Abstract
The cannabinoid signaling system is located during brain development in a position concordant with playing a modulatory function in the regulation of neuronal and glial cell proliferation and migration, survival of neural progenitors, axonal elongation and synaptogenesis and differentiation of oligodendrocytes and formation of myelin. This assumption is based on the fact that CB1 receptors and their ligands emerge early in brain development and are transiently expressed in certain brain regions that play key roles in these processes. We have recently proposed that this modulatory action might be exerted through regulating L1 and other cell adhesion molecules, that are also key elements for those processes. The present commentary will address these two questions trying to summarize all the available evidence and to suggest the future directions for research.
Key words: cannabinoid signaling system, CB1 receptors, brain development, neural cell proliferation, migration and differentiation, cell adhesion molecules
The study of the molecular mechanisms underlying the psychoactive effects of Cannabis sativa led to the discovery of the “endogenous cannabinoid system”, an intercellular signaling system that plays modulatory functions in brain synapses and also in the periphery. It consists of multiple endocannabinoid ligands, their membrane receptors (CB1, CB2 and others), anabolic and catabolic enzymes, as well as a membrane-transport mechanism. The function(s) of this system has (have) been extensively studied in adult mammals pointing to an important role in the regulation of numerous neurobiological processes. Studies conducted during the last decade, which addressed the ontogeny of this system in the brain, led to the assumption that the endocannabinoid system might also play relevant modulatory functions during brain development. This assumption derived from certain particularities found in the ontogenic pattern of the endocannabinoid system, which mimicked similar results found for neurotransmitters having a neurotrophic function.1 Thus, the endo cannabinoid signaling system, in particular the CB1 receptors: (1) emerge early in brain development,1–5 (2) are particularly abundant in forebrain subventricular zones and cortical structures,1,4 which play a key role in cell proliferation and migration, respectively, and (3) and are transiently located, during restricted ontogenic periods, in forebrain white matter structures, in particular transverse commissural tracts,1,2,4 which are essential for cell migration and axonal elongation. The fact that this “atypical” distribution of the CB1 receptor disappears coinciding with the conclusion of the establishement of synaptic communication and postsynaptic target selection1,6 is highly suggestive of a specific role of the cannabinoid signaling in these processes.
Regulation by Endocannabinoids of Neural Cell Proliferation, Migration, Differentiation, Survival and Establishement of Synaptic Communication
One of the first functions proposed for endocannabinoids, after seeing the particular location of their CB1 receptors in subventricular zones during brain development, was the control of neural cell proliferation. Subventricular zones are enriched in progenitors for neurons and glial cells, and several studies have strongly demonstrated that these neural progenitors express functional cannabinoid receptors and the catabolic enzyme FAAH, and that they are able to synthesize endocannabinoids.7 These observations suggested that the endocannabinoid signaling might regulate the proliferation of progenitor cells and promote their differentiation into glial cells,8 in an attempt to maintain the neuron/glia balance during brain development. In addition, the endocannabinoid signaling also plays a role in the generation of neurons from neural progenitors although the type of control exerted in this case, facilitatory or inhibitory, has remained controversial to date.9,10 It is possible that the type of effect depends on specific neuronal subpopulation under investigation, being facilitatory, for example, in the case of pyramidal cells, whose generation from neural progenitors, as well as their migration and differentiation, was stimulated by the endocannabinoid signaling.11
Endocannabinoids and their receptors are also operational in the migration of neural cells, in particular immature neurons, for which they could play an instructive and permissive role presumably by modifying the adhesive interactions between neurons and other substrates (e.g., radial glia).6 This function is concordant with the location of CB1 receptors in specific white matter structures2,4 and with the presence of FAAH enzyme in radial glia,6,8 given the role played by both cellular elements in neuronal migration.1 Thus, the activation of CB1 receptors: (1) facilitated the migration of interneurons through the cerebral cortex,12 an effect thar was additive with BDNF, and (2) promoted the radial migration of immature pyramidal cells.11
In parallel to the processes of cell proliferation and subsequent migration, neural cells do also experience a process of differentiation which, in several phases, is regulated by endocannabinoids. In fact, this function might already include the effects of endocannabinoids on the generation of immature neurons or glial cells from neural progenitors6–8 that have been addressed before, and also their participation in the differentiation of oligodendrocytes and, subsequently, in the formation of myelin.13 In addition, recent studies demonstrated that it also includes further steps in the process of acquisition of neuronal or glial identity. For example, in the specific case of neurons, endocannabinoids have been involved in the control of neuritic elongation, the establishment of synaptic communication, and the acquisition of a specific neurotransmitter phenotype.1,6 That the endocannabinoid system plays a role in axonal elongation and guidance is supported by the presence of CB1 receptors in forebrain white matter tracts, since it was demonstrated that this receptor was located in axons elongating through these tracts to reach their final fields for synaptic processes.1,2,4 In these axons, CB1 receptor immunostaining perfectly overlaps with markers of growth cones such as GAP-43.14 Similar results were found by Berghuis et al.15 in growth cones of cortical GABAergic interneurons during late gestation, whereas other authors found a parallelism between the generation of endocannabinoids and the activation of CB1 receptors within the growth cone.6,16,17 By contrast, significant deficits in axonal fasciculation were found in mice lacking CB1 receptors or after pharmacological blockade of CB1 receptors.11,18 Overall, these data support that the endocannabinoid system plays an important role in the regulation of specific aspects of growth cone differentiation and axonal guidance.6 The cannabinoid signaling system has been also involved in the control of synaptic communication.19 In fact, mice lacking CB1 receptors showed an impaired target selection by cortical GABAergic interneurons.15 However, the function of CB1 receptors on synaptogenesis would be restricted to the times immediately before the establishment of synaptic contacts, since there is evidence that CB1 receptors disappear in axons once synaptogenesis and selection of postsynaptic targets have concluded.6 This explains the lack of colocalization between synaptophysin, a marker of synaptic vesicles in neuronal axons, and CB1 receptors in forebrain white matter structures of rat fetuses.14 The formation of synaptic vesicles and their secretory activity in elongating axons start with the establishment of synaptic contacts, so that the fact that CB1 receptors do not colocalize with synaptophysin situate this receptor in neurons in an earlier stage of maturation.14 In parallel to this function of the cannabinoid signaling in the establishment of synaptic communication, this system would also participate in the acquisition of a specific neurotransmitter phenotype by selected subpopulations of neurons.1,20 This effect would be presumably exerted through an action of endocannabinoids and their receptors on several key genes for specific neurotransmitters, such as the case of genes encoding for the enzyme tyrosine hydroxylase1,21 or for opioid peptide precursors.22,23
A last event related to neural development that was reported to be susceptible of endocannabinoid regulation is the control of cell death/survival processes that occur during different phases of neural development. These processes are always physiologically regulated by different types of endogenous signals, e.g., neurotrophins. Recent evidence include endocannabinoids and their receptors among these endogenous signals. Thus, the endocannabinoid system might participate in the regulation of death/survival decision of neural progenitors, as has been already addressed before,6–8 in an attempt to maintain an adequate number of proliferating neural cells during brain development. In addition, the endocannabinoid system might also play a role in eliciting the apoptosis of exceeding neurons or of specific subpopulations of neurons with a neurotrophic function.1,20 The presence of endocannabinoid elements, in particular the CB1 receptor, in developing neuronal subpopulations, such as tyrosine hydroxylase-containing mesencephalic neurons,21 that do not contain this receptor type in the adult brain, supports this apoptotic function of the endocannabinoid system.
Importance of Cell Adhesion Molecules in the Neurodevelopmental Effects of Endocannabinoids
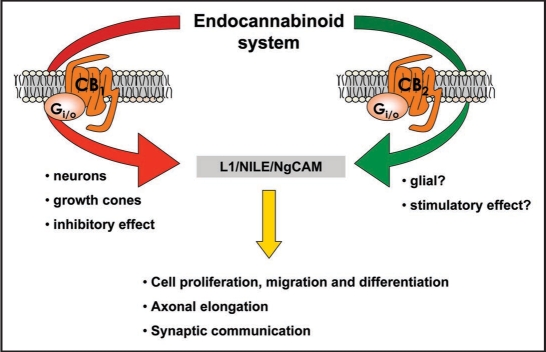
During the last years, various laboratories have provided sufficient experimental evidence to ensure that the endocannabinoid system is involved in the regulation of these neurodevelopmental events. However, the key issue now is the elucidation of the molecular and cellular substrates involved in these modulatory actions. In this context, we recently proposed a role of endocannabinoids by regulating the neural cell adhesion molecules (e.g., L1),14,24,25 which, given their capability to mediate cell-cell and cell-matrix interactions, have been strongly linked to cell proliferation, migration and differentiation, neuritic elongation and guidance, synaptogenesis, neuron-glia interactions and myelinogenesis, exactly the same processes that were proposed for the ontogenic function of endocannabinoids. Our proposal was based on the fact that: (1) those regions where cannabinoid agonists altered L1,24,25 were the same white matter regions where CB1 receptors were transiently expressed during brain development,2,4 (2) these effects were always restricted to specific periods (late gestation and early posnatal life) during brain development, but later on the cannabinoid system would loss its capability to influence the function of L124,25 and (3) different elements of the cannabinoid signaling (e.g., CB1 and CB2 receptors) are expressed in developing neurons or glial cells1,6,14 (e.g., growth cones, elongating axons, immature migrating cells) in close vicinity with L1. We think that the activation of either CB1 or CB2 receptors might have a dual effect on this cell-adhesion protein depending on the type of cells where both cannabinoid receptor types are located (see Fig. 1), which is concordant with the idea of “multiple spatial and temporal regulation” exerted by other molecules having a neurotrophic action. Thus, a stimulation of L1 effects might be attained through the activation of CB2 receptors located on glial cells, since these are the major cell subpopulation having their nuclei within the white matter structures where cannabinoid agonists enhanced L1 expression.24 The presence of CB2 receptors in various types of developing neural cells has been recently documented26,27 and we have also preliminary evidence that these receptors are present in the rat fetal brain. By contrast, an inhibition of L1 by cannabinoids would be an event restricted to CB1 receptors located on neuronal cells, a fact supported by the following observations: (1) L1 and CB1 receptors co-localize in neuronal elements in forebrain white matter structures of rat fetuses,14 (2) these neuronal elements are preferentially elongating axons and not migrating neurons given the overlapping found for this receptor type with markers of growth cones14 and (3) L1 synthesis is enhanced in CB1 receptor-deficient mice (unpublished results).
Figure 1.
Mechanisms proposed for the action of the cannabinoid signaling on L1-dependent processes during brain development.
Acknowledgements
The present study has been supported with grants from CIBERNED (CB06/05/0089), MEC (SAF2006/11333) and CAM (S-SAL-0261/2006).
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/6749
References
- 1.Fernández-Ruiz J, Berrendero F, Hernández ML, Ramos JA. The endogenous cannabinoid system and brain development. Trends Neurosci. 2000;23:14–20. doi: 10.1016/s0166-2236(99)01491-5. [DOI] [PubMed] [Google Scholar]
- 2.Romero J, García-Palomero E, Berrendero F, García-Gil L, Hernández ML, Ramos JA, Fernández-Ruiz J. Atypical location of cannabinoid receptors in white matter areas during rat brain development. Synapse. 1997;26:317–323. doi: 10.1002/(SICI)1098-2396(199707)26:3<317::AID-SYN12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 3.Buckley NE, Hansson S, Harta G, Mezey E. Expression of the CB1 and CB2 receptor messenger RNAs during embryonic development in the rat. Neuroscience. 1998;82:1131–1149. doi: 10.1016/s0306-4522(97)00348-5. [DOI] [PubMed] [Google Scholar]
- 4.Berrendero F, García-Gil L, Hernández ML, Romero J, Cebeira M, de Miguel R, et al. Localization of mRNA expression and activation of signal transduction mechanisms for cannabinoid receptor in rat brain during fetal development. Development. 1998;125:3179–3188. doi: 10.1242/dev.125.16.3179. [DOI] [PubMed] [Google Scholar]
- 5.Berrendero F, Sepe N, Ramos JA, Di Marzo V, Fernández-Ruiz J. Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse. 1999;33:181–191. doi: 10.1002/(SICI)1098-2396(19990901)33:3<181::AID-SYN3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 6.Harkany T, Guzmán M, Galve-Roperh I, Berghuis P, Devi LA, Mackie K. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol Sci. 2007;28:83–92. doi: 10.1016/j.tips.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Aguado T, Monory K, Palazuelos J, Stella N, Cravatt B, Lutz B, et al. The endocannabinoid system drives neural progenitor proliferation. FASEB J. 2005;19:1704–1706. doi: 10.1096/fj.05-3995fje. [DOI] [PubMed] [Google Scholar]
- 8.Aguado T, Palazuelos J, Monory K, Stella N, Cravatt B, Lutz B, et al. The endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cells. J Neurosci. 2006;26:1551–1561. doi: 10.1523/JNEUROSCI.3101-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rueda D, Navarro B, Martínez-Serrano A, Guzmán M, Galve-Roperh I. The endocannabinoid anandamide inhibits neuronal progenitor cell differentiation through attenuation of the Rap1/B-Raf/ERK pathway. J Biol Chem. 2002;277:46645–46650. doi: 10.1074/jbc.M206590200. [DOI] [PubMed] [Google Scholar]
- 10.Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji SP, Bai G, Zhang X. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J Clin Invest. 2005;115:3104–3116. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulder J, Aguado T, Keimpema E, Barabás K, Ballester Rosado CJ, Nguyen L, et al. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc Natl Acad Sci USA. 2008;105:8760–8785. doi: 10.1073/pnas.0803545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berghuis P, Dobszay MB, Wang X, Spano S, Ledda F, Sousa KM, et al. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc Natl Acad Sci USA. 2005;102:19115–19120. doi: 10.1073/pnas.0509494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arévalo-Martín A, García-Ovejero D, Rubio-Araiz A, Gómez O, Molina-Holgado F, Molina-Holgado E. Cannabinoids modulate Olig2 and polysialylated neural cell adhesion molecule expression in the subventricular zone of post-natal rats through cannabinoid receptor 1 and cannabinoid receptor 2. Eur J Neurosci. 2007;26:1548–1559. doi: 10.1111/j.1460-9568.2007.05782.x. [DOI] [PubMed] [Google Scholar]
- 14.Gómez M, Hernández ML, Pazos MR, Tolón RM, Romero J, Fernández-Ruiz J. Colocalization of CB1 receptors with L1 and GAP-43 in forebrain white matter regions during fetal rat brain development: Evidence for a role of these receptors in axonal growth and guidance. Neuroscience. 2008;153:687–699. doi: 10.1016/j.neuroscience.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 15.Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, et al. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- 16.Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams EJ, Walsh FS, Doherty P. The FGF receptor uses the endocannabinoid signaling system to couple to an axonal growth response. J Cell Biol. 2003;160:481–486. doi: 10.1083/jcb.200210164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watson S, Chambers D, Hobbs C, Doherty P, Graham A. The endocannabinoid receptor, CB1, is required for normal axonal growth and fasciculation. Mol Cell Neurosci. 2008;38:89–97. doi: 10.1016/j.mcn.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Bernard C, Milh M, Morozov YM, Ben-Ari Y, Freund TF, Gozlan H. Altering cannabinoid signaling during development disrupts neuronal activity. Proc Natl Acad Sci USA. 2005;102:9388–9393. doi: 10.1073/pnas.0409641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernández-Ruiz J, Gómez M, Hernández ML, de Miguel R, Ramos JA. Cannabinoids and gene expression during brain development. Neurotox Res. 2004;6:389–401. doi: 10.1007/BF03033314. [DOI] [PubMed] [Google Scholar]
- 21.Hernández ML, Berrendero F, Suárez I, García-Gil L, Cebeira M, Mackie K, et al. Cannabinoid CB1 receptors colocalize with tyrosine hydroxylase in cultured fetal mesencephalic neurons and their activation increases the levels of this enzyme. Brain Res. 2000;857:56–65. doi: 10.1016/s0006-8993(99)02322-7. [DOI] [PubMed] [Google Scholar]
- 22.Pérez-Rosado A, Manzanares J, Fernández-Ruiz J, Ramos JA. Prenatal Δ9-tetrahydrocannabinol exposure modifies proenkephalin gene expression in the fetal rat brain: sex-dependent differences. Dev Brain Res. 2000;120:77–81. doi: 10.1016/s0165-3806(99)00170-4. [DOI] [PubMed] [Google Scholar]
- 23.Pérez-Rosado A, Gómez M, Manzanares J, Ramos JA, Fernández-Ruiz J. Changes in prodynorphin and POMC gene expression in several brain regions of rat fetuses prenatally exposed to Δ9-tetrahydrocannabinol. Neurotox Res. 2002;4:211–218. doi: 10.1080/10298420290023936. [DOI] [PubMed] [Google Scholar]
- 24.Gómez M, Hernández ML, Johansson B, de Miguel R, Ramos JA, Fernández-Ruiz J. Prenatal cannabinoid and gene expression for neural adhesion molecule L1 in the fetal rat brain. Dev Brain Res. 2003;147:201–207. doi: 10.1016/j.devbrainres.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Gómez M, Hernández ML, Fernández-Ruiz J. The activation of cannabinoid receptors during early postnatal development reduces the expression of cell adhesion molecule L1 in the rat brain. Brain Res. 2007;1145:48–55. doi: 10.1016/j.brainres.2007.01.102. [DOI] [PubMed] [Google Scholar]
- 26.Palazuelos J, Aguado T, Egia A, Mechoulam R, Guzmán M, Galve-Roperh I. Non-psychoactive CB2 cannabinoid agonists stimulate neural progenitor proliferation. FASEB J. 2006;20:2405–2407. doi: 10.1096/fj.06-6164fje. [DOI] [PubMed] [Google Scholar]
- 27.Molina-Holgado F, Rubio-Araiz A, García-Ovejero D, Williams RJ, Moore JD, Arévalo-Martin A, et al. CB2 cannabinoid receptors promote mouse neural stem cell proliferation. Eur J Neurosci. 2007;25:629–634. doi: 10.1111/j.1460-9568.2007.05322.x. [DOI] [PubMed] [Google Scholar]