integrin chains in cell-matrix interactions. Proc Natl Acad Sci USA. 1993;90:1795–1799. doi: 10.1073/pnas.90.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isaji T, Sato Y, Zhao Y, Miyoshi E, Wada Y, Taniguchi N, Gu J. N-glycosylation of the beta-propeller domain of the integrin alpha5 subunit is essential for alpha5beta1 heterodimerization, expression on the cell surface, and its biological function. J Biol Chem. 2006;281:33258–33267. doi: 10.1074/jbc.M607771200. [DOI] [PubMed] [Google Scholar]
- 23.Zhuo Y, Chammas R, Bellis SL. Sialylation of beta1 integrins blocks cell adhesion to galectin-3 and protects cells against galectin-3-induced apoptosis. J Biol Chem. 2008 doi: 10.1074/jbc.M8000015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seales EC, Jurado GA, Brunson BA, Wakefield JK, Frost AR, Bellis SL. Hypersialylation of beta1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by upregulating cell motility. Cancer Res. 2005;65:4645–4652. doi: 10.1158/0008-5472.CAN-04-3117. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimura M, Ihara Y, Matsuzawa Y, Taniguchi N. Aberrant glycosylation of E-cadherin enhances cell-cell binding to suppress metastasis. J Biol Chem. 1996;271:13811–13815. doi: 10.1074/jbc.271.23.13811. [DOI] [PubMed] [Google Scholar]
- 26.Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol. 1998;153:333–339. doi: 10.1016/S0002-9440(10)65575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akama R, Sato Y, Kariya Y, Isaji T, Fukuda T, Lu L, et al. N-Acetylglucosaminyltransferase III Expression Is Regulated by Cell-Cell Adhesion via the E-cadherin-catenin-actin Complex. Proteomics. 2008;8:3221–3228. doi: 10.1002/pmic.200800038. [DOI] [PubMed] [Google Scholar]
- 28.Iijima J, Zhao Y, Isaji T, Kameyama A, Nakaya S, Wang X, et al. Cell-cell interaction-dependent regulation of N-acetylglucosaminyltransferase III and the bisected N-glycans in GE11 epithelial cells. Involvement of E-cadherin-mediated cell adhesion. J Biol Chem. 2006;281:13038–13046. doi: 10.1074/jbc.M601961200. [DOI] [PubMed] [Google Scholar]
- 29.Freeze HH. Genetic defects in the human glycome. Nat Rev Genet. 2006;7:537–551. doi: 10.1038/nrg1894. [DOI] [PubMed] [Google Scholar]