Alterations in synaptic strength are proposed to underlie learning and memory, and two major mechanisms utilized by neurons to bring about such changes involve regulating the number of AMPARs found at the synaptic plasma membrane, and altering the size and/or shape of the specialized postsynaptic compartment, the dendritic spine. It is now well-established that control of receptor number is brought about by precise modulation of vesicle trafficking, although the details of this crucial process are still far from clear. Regulation of spine size involves dynamic alterations in the spine actin cytoskeleton, but recent studies suggest that trafficking events may also play a role. In this review, I will summarize some recent findings about AMPAR trafficking pathways, and highlight the idea that membrane trafficking events not only regulate the complement of AMPARs at the synaptic plasma membrane, but also contribute to spine morphogenesis, either by regulating the plasma membrane content of spines, or as a result of changes in AMPAR trafficking.
Trafficking Pathways During LTP, LTD and Constitutive Cycling
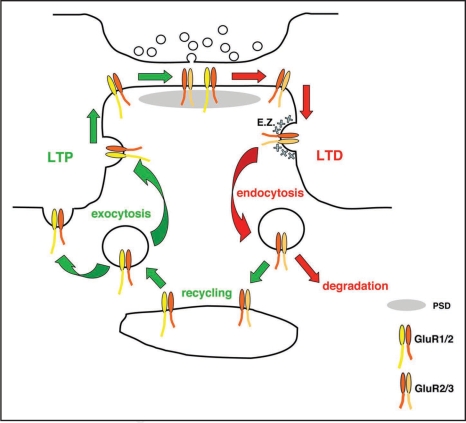
AMPARs undergo both constitutive and regulated exocytosis, endocytosis and recycling, as well as lateral diffusion in the plane of the plasma membrane. A popular model is that Long-Term Potentiation of synaptic transmission (LTP) involves the insertion of additional AMPA receptors into the postsynaptic membrane by a combination of exocytosis and lateral diffusion. Long-Term Depression (LTD) involves AMPAR removal, also by lateral diffusion followed by internalization, and basal AMPAR numbers are maintained by constitutive rounds of endocytosis and exocytosis. Although the fundamentals behind this basic model (Fig. 1) are essentially correct, recent work has demonstrated that the real picture is considerably more complex. Furthermore, there remains some disagreement surrounding issues such as the precise site of AMPAR exocytosis during LTP, which may be relevant to the mechanisms of membrane delivery during spine enlargement. The vast majority of studies have been carried out in hippocampal pyramidal cells; therefore this review will discuss mechanisms relevant to these principal neurons.
Figure 1.
AMPA receptor trafficking pathways during synaptic plasticity. Long-term potentiation involves the exocytosis of GluR1-containing AMPARs, that originate from recycling endosomes, at sites either on the dendritic spine or shaft. They subsequently drift laterally in the plane of the plasma membrane to reach the postsynaptic density. Long-term depression involves the lateral movement of GluR2-containing AMPARs from the PSD to designated endocytic zones (E.Z.) on the dendritic spine. Following internalization by clathrin-mediated endocytosis, AMPARs are sorted to lysosomes for degradation. During constitutive trafficking, AMPARs are internalized from E.Z.s, and traffic via recycling endosomes back to the plasma membrane.
AMPARs are tetrameric complexes made up from subunits GluR1–4.1 GluR4 in hippocampal neurons is predominantly expressed early in development,2 so will not be discussed in detail here. Hippocampal AMPARs are mainly heteromers of GluR1/2 and GluR2/3, with a minority of GluR1 homomers;3 most studies have focussed on the behaviour of GluR1 and GluR2.
Early evidence that LTP requires the exocytosis of plasma membrane proteins was provided by experiments using agents to disrupt the function of the SNARE complex and their associated factors.4,5 It was also shown that the AMPAR subunit GluR1 is restricted from synapses under basal conditions, but is functionally inserted into synapses following LTP stimuli.6 Similarly, agents that disrupt clathrin-mediated endocytosis were shown to inhibit LTD,7,8 indicating that LTD requires endocytosis. In addition, it was shown that AMPAR internalization could be stimulated by the same signalling pathways that mediate LTD.9 Some important questions arose from these pioneering studies, leading to very active research into investigating the exact sites on the dendritic surface for endocytosis and exocytosis, and precisely which stages of the trafficking pathways are regulated. In addition, with the increased attention given to the structural plasticity of dendritic spines, a link between AMPAR trafficking and spine morphogenesis has also been explored.
Sites of AMPAR exocytosis
The demonstration that AMPARs diffuse rapidly in the plane of the plasma membrane10 led to the novel concept that receptor vesicle trafficking need not be directed immediately to the PSD. Early studies using fixed-cell assays suggested that recombinant GluR1 subunit is inserted initially at extrasynaptic sites, whereas GluR2 is inserted directly at synapses.11 Recombinant heteromers were shown to behave in a similar manner to GluR1 homomers, suggesting that GluR1 controls receptor insertion. Using more elaborate live imaging techniques, it has been suggested that “chemical LTP” stimuli result in insertion of GluR1 directly into the spine head. Super-ecliptic pHluorin (SEP), a pH-sensitive variant of GFP that exhibits strong fluorescence when exposed to the extracellular medium, but not in trafficking vesicles, has recently been used as a tool to study the surface expression of AMPAR subunits. Following a chemical LTP protocol, SEP-GluR1 was seen to accumulate in dendritic spines, with no preceding increase in fluorescence on the dendritic shaft.12 Again, this supports the notion that AMPAR subunits are inserted directly into synaptic sites. However, it should be noted that the spatial resolution in this study may not have allowed the distinction between PSD and extrasynaptic sites on the spine surface, so SEP-GluR1 may be inserted at a perisynaptic location, followed by lateral drift to the PSD. Some indirect evidence from Ehlers and colleagues also suggests insertion within the spine. Having demonstrated that recycling endosomes supply AMPARs for LTP;13 see later section), a recent paper from the same group elegantly shows that following the same chemical-LTP stimulus, recycling endosomes move into spines to contribute membrane to spine growth.14 Although AMPAR subunits were not specifically analyzed, this would suggest that AMPARs are inserted into spines during LTP. However, the issue of whether the insertion occurs at the PSD, or at extrasynaptic sites on the spine head was not addressed. A more recent study that utilized SEP-tagged-GluR1 (but different microscopy techniques) produced contrasting results. SEP-GluR1 was shown to insert in discrete puncta at sites on the dendritic shaft and soma, but not at spines. The inserted SEP-GluR1 was often seen to spread to nearby spines by lateral diffusion.15
As well as LTP-stimulated conditions, several studies have addressed the location of receptor insertion under basal conditions. Again, conflicting results have arisen. Early fixed-cell studies suggested that, similar to LTP-induced trafficking, basal exocytosis of GluR1 occurs at extrasynaptic dendritic sites. A more radical model has been suggested following experiments using the photoactivatable AMPAR antagonist ANQX to rapidly silence surface receptors, so that re-emerging endogenous receptors can be detected.16 This study suggests that AMPARs are inserted at the neuronal cell body and then diffuse along the dendritic plasma membrane to reach synapses. Since this process is very slow (∼16 h), it seems unlikely that this kind of trafficking could mediate LTP, unless the final stages of lateral diffusion to the PSD, rather than exocytosis per se were the regulated step. More recently it has been demonstrated that endogenous AMPARs under basal conditions accumulate just beneath the PSD, when mutants of the exocyst complex are expressed in neurons.17 Based on the assumption that this treatment holds up exocytic cargo, including AMPARs, just before the point of exocytosis, this report suggests that receptors are actually inserted directly at the PSD.
In summary, despite considerable effort, the precise sites of AMPAR exocytosis remain unclear. Perhaps there are no designated exocytic zones, and AMPARs can be inserted at multiple sites on the neuronal plasma membrane. In this case, the type of preparation, stimulus, or age of neurons studied could influence the result. However, given the extremely densely-packed protein network at the PSD, it would seem less likely that exocytosis would occur directly through this structure, purely on the grounds of physical hindrance. However, whether AMPARs are inserted into extrasynaptic spine regions, the dendritic shaft, or the soma, remains to be determined.
Sites of AMPAR endocytosis.
In contrast, there appears to be greater agreement over the sites of AMPAR endocytosis, although this could in part be due to the smaller number of labs who have so far addressed the issue. Experiments using GFP-tagged clathrin expressed in hippocampal neurons demonstrated the existence of designated functional endocytic zones (EZs) in dendritic spines, adjacent to the PSD.18 This was also supported by immunogold labelling EM studies analysing the localisation of endogenous clathrin.19 Although these reports did not investigate whether AMPARs are internalized at these specific sites, the proximity to the PSD strongly suggested that this would be the case. Indeed, a follow-up report from the same group demonstrated that blocking the physical association of the EZ with the PSD disrupted basal endogenous AMPAR cycling, indicating that at least under unstimulated conditions, AMPARs are internalized at perisynaptic sites in the spine head, implying that receptors drift laterally prior to endocytosis.20 This study describes a surprising mechanism whereby AMPAR endocytosis at the EZ is required to maintain synaptic AMPAR number, by capturing laterally-drifting receptors and recycling them back to the plasma membrane. Disruption of the PSD-EZ interaction results in a loss of synaptic AMPARs because they are not recycled.20
AMPAR internalization in response to chemical-LTD (NMDAR activation) has been directly visualized using SEP-tagged-GluR2 subunit. Interestingly, SEP-GluR2 rapidly internalizes from extra- synaptic sites in response to NMDA treatment, followed by a delayed removal from synaptic sites, again suggesting that AMPARs move from synapses in the plane of the plasma membrane, prior to extrasynaptic endocytosis.21 In this study, the extrasynaptic sites for endocytosis appear to be on the dendritic shaft rather than on spines.
Regulation of intracellular AMPAR trafficking pathways.
AMPARs traffic constitutively, i.e., they are endocytosed from the plasma membrane, recycled, and reinserted at the surface. As discussed in the previous section, this process is likely to also involve some lateral movement in the plane of the plasma membrane between the PSD and the sites of endo- or exocytosis. The reduced number of synaptic AMPARs following LTD induction could be a result of enhanced lateral movement from the PSD, enhanced endocytosis, or reduced recycling back to the plasma membrane. Chemical LTD protocols result in an accumulation of AMPARs in intracellular compartments, as analyzed by antibody-feeding assays, using antibodies that bind to the extracellular domains of receptor subunits on live cells, and are taken into the endocytic pathway upon AMPAR endocytosis.7,9,22–24 The simplest explanation for the observed increase in internalized receptor is that endocytosis increases as a result of chemical LTD. However, it has been suggested that a sorting step at the level of the recycling endosome determines whether endocytosed AMPARs are recycled back to the plasma membrane or targeted for degradation.22,23 NMDAR activation results in the sorting of AMPARs to late endosomes and ultimately lysosomes, hence reducing the number of receptors recycled back to the surface and therefore at the synapse. The enhanced accumulation of internalized AMPARs following NMDA treatment would therefore be consistent with this model because receptors are not recycled back to the cell surface. However, studies using SEP-GluR2 demonstrate a rapid and dramatic reduction in surface-localised receptor as a direct result of NMDA application, suggesting that GluR2 endocytosis per se is indeed enhanced during chemical LTD.21,25 The third possibility is that AMPARs could be mobilised to leave the PSD by lateral movement, resulting in a greater number of receptors associating with the perisynaptic endocytic zone. Although there is no direct evidence to support the idea that lateral movement is regulated specifically in response to NMDAR activation, glutamate application has been shown to increase the mobility of synaptic AMPARs so that they move to perisynaptic sites.26 However, it is possible that this occurs as a secondary effect of membrane flow induced by the proximity of internalizing endocytic zones. Taken together, there is evidence to suggest that AMPAR internalization may be regulated at more than one checkpoint.
Similarly, the increase in receptor number observed at the synapse following LTP could be a result of enhanced exocytosis, enhanced lateral movement towards the synapse, or reduced lateral movement away from the synapse. Although there is good evidence that blocking exocytosis inhibits LTP,4,5 this does not necessarily mean that this is the regulated step. An important addition to the model for LTP is that the receptors inserted during LTP emerge via recycling endosomes, and therefore originate from the neuronal surface, not from de novo synthesis.13 This adds the possibility that regulation of AMPAR recycling is also a critical component in LTP. This study, in conjunction with a later report from the same group,14 provides evidence that chemical LTP not only promotes the recycling of AMPARs, but also of transferrin receptor, and of membrane in general. Since recycling compartments were seen to enter spine necks as a result of a chemical LTP stimulus, these experiments suggest regulation at the level of the recycling endosome. In this scenario, presumably the fusion of recycled AMPAR-containing vesicles with the plasma membrane must also be increased to prevent build-up in the spine neck. In the case of chemical-LTP-induced exocytic events occurring at sites on dendritic shafts, recombinant GluR1 was seen to diffuse to some neighboring spines, but not all,15 suggesting that lateral mobility is not random, but may be regulated in some way, possibly in response to LTP stimuli. This question was partly addressed by Choquet's group in experiments where KCl treatment, which has been shown to stimulate the accumulation of surface AMPAR puncta in an NMDAR-dependent manner,27 increased the lateral mobility of extrasynaptic AMPARs.28
Linking AMPAR Trafficking with Spine Morphogenesis
Dendritic spines are highly specialized subcellular compartments that contain the postsynaptic protein machinery of most excitatory synapses. They concentrate and compartmentalise biochemical signals such as Ca2+, and synaptic protein machinery such as neurotransmitter receptors, providing the synaptic specificity required for plasticity.29–32 In addition to regulating the number of synaptic AMPARs by trafficking, changes in synaptic strength correlate with corresponding changes in dendritic spine size, and possibly shape.33–38 LTD stimuli result in spine shrinkage and retraction,35,38 whereas LTP leads to the formation of new spines, or the growth of existing ones.33,38
Actin cytoskeleton.
Spines are extremely rich in dynamic actin filaments, which underlie these activity-dependent changes in spine size. LTP is thought to promote actin polymerization resulting in an increase in spine F-actin, and LTD results in reduced F-actin via actin depolymerization.39 The signalling pathways that lead to these changes in actin polymerization to bring about spine growth or shrinkage have become a major research focus in their own right29,40,41 and will not be discussed in great detail here. However, some interesting recent developments have suggested that AMPAR trafficking and spine morphogenesis may be linked.
Early studies demonstrated that AMPAR localisation and trafficking are dependent on the polymerization state of actin. Enhanced actin polymerization and stabilised actin filaments (F-actin) favour surface expression of AMPARs.42 Actin depolymerization stimulates a removal of AMPARs from synaptic sites.42,43 This has been supported by a more recent study that provides a mechanism involving PICK1 as a regulator of actin polymerization during AMPAR internalization.24 It has also been suggested that AMPARs are “anchored” in some way to F-actin at the synaptic plasma membrane, although current models of a dynamic, continually cycling AMPAR population may be inconsistent with this notion. An additional explanation is provided by the observation that forward traffic of AMPARs into spines involves the actin-based motor protein myosin Va,44 and the α-actinin binding protein RIL,45 both of which require F-actin. Although these observations do not give direct evidence for a connection between spine morphogenesis and AMPAR trafficking, they are consistent with the concept that these two processes both utilise actin regulation as an underlying cell biological mechanism.
A very surprising development came when Sheng's group identified an N-terminal domain (NTD) of GluR2 as a direct modulator of spine morphogenesis.46 GluR2 overexpression in hippocampal neurons results in increased size and number of spines, and GluR2 knockdown by RNAi inhibits spine morphogenesis. The mechanism for this effect appears to be via an interaction between GluR2 NTD and N-Cadherin,47 although the precise details are unclear. One possibility is that the presence of GluR2 may enhance N-Cadherin synaptic localisation, leading to intracellular signalling events that alter actin dynamics. This led to the intriguing possibility that plasticity-associated trafficking of GluR2-containing AMPARs, could itself trigger the changes in spine size that occur following LTP and LTD. Since LTD involves internalization of GluR2-containing AMPARs,23 AMPAR endocytosis may be a direct stimulus for spine shrinkage that is associated with LTD. However, it has recently been suggested that during LTD, AMPAR internalization and spine shrinkage are quite separate events.48 Blockade of trafficking does not inhibit spine shrinkage, and inhibition of cofilin-mediated actin depolymerization, which blocks spine shrinkage, does not inhibit LTD. However, interfering with actin depolymerization with the F-actin-stabilizing drug Jasplakinolide blocks both LTD and spine shrinkage,48 suggesting that AMPAR trafficking does require actin rearrangement, but this may be via a mechanism distinct from the control of spine size. Furthermore, the recent observation that LTP may involve a transient removal of GluR2 from stimulated synapses49,50 obscures a clear mechanism involving GluR2 in spine size regulation. The reduction in GluR2 is suggested to last for around 20 minutes following LTP induction. Stable increases in spine volume have been reported less than ten minutes following stimulation.33
GluR1 subunit has also been implicated in spine morphogenesis. Overexpression of a mutant GluR1 that is not phosphorylated at functionally-relevant sites and therefore does not enter synapses in response to chemical LTP stimuli, blocks LTP-induced spine enlargement.51 Furthermore, this study demonstrated that the isolated C-terminal tail peptide of GluR1 is driven to spines during chemical LTP, and is sufficient to stimulate spine enlargement, suggesting that GluR1 could directly interact with intracellular signaling machinery to bring about appropriate cytoskeletal changes. Further work is needed to identify the downstream pathways regulated by GluR1 that bring about changes in spine morphology. A significant step in this direction may have been provided by Penzes and colleagues who identified the Rac GEF kalirin-7 as a GluR1 interactor.52 Activation of Rac by kalirin leads to spine growth, presumably via the well-known function of Rac1 in regulation of the actin cytoskeleton. However, this study suggests that kalirin-7 controls AMPAR recruitment, rather than AMPARs regulate kalirin,52 so it may not provide the mechanistic explanation for how GluR1 signals to spine morphogenesis. Regulation of spine morphogenesis by kalirin-7 is activated by trans-synaptic ephrin-Eph receptor signalling.53 Although no direct evidence exists for a role of ephrin-Eph signaling in AMPAR trafficking, it has been suggested that this trans-synaptic interaction is involved in LTP.54 Since ephrins, Eph receptors and AMPAR subunit GluR2 can bind the PDZ domain proteins GRIP and PICK1,55 it is possible that recruitment of these proteins to the synapse to regulate AMPAR trafficking may also influence kalirin-7 via ephrin signaling to regulate spine morphogenesis. Additional GluR1 interactors may also play a role in regulating spine morphogenesis. The F-actin-binding protein 4.1N interacts directly with a membrane-proximal region of GluR1, and promotes the surface expression of GluR1-containing AMPARs.56 The PDZ protein SAP97 binds GluR1 and is involved in the forward traffic of AMPARs to synaptic sites.57,58 Both 4.1N and SAP97 have been implicated in regulating spine morphology.57,59 Interestingly, SAP97 binds directly to protein 4.1N,56 suggesting that they may form a functional complex in neurons. However, there is currently no evidence for a direct link between AMPAR trafficking and spine morphogenesis via protein 4.1N, SAP97 or a complex containing both proteins.
A key function of the Rho family GTPases (most commonly Rac1, RhoA, Cdc42) is regulation of the actin cytoskeleton. Rac1 enhances spine size, most likely via regulation of the WAVE complex that stimulates actin polymerization mediated by the Arp2/3 complex.60,61 Rac1 also enhances synaptic clustering of AMPARs in mature spines.62 This study suggests that the effects of Rac1 on AMPAR clustering are not secondary to enhanced spine size, as Rac overexpression induces AMPAR clusters on somatic and dendritic shaft regions of immature non-spiny neurons. In addition, Rac1 enhances AMPAR clustering on pre-existing spines.62 It is possible that the enhanced F-actin induced by Rac1 provides an efficient path for RIL and myosin Va-dependent trafficking of AMPARs to synaptic sites,44,45 or perhaps Rac1 has alternative effects on AMPAR trafficking.
Rap1, a GTPase of the Ras family, is involved in AMPAR trafficking, and triggers the removal of receptors from synaptic sites.63 Rap1 has also been implicated in regulating spine size.64 In this report, NMDAR activation was shown to activate Rap1, resulting in longer, thinner spines with fewer AMPARs, which is consistent with a role for Rap1 activation in AMPAR removal from synapses.63 Inactivation of Rap1 via RapGAP results in larger spines with increased AMPAR content. It is unclear whether Rap1 influences spine morphology directly, or indirectly as a consequence of AMPAR trafficking. The influence of Rap1 on spines is mediated by the PDZ protein AF-6;64 it would be of great interest to directly investigate the role of AF-6 in AMPAR trafficking.
Regulation of spine plasma membrane area
In addition to cytoskeletal dynamics, changes in spine size must also involve changes in the surface area of plasma membrane associated with the postsynaptic compartment; smaller spines must have less plasma membrane than larger spines. An important question therefore, is how are these changes in membrane surface area brought about? It has recently been shown that spine growth stimulated by chemical LTP requires transport of recycling endosomes into spines.14 Disrupting the recycling endosome pathway results in spine retraction, and blocks LTP-induced spine growth. Furthermore, recycling endosomes entering dendritic spines from the shaft, followed by exocytosis at the spine head can be observed during LTP stimuli.14 Since recycling endosomes also provide synapses with GluR1-containing AMPARs during LTP,13 it appears that neurons utilise precisely the same process to deliver both AMPARs and the necessary membrane to house them, at the same time. For this model to work efficiently, AMPAR-containing vesicles must be inserted directly into the spine. As discussed in the previous sections, whether this occurs is still a matter of debate. However, transferrin receptors, not AMPARs were used as a marker for exocytosing cargo from recycling endosomes in this study,14 so the possibility remains that AMPARs are exocytosed to a non-spine region as a separate event from membrane delivery.
It might reasonably be proposed that, in an equivalent manner, LTD stimuli would remove both AMPARs and membrane during the same endocytic event. Evidence has not yet been provided for this, in fact as discussed above, it has recently been suggested that during LTD, endocytosis and spine shrinkage are separate events.48
Concluding Remarks
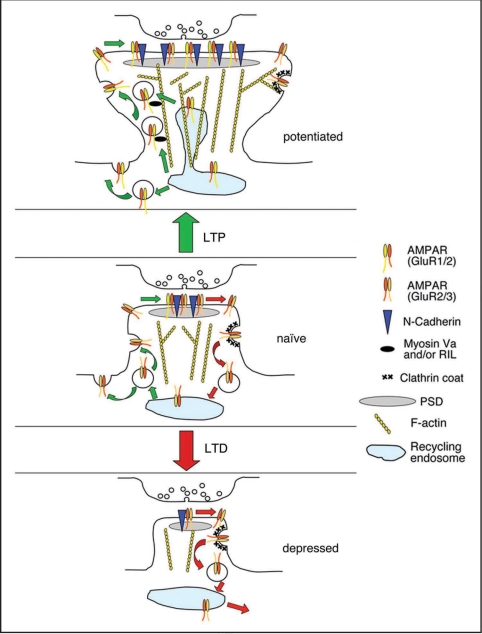
There is a close correlation between dendritic spine size and PSD size65 and between PSD size and AMPAR density at the synapse.66 Therefore small spines have fewer AMPARs. It therefore makes sense for regulation of spine size to be linked in some way to regulation of AMPAR number by vesicle trafficking. A model summarizing this idea is shown in Figure 2. Some initial studies have suggested that this may indeed be the case. Some evidence points towards a model whereby AMPAR trafficking is the primary driving force, and spine size changes as a result of the altered complement of AMPARs at the synapse. Alternatively, direct control of the spine actin cytoskeleton, most likely via small GTPases and their associated actin-regulating machinery, may also be important. This could then influence AMPAR trafficking, which is known to depend on actin dynamics. In addition, spine plasma membrane surface area may be regulated by the very same vesicles that transport AMPARs, providing a further way in which these important neuronal phenomena are linked.
Figure 2.
Relationship between AMPA receptor trafficking, actin dynamics and spine size during synaptic plasticity. Under basal conditions, AMPARs undergo successive rounds of endocytosis, recycling and reinsertion. Spine structure is supported by the underlying actin cytoskeleton. This may in part be maintained by the GluR2/N-Cadherin complex at the PSD. A balance between endocytosis and exocytosis preserves the spine plasma membrane area. During LTP, actin polymerisation is enhanced, resulting in increased F-actin in spines, enlarging the spine head. These actin filaments may provide transport routes for GluR1-containing AMPARs trafficking towards the plasma membrane, via myosin Va or RIL. A shift towards actin polymerization also reduces AMPAR endocytosis. The increase in surface AMPARs leads to spine enlargement via the GluR2/Cadherin complex, possibly by signaling to the actin cytoskeleton. The increase in AMPAR insertion from recycling endosomes contributes additional membrane to the surface of the growing spine. During LTD, a shift towards actin depolymerization results in less F-actin and hence a smaller spine head. Therefore, fewer actin filaments are present to support actin-based trafficking towards the plasma membrane. Actin depolymerization promotes PICK1-mediated AMPAR internalisation. The reduced surface GluR2 contributes to spine shrinkage, possibly via reduced signaling from N-Cadherin. The increase in AMPAR internalization may also result in a net internalization of plasma membrane, reducing the surface membrane of the shrinking spine.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/6510
References
- 1.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 2.Zhu JJ, Esteban JA, Hayashi Y, Malinow R. Postnatal synaptic potentiation: delivery of GluR4containing AMPA receptors by spontaneous activity. Nat Neurosci. 2000;3:1098–1106. doi: 10.1038/80614. [DOI] [PubMed] [Google Scholar]
- 3.Wenthold RJ, Petralia RS, Blahos J, II, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lledo PM, Zhang X, Sudhof TC, Malenka RC, Nicoll RA. Postsynaptic membrane fusion and long-term potentiation. Science. 1998;279:399–403. doi: 10.1126/science.279.5349.399. [DOI] [PubMed] [Google Scholar]
- 5.Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 6.Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, et al. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 7.Luscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, et al. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- 8.Man HY, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, et al. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron. 2000;25:649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- 9.Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, et al. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- 10.Borgdorff AJ, Choquet D. Regulation of AMPA receptor lateral movements. Nature. 2002;417:649–653. doi: 10.1038/nature00780. [DOI] [PubMed] [Google Scholar]
- 11.Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- 12.Kopec CD, Li B, Wei W, Boehm J, Malinow R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J Neurosci. 2006;26:2000–2009. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- 14.Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, et al. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52:817–830. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yudowski GA, Puthenveedu MA, Leonoudakis D, Panicker S, Thorn KS, Beattie EC, et al. Real-time imaging of discrete exocytic events mediating surface delivery of AMPA receptors. J Neurosci. 2007;27:11112–11121. doi: 10.1523/JNEUROSCI.2465-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adesnik H, Nicoll RA, England PM. Photoinactivation of native AMPA receptors reveals their real-time trafficking. Neuron. 2005;48:977–985. doi: 10.1016/j.neuron.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 17.Gerges NZ, Backos DS, Rupasinghe CN, Spaller MR, Esteban JA. Dual role of the exocyst in AMPA receptor targeting and insertion into the postsynaptic membrane. EMBO J. 2006;25:1623–1634. doi: 10.1038/sj.emboj.7601065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanpied TA, Scott DB, Ehlers MD. Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron. 2002;36:435–449. doi: 10.1016/s0896-6273(02)00979-0. [DOI] [PubMed] [Google Scholar]
- 19.Racz B, Blanpied TA, Ehlers MD, Weinberg RJ. Lateral organization of endocytic machinery in dendritic spines. Nat Neurosci. 2004;7:917–918. doi: 10.1038/nn1303. [DOI] [PubMed] [Google Scholar]
- 20.Lu J, Helton TD, Blanpied TA, Racz B, Newpher TM, Weinberg RJ, et al. Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer. Neuron. 2007;55:874–889. doi: 10.1016/j.neuron.2007.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashby MC, De La Rue SA, Ralph GS, Uney J, Collingridge GL, Henley JM. Removal of AMPA receptors (AMPARs) from synapses is preceded by transient endocytosis of extrasynaptic AMPARs. J Neurosci. 2004;24:5172–5176. doi: 10.1523/JNEUROSCI.1042-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- 23.Lee SH, Simonetta A, Sheng M. Subunit rules governing the sorting of internalized AMPA receptors in hippocampal neurons. Neuron. 2004;43:221–236. doi: 10.1016/j.neuron.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Rocca DL, Martin S, Jenkins EL, Hanley JG. Inhibition of Arp2/3-mediated actin polymerization by PICK1 regulates neuronal morphology and AMPA receptor endocytosis. Nat Cell Biol. 2008;10:259–271. doi: 10.1038/ncb1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin DT, Huganir RL. PICK1 and phosphorylation of the glutamate receptor 2 (GluR2) AMPA receptor subunit regulates GluR2 recycling after NMDA receptor-induced internalization. J Neurosci. 2007;27:13903–13908. doi: 10.1523/JNEUROSCI.1750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tardin C, Cognet L, Bats C, Lounis B, Choquet D. Direct imaging of lateral movements of AMPA receptors inside synapses. EMBO J. 2003;22:4656–4665. doi: 10.1093/emboj/cdg463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pickard L, Noel J, Duckworth JK, Fitzjohn SM, Henley JM, Collingridge GL, et al. Transient synaptic activation of NMDA receptors leads to the insertion of native AMPA receptors at hippocampal neuronal plasma membranes. Neuropharmacology. 2001;41:700–713. doi: 10.1016/s0028-3908(01)00127-7. [DOI] [PubMed] [Google Scholar]
- 28.Groc L, Heine M, Cognet L, Brickley K, Stephenson FA, Lounis B, Choquet D. Differential activity-dependent regulation of the lateral mobilities of AMPA and NMDA receptors. Nat Neurosci. 2004;7:695–696. doi: 10.1038/nn1270. [DOI] [PubMed] [Google Scholar]
- 29.Carlisle HJ, Kennedy MB. Spine architecture and synaptic plasticity. Trends Neurosci. 2005;28:182–187. doi: 10.1016/j.tins.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy MB, Beale HC, Carlisle HJ, Washburn LR. Integration of biochemical signalling in spines. Nat Rev Neurosci. 2005;6:423–434. doi: 10.1038/nrn1685. [DOI] [PubMed] [Google Scholar]
- 31.Lippman J, Dunaevsky A. Dendritic spine morphogenesis and plasticity. J Neurobiol. 2005;64:47–57. doi: 10.1002/neu.20149. [DOI] [PubMed] [Google Scholar]
- 32.Bloodgood BL, Sabatini BL. Ca(2+) signaling in dendritic spines. Curr Opin Neurobiol. 2007;17:345–351. doi: 10.1016/j.conb.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuzaki M. Factors critical for the plasticity of dendritic spines and memory storage. Neurosci Res. 2007;57:1–9. doi: 10.1016/j.neures.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol. 2006;16:95–101. doi: 10.1016/j.conb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Schubert V, Dotti CG. Transmitting on actin: synaptic control of dendritic architecture. J Cell Sci. 2007;120:205–212. doi: 10.1242/jcs.03337. [DOI] [PubMed] [Google Scholar]
- 38.Nagerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44:759–767. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 39.Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004;7:1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- 40.Ethell IM, Pasquale EB. Molecular mechanisms of dendritic spine development and remodeling. Prog Neurobiol. 2005;75:161–205. doi: 10.1016/j.pneurobio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Sekino Y, Kojima N, Shirao T. Role of actin cytoskeleton in dendritic spine morphogenesis. Neurochem Int. 2007;51:92–104. doi: 10.1016/j.neuint.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 42.Allison DW, Gelfand VI, Spector I, Craig AM. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J Neurosci. 1998;18:2423–2436. doi: 10.1523/JNEUROSCI.18-07-02423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Q, Xiao M, Nicoll RA. Contribution of cytoskeleton to the internalization of AMPA receptors. Proc Natl Acad Sci USA. 2001;98:1261–1266. doi: 10.1073/pnas.031573798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Correia SS, Bassani S, Brown TC, Lise MF, Backos DS, El-Husseini A, et al. Motor protein-dependent transport of AMPA receptors into spines during long-term potentiation. Nat Neurosci. 2008;11:457–466. doi: 10.1038/nn2063. [DOI] [PubMed] [Google Scholar]
- 45.Schulz TW, Nakagawa T, Licznerski P, Pawlak V, Kolleker A, Rozov A, et al. Actin/alpha-actinin-dependent transport of AMPA receptors in dendritic spines: role of the PDZ-LIM protein RIL. J Neurosci. 2004;24:8584–8594. doi: 10.1523/JNEUROSCI.2100-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Passafaro M, Nakagawa T, Sala C, Sheng M. Induction of dendritic spines by an extracellular domain of AMPA receptor subunit GluR2. Nature. 2003;424:677–681. doi: 10.1038/nature01781. [DOI] [PubMed] [Google Scholar]
- 47.Saglietti L, Dequidt C, Kamieniarz K, Rousset MC, Valnegri P, Thoumine O, et al. Extracellular interactions between GluR2 and N-cadherin in spine regulation. Neuron. 2007;54:461–477. doi: 10.1016/j.neuron.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 48.Wang XB, Yang Y, Zhou Q. Independent expression of synaptic and morphological plasticity associated with long-term depression. J Neurosci. 2007;27:12419–12429. doi: 10.1523/JNEUROSCI.2015-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, et al. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci. 2006;9:602–604. doi: 10.1038/nn1678. [DOI] [PubMed] [Google Scholar]
- 50.Terashima A, Pelkey KA, Rah JC, Suh YH, Roche KW, Collingridge GL, et al. An essential role for PICK1 in NMDA receptor-dependent bidirectional synaptic plasticity. Neuron. 2008;57:872–882. doi: 10.1016/j.neuron.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kopec CD, Real E, Kessels HW, Malinow R. GluR1 links structural and functional plasticity at excitatory synapses. J Neurosci. 2007;27:13706–13718. doi: 10.1523/JNEUROSCI.3503-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, Woolfrey KM, et al. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56:640–656. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Penzes P, Beeser A, Chernoff J, Schiller MR, Eipper BA, Mains RE, et al. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–274. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- 54.Calo L, Cinque C, Patane M, Schillaci D, Battaglia G, Melchiorri D, et al. Interaction between ephrins/Eph receptors and excitatory amino acid receptors: possible relevance in the regulation of synaptic plasticity and in the pathophysiology of neuronal degeneration. J Neurochem. 2006;98:1–10. doi: 10.1111/j.1471-4159.2006.03844.x. [DOI] [PubMed] [Google Scholar]
- 55.Torres R, Firestein BL, Dong H, Staudinger J, Olson EN, Huganir RL, et al. PDZ proteins bind, cluster, and synaptically colocalize with Eph receptors and their ephrin ligands. Neuron. 1998;21:1453–1463. doi: 10.1016/s0896-6273(00)80663-7. [DOI] [PubMed] [Google Scholar]
- 56.Shen L, Liang F, Walensky LD, Huganir RL. Regulation of AMPA receptor GluR1 subunit surface expression by a 4.1N-linked actin cytoskeletal association. J Neurosci. 2000;20:7932–7940. doi: 10.1523/JNEUROSCI.20-21-07932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rumbaugh G, Sia GM, Garner CC, Huganir RL. Synapse-associated protein-97 isoform-specific regulation of surface AMPA receptors and synaptic function in cultured neurons. J Neurosci. 2003;23:4567–4576. doi: 10.1523/JNEUROSCI.23-11-04567.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakagawa T, Futai K, Lashuel HA, Lo I, Okamoto K, Walz T, Hayashi Y, Sheng M. Quaternary structure, protein dynamics, and synaptic function of SAP97 controlled by L27 domain interactions. Neuron. 2004;44:453–467. doi: 10.1016/j.neuron.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 59.Li H, Khirug S, Cai C, Ludwig A, Blaesse P, Kolikova J, et al. KCC2 interacts with the dendritic cytoskeleton to promote spine development. Neuron. 2007;56:1019–1033. doi: 10.1016/j.neuron.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 60.Soderling SH, Guire ES, Kaech S, White J, Zhang F, Schutz K, Langeberg LK, et al. A WAVE-1 and WRP signaling complex regulates spine density, synaptic plasticity, and memory. J Neurosci. 2007;27:355–365. doi: 10.1523/JNEUROSCI.3209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soderling SH, Scott JD. WAVE signalling: from biochemistry to biology. Biochem Soc Trans. 2006;34:73–76. doi: 10.1042/BST0340073. [DOI] [PubMed] [Google Scholar]
- 62.Wiens KM, Lin H, Liao D. Rac1 induces the clustering of AMPA receptors during spinogenesis. J Neurosci. 2005;25:10627–10636. doi: 10.1523/JNEUROSCI.1947-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu Y, Pak D, Qin Y, McCormack SG, Kim MJ, Baumgart JP, et al. Rap2-JNK removes synaptic AMPA receptors during depotentiation. Neuron. 2005;46:905–916. doi: 10.1016/j.neuron.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 64.Xie Z, Huganir RL, Penzes P. Activity-dependent dendritic spine structural plasticity is regulated by small GTPase Rap1 and its target AF-6. Neuron. 2005;48:605–618. doi: 10.1016/j.neuron.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 65.Harris KM, Stevens JK. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J Neurosci. 1989;9:2982–2997. doi: 10.1523/JNEUROSCI.09-08-02982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takumi Y, Ramirez-Leon V, Laake P, Rinvik E, Ottersen OP. Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat Neurosci. 1999;2:618–624. doi: 10.1038/10172. [DOI] [PubMed] [Google Scholar]