Abstract
A lectin from the lichen Evernia prunastri developing arginase activity (EC. 3.5.3.1) binds to the homologous algae that contain polygalactosilated urease (EC. 3.5.1.5) in their cell walls acting as a lectin ligand. The enzyme bound to its ligand shows to be inactive to hydrolyze of arginine. Hydrolysis of the galactoside moiety of urease in intact algae with α-1,4-galactosidase (EC. 3.2.1.22) releases high amount of D-galactose and impedes the binding of the lectin to the algal cell wall. However, the use of β-,4-galactosidase (EC.3.2.1.23) releases low amounts of D-galactose from the algal cell wall and does not change the pattern of binding of the lectin to its ligand. The production of glycosilated urease is restricted to the season in which algal cells divide and this assures the recognition of new phycobiont produced after cell division by its fungal partner.
Key Words: arginase, cell wall, evernia prunastri, lectin ligand, phycobiont, urease
Introduction
Lichens are symbiotic associations between a fungus and a cyanobacterium (cyanolichens) or a green alga (phycolichens), joined to form a new biological entity different from its individual components. Both bionts appear in nature among a mixture of millions of nonsymbiotic microorganisms and they have to select each other for a compatible combination.1 Thus, specificity seems to be required for the lichen association.
Recognition mechanisms used by lichens are based on the production and secretion of fungal lectins as signaling molecules2 which, therefore develop arginase enzymatic activity.3 Several conditions have to be met, according to Galun and Kardish,1 to define a protein as a lectin:
The lectin should be produced by the fungus independently on its symbiotic situation.
It should be a surface protein to possibilite the direct steric contact with potential phycobionts (or cyanobionts).
This contact requires the occurrence of receptor sites on the surface of the potential photobions, to which the lectin molecule binds.
Via this interaction, the lectin should be able to discriminate between compatible and incompatible photobionts.
Compatible photobionts must be in the immediate environment of the potential fungal partner. The receptor of lichen lectins (the ligand) has turned out to be a glycosilate durease.
The lack of the adequate lectin ligand makes incompatible the alga cell. Consequently, the lectin enters the algal cell, the concentration of polyamines increases,4 as derived from the arginase activity of the signaling protein, and these polyamines, mainly putrescine, induce ultrastructure damages such as chloroplast disorganization, hydrolysis of the cell wall and, finally, cell death.5 On the contrary, the occurrence of the ligand at the surface of algae retains the lectin outside the cell and preserves both structure and function of the recognized phycobiont.6
The binding of fungal arginase to glycosylated urease in the algal cell wall implies an affinity mechanism between some specific amino acid residues of the lectin and specific sugar residues of the glycoside moiety of the ligand7,8 that, in Evernia prunastri, is a polygalactose chain attached to the urease polypeptide.9 This mechanism is almost identical to that described for other lectins from different origins.10 In addition, the binding of the lectin to its ligand results in a complete loss of both arginase and urease activities.3 However, the nature of the chemical bond between galactose units in the ligand is yet unknown.
In the present study, some experiments have been performed to find out the class of glycoside linkage between galactose residues of the ligand using the ability of the lectin to bind to the glycoside moiety of the algal urease. If only one from several galactosidases, added to a suspension of algal cells before the contact with the corresponding lectin, incapacitates the cell wall receptor of the phycobiont to be recognized by the fungal lectin, it will turn to be incompatible and the lectin would enter the algal cell.
Material and Methods
Plant material.
The lichen Evernia prunastri (L.) Ach (Parmaliaceae), containing Trebouxia excentrica as a phycobiont and growing on Quercus rotundifolia Willd., was collected in La Quinta (El Pardo, Madrid, Spain) during two different time periods, because urease is a nonconstitutive enzyme and, in natural conditions, it cannot be continuously detected in lichen thalli along the year. In fact, urease activity can only be detected during the end of autumn and the first weeks of the winter, decreases along the spring, completely disappears in the summer and a new biosynthetic process is started at the end of the autumn.11 Then, compatibility defined on the basis of affinity binding between glycosylated arginase and polygatactosylated urease previously required the induction of the cell wall ligand. Urease is currently induced by urea in laboratory conditions.12
Arginase induction.
Samples of 1.0 g of thalli of E. prunastri were floated on 20 ml of 0.1 M Tris-HCl buffer (pH 9.15) containing 40 mM L-arginine for 8 h in the dark. The medium was then filtered through Whatman 3 MM filter paper, dialyzed during 8 h against 10 mM Tris-HCl buffer (pH 9.15) at 4°C and purified according to Legaz et al.13
Assay of arginase activity.
Assay of arginase activity was performed according to Legaz and Vicente14 by coupling to arginase reaction a crystalline urease to break the urea produced into carbon dioxide and ammonia. This last was recovered by microdifussion on sulfuric acid15 and spectrophotometrically measured. Reaction mixtures contained 10 µmol Tris-HCl buffer (pH 9.15), 7.5 µmol maleic acid, 5.0 µmol Mn2+, 0.4 µmol arginine, 8.3 mg crystalline urease and 50 µg protein in a final volume of 2.5 ml. The reaction was carried out at 37°C for 25 min and stopped by addition of 0.5 ml of saturated potassium carbonate solution. Microdifussion of ammonia lasted for 2 h at 37°C. Protein was estimated according to Lowry et al.16 A unit of enzyme activity was defined as 1.0 µmol of ammonia produced per mg protein per min.
Isolation of Evernia prunastri phycobionts.
Phycobionts were isolated from thalli of E. prunastri according to Fontaniella et al.17 Samples of dry thalli (0.5 g ) were rinsed in distilled water to remove superficial contaminations. Samples were then macerated in a mortar with 10 ml distilled water. Homogenates were filtered through six layers of cheese-cloth and both myco and phycobionts were separated by centrifugation in sucrose: potassium iodide and phosphate:sucrose gradients, as described.
Cytochemical detection of urease activity.
Urease breaks urea into NH3 and CO2, this one turns to CO3Co after reacting with CoCl2. Cobalt carbonate produces black and insoluble precipitates of CoS with (NH4)2S, indicating the presence of urease in the phycobiont.18,19
Algal suspension (0.5 ml) was incubated with 0.5 ml of 8% (w:v) Fast-Green in distilled water to block phenols in order to avoid Co2+ chelation.20 The suspension was centrifuged at 10000 × g for 5 min and cells were washed with distilled water. Algae were resuspended in 1.0 ml of 10 mM CoCl2 only, as a control, or in 1.0 ml of 10 mM CoCl2 and 40 mM urea and maintained at 37°C for 30 min. After centrifuging and washing, (NH4)2S was added on each sample, maintained for 30 s newly washed with distilled water and observed under light microscope.
Modifications of algal surface for binding the lectin to the cell wall ligand.
Algal cells isolated from lichen thalli collected during spring (May, 2004) were incubated 40 mM urea during 8 h to induce the cell wall receptor,6 whereas those isolated from lichen thalli collected during the autumn (November, 2004) naturally contained urease in their cell walls. Both algal suspensions (1.0 ml each one) were incubated with 1.0 ml of α- or β-galactosidase (25 units ml−1) whereas a control was incubated with 1.0 ml of 10 mM phosphate buffer during 2 h at 30°C. Cells were removed by centrifugation at 10000 × g for 5 min and supernatants were used to quantify sugars removed by hydrolysis. Galactose was estimated by specific galactose oxidase reaction21 which oxydized the primary alcohol to the corresponding aldehyde coupled to the reduction of dioxygen to hydrogen peroxide.22 The amount of peroxide produced was estimated by coupling to the first reaction a horseradish peroxidase that used guayacol as a substrate.23 Then, algae were incubated with arginase for 2 h at 30°C. Cells were newly removed by centrifugation and arginase activity was assayed in the supernatant.
Labelling of fungal arginase with fluorescein isothiocyanate and binding to phycobiont cells.
Purified arginase was labelled was fluorescein isothiocyanate (FITC) by mixing protein and fluorophore at a ratio of 10 µg FITC per µg of protein for 2 h at 30°C with continuous shaking. The mixture was then dialyzed against 10 mM Tris-HCl buffer (pH 9.15) to remove free fluorophore. All these processes were done in the dark.
Algal cells freshly isolated from samples of 0.5 g of lichen thalli were incubated with α or β-galactosidase (25 units ml−1) and then with fluorescent arginase 2 h at 30°C with shaking. The fraction of protein not retained by algal cells was independently estimated in supernatants for fluorescence emission using a spectrofluorimeter Kontron SF25 (Kontron Instruments A6, Zurich, Switzerland). Samples were excited at 468 nm and the fluorescence emission was measured at 512 nm.6
Then, cells were incubated for 1 h with 100 mM D-galactose to desorb bound arginase, removed by centrifugation and fluorescence emission was measured in the supernatant. Cells recovered after centrifugation were washed with sufficient distilled water and subjected to mechanical disruption with alumin. Homogenates were centrifuged at 12,000 × g for 15 min at 2°C, pellets were discarded and supernatants were used to measure fluorescence emission.
To verify the lectin binding to its cell wall ligand, samples of collected cells were immobilized on a polylysine-coated slide for 12 h, embedded with Mowiol-DABCO (Calbiochem, La Jolla, CA, USA) to avoid fluorescence decay, and examined in a Olympus BX51 fluorescence microscope to visualize the binding of fluorescent arginase to the phycobionts.
Results
Detection of the cell wall ligand for arginase.
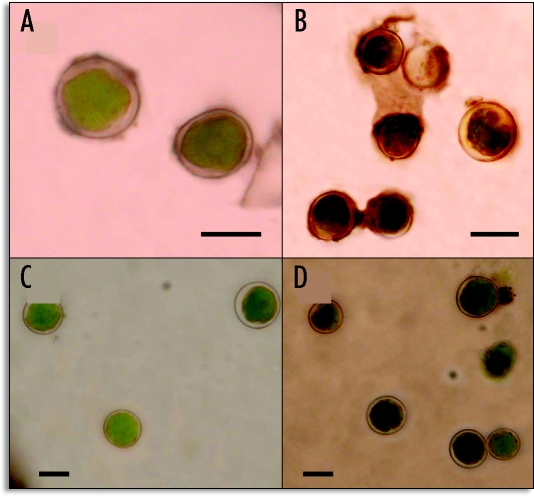
Isolated phycobionts from the lichen E. prunastri collected during spring or autumn were resuspended in 3.0 ml distilled water or 3.0 ml 40 mM urea for 2 h at 30°C. After this, algal cells were recovered by centrifugation and used for the cytochemical assay of urease. Algae from lichen thalli collected in November (Fig. 1A–B) produce deposits of CoS on the cell wall darker than the those collected in May (Fig. 1C and D). although their preincubation on urea enhanced urease detection. Phycobionts from spring samples were green and round with a clear and well-differentiated wall for the untreated sample due to the lack of the cell wall receptor (Fig. 1C). However, the preincubation with urea produced incipient plasmolysis of cells and light black deposits in the wall formed by SCo (Fig. 1D).
Figure 1.
Detection of the lectin ligand (urease) at the cell wall of Evernia phycobionts as a black deposit of CoS. (A) Control without urea and (B) reaction with urea of isolated algae from lichen thalli collected in November. (C) Control without urea and (D) with urea of algae isolated from lichen thalli collected in May. Bar = 5.0 µm.
Binding of lichen arginase to algal cells.
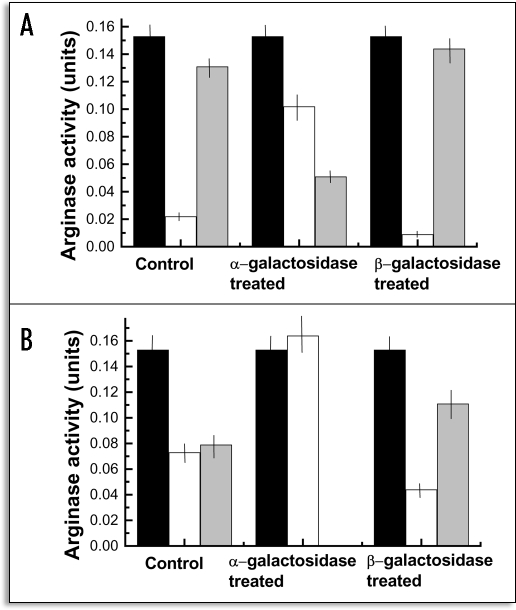
After incubating with arginase untreated algae, just isolated from thalli collected in November, about 85% of the enzyme bound to the ligand since only 13.3% of the total activity was recovered in the supernatant after incubation (Fig. 2). However, the amount of arginase bound to the cells drastically decreased when phycobionts were previously treated with α-1,4-galactosidase before arginase addition, since about 67% of the total activity was recovered in the supernatant after incubation. In contrast, pretreatment of cells with β-1,4-galactosidase provided a amount of bound arginase similar to that found for the control samples (Fig. 2A).
Figure 2.
Loss of arginase activity after binding of the enzyme to the cell wall ligand. (A) binding to algal naturally containing the ligand (collected in November); (B) binding to algal cells containing the ligand after experimental induction by urea (collected in May). Black rectangles: total arginase added to cell suspension; white rectangles, arginase activity recovered in the supernatant after incubation with algal cells; grey rectangles; activity lost after binding to the ligand. Values are the mean of three replicates. Vertical bars give standard error.
Enzymatically-untreated, control cells retained about a half of the total arginase added (Fig. 2B) when algae isolated from thalli collected in the spring were used after urease induction, pretreatment with α-galactosidase completely nullified arginase adhesion to algae whereas the amount of arginase bound to β-galactosidase-treated cells increases up to 73% of the total lectin added (Fig. 2B).
Effect of galactosidase treatment.
To verify the supposed action of both α- and β-galactosidase on isolated phycobionts, the amount of sugar released after enzymatic treatment was measured by specific oxidation of the hexose with galactose oxidase (D-galactose:oxygen 6oxidoreductase). As it is shown in Table 1, the highest amount of galactose was released from algal cells naturally containing the lectin ligand and, in addition, α-galactosidase treatment always released an amount of galactose higher than that produced by β-galactosidase action. In addition, much more galactose was released from algal cells that previously contained the lectin ligand (those collected in November) after treatment with α-galactosidase.
Table 1.
Measurement of galactose released from Evernia phycobiont treated with α- and β-galactosidase
| Photobionts Isolated From | Enzyme Treatment | Galactose (µg ml-1 of Algal Suspension) |
| Thalli with naturally induced ligand | Untreated | 129.6 ± 9.7 |
| α-Galactosidase | 205.2 ± 17.6 | |
| β-Galactosidase | 64.8 ± 5.8 | |
| Thalli without ligand but experimentally induced | Untreated | 57.6 ± 5.3 |
| α-Galactosidase | 90.0 ± 7.9 | |
| β-Galactosidase | 61.2 ± 5.4 |
Binding of FITC-labelled arginase to the phycobiont.
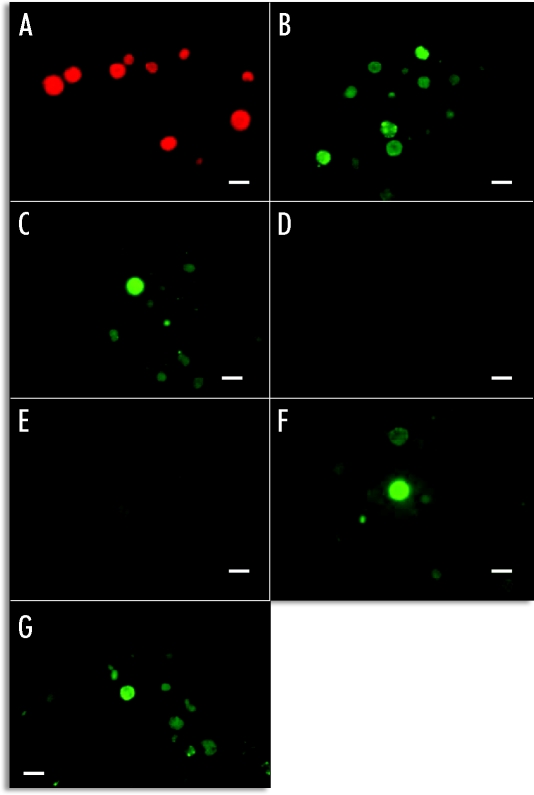
Untreated algae did not show autofluorescence emission from their cell walls in absence of the added labelled arginase (Fig. 3A). Only red fluorescence emitted from their photo-excited chlorophylls could be observed. When algal cells were incubated with FITC arginase, small but numerous dots of intense green fluorescence in their walls were evident (Fig. 3B and C). No fluorescence emission was observed neither from algae containing the lectin ligand experimentally induced by urea (Fig. 3E) nor from those cells that previously contained urease in their cell walls (Fig. 3D) when these algae were treated with α-galactosidase prior to labelled arginase addition. The action of β-galactosidase on the cell wall did not significantly decrease the binding of labelled lectin to its ligand, since algae showed quite fluorescence at microscope (Fig. 3F and G).
Figure 3.
Binding of FITC-arginase to lichen phycobionts. (A) control without incubation with FITC-arginase; (B) phycobionts from thalli collected in November, treated with FITC-arginase and observed during irradiation with blue light; (C) phycobionts from thalli collected in November, previously incubated with urea and, after this, treated with FITC-arginase and observed during irradiation with blue light; (D) the same cells than in B incubated with α-galactosidase, then treated with FITC-arginase and observed during irradiation with blue light;E) the same cells than in (C) incubated with α-galactosidase, then treated with FITC-arginase and observed during irradiation with blue light; (F) the same cells than in (B) incubated with β-galactosidase, then treated with FITC-arginase and observed during irradiation with blue light; (G) the same cells than in (C) incubated with β-galactosidase, then treated with FITC-arginase and observed during irradiation with blue light; Bar = 5.0 µm.
Therefore, the spectrofluorimetric measurement of fluorescence emission after desorbing the labelled lectin with 100 mM D-galactose from galactosidase-untreated cells, isolated from thalli collected in November, indicated that a significant part of labelled arginase was superficially bound to the wall of algae (Fig. 4). About 50% of the FITC-arginase added to recently isolate, untreated cells was retained on the cell wall and practically desorbed by 100 mM D-galactose. However, only a 20% of the total labelled arginase added to α-galactosidase-treated cells was retained, 6% of which could be desorbed by galactose, the rest being recovered after disruption of algal cells (Fig. 4). The patterns of retention by, and desorption from β-galactosidase-treated cells was similar to those found for control cells.
Figure 4.
Binding of FITC-labelled secretable arginase to the cell wall ligand of algae isolated from E. prunastri thalli collected in November. Values are the mean of three replicates. Bars give standard error.
Discussion
Arginase behaves as a lectin for algal recognition.
Currently, the binding of a lectin to its specific ligand is measured by using the protein labelled with a known fluorophore, usually fluorescein or fluorescamine, and visualizing the fluorescence emission from the cell surface after binding. Many plant lectins develop enzymatic activities that are not related to their ability to link to their ligand, and these activity are lost after binding. This has been found for arginase lectins from the lichens E. prunastri6 and Xanthoria parietina.3 In this way, the lost of the corresponding enzymatic activity can be seen as an indirect probe of the binding. In fact, retention of FITC-arginase by Evernia phycobionts, as shown in Figure 4, measured as the decrease of fluorescence emission after the binding of FITC-arginase can be correlated to the lost of arginase activity after binding to the same phycobionts (Fig. 2).
The nature of the glycoside bond of the lectin ligand.
Since arginase lectin binds to the polygalactoside moiety of a cell wall pelletable-urease,6,9 the rationale supporting the treatment of phycobiont cells with α- or β-galactosidases consists to relate the occasional lost of the binding capability of the lectin to the partial of total enzymatic hydrolysis of the polygactoside rest of the ligand. These hydrolases not only break the glycoside bond of disaccarides (lactose, melibiose) or tri- and tetrasaccharides (raffinose, stachyose) containing galactose. Galactomannans, for example, release galactose after incubation with tomato α-galactosidase24 since α-galactosidases specifically hydrolyse the terminal, nonreducing α-D-galactose residues in α-D-galactosides.25 Moreover, β-galactosidase catalyzes degactosylation of an agglutinin-glycoprotein from peanut and release galactose from galactosyl-fetuin.26 Thus, it can be expected that the incubation of Evernia phycobionts with α- or β-galactosidases will produce degalactosylation of some galactosyl proteins in the surface of the cell wall. As it is shown in Table 1, α-galactosidase removes three times more galactose than β-galactosidase from phycobiont cells naturally containing the lectin ligand (collected in November) and 1.5 times more from those containing experimentally induced ligand (collected in May). In parallel to the loss of α-1,4-D-galactosides from the surface of the cell wall, lectin does not bind to its specific ligand (Figs. 2 and 4). The low level of fluorescence emission shown in Figure 4 as well as in Figure 3D and E can be explained if some FITC-arginase molecules enter the cell in absence of the α-1,4-polygalactoside after hydrolysis, very different from the intense fluorescence shown by cells with the labelled lectin adhered to the cell surface. Anyway, lectin enters the cells in some extent even when their cell walls contained the unmodified ligand (Fig. 4). This fact could be due to the occurrence of a limited number of ligand sites in the algal cell wall that saturates at low amount of labelled lectin. According to Legaz et al.6 other minor α-galatosilated proteins different from urease could act as ligands for the lectin after urease induction in laboratory conditions. This hypothesis could be in agree with the results shown in Figure 2A but, as deduced from results shown in Figure 2B, seasonally synthesized urease is the unique ligand for fungal arginase, since the binding of the lectin to isolated algal cells is completely nullified by a previous treatment with specific α-galactosidase. Thus, it can be conclude that glycosilated urease acting as the main (or unique) ligand for fungal arginase contains an α-1,4-D-galactoside attached to the polypeptide.
Recognition of compatible algal cells in symbiosis.
Recognition mechanisms of a compatible phycobiont by its potential fungal partner is absolutely required not only for de novo formation of new associations, but also for the maintenance of the symbiotic equilibrium in the lichen symbiosis. When the algal cells multiply inside a growing thallus, daughter cells are enveloped by fungal hyphae, which recognize new cells as compatible.27 Thus, production of the lectin ligand might be coincident in time with algal reproduction. In natural conditions, urease synthesis by E. prunastri is restricted to the end of the autumn and the first weeks of the winter.11 A study of Flavoparmelia caperata showed that during the period July–September and January–March, there were few dividing cells in the Trebouxia phycobiont population.28 On the other hand, the number of Trebouxia cells from Hypogymnia physodes collected in January was 2.5 times higher than in June and their size average was small whereas from March to the end of October, the majority of cells had reached their highest size.29 Similar seasonnal differences were found by Czezuga and Krukowska30 for Cetraria islandica, Cladonia arbuscula, H. physodes, Parmelia sulcata and Peltigera rufescens. Thus, the maximal production of urease is achieved in parallel to the algal division and this fact must be interpreted as a mechanism that assures the occurrence of the lectin ligand in the cell wall of new algae inside the thallus possibiliting their recognition.
Acknowledgements
This work was supported by a grant from The Ministerio de Ciencia y Tecnología (Spain) BFI2003-06234.
Abbreviations
- FITC
fluorescein isothiocyanate
- Tris-HCl
tris (hydroxymethyl) aminomethane hydrochloride
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=2276
References
- 1.Galun M, Kardish N. Lectins as determinants of symbiotic specificity in lichens. Cryptog Bot. 1995;5:144–148. [Google Scholar]
- 2.Kardish N, Silberstein L, Flemminger N, Galun M. Lectin from the lichen Nephroma laevigatum. Localization and function. Symbiosis. 1991;11:47–62. [Google Scholar]
- 3.Molina MC, Muñiz E, Vicente C. Enzymatic activities of algal-binding protein and its algal cell wall receptor in the lichen Xanthoria parietina. An approach to the parasitic basis of mutualism. Plant Physiol Biochem. 1993;31:131–142. [Google Scholar]
- 4.Molina MC, Vicente C. Correlationships between enzymatic activity of lectins, putrescine content and chloroplast damage in Xanthoria parietina phycobionts. Cell Adh Commun. 1995;3:1–12. doi: 10.3109/15419069509081274. [DOI] [PubMed] [Google Scholar]
- 5.Molina MC, Stocker-Wörgötter E, Turk R, Bajon C, Vicente C. Secreted, glycosylated arginase from Xanthoria parietina thallus induces loss of cytoplasmic material from Xanthoria photobionts. Cell Adh Commun. 1998;6:481–490. doi: 10.3109/15419069809010796. [DOI] [PubMed] [Google Scholar]
- 6.Legaz ME, Fontaniella B, Millanes AM, Vicente C. Secreted arginases from phylogenetically far-related lichens species act as cross-recognition factors for two different algal cells. Eur J Cell Biol. 2004;83:435–446. doi: 10.1078/0171-9335-00384. [DOI] [PubMed] [Google Scholar]
- 7.Fontaniella B, Millanes AM, Vicente C, Legaz ME. Concanavalin A binds to mannose-containing ligand in the cell wall of some lichen phycobionts. Plant Physiol Biochem. 2004;42:773–779. doi: 10.1016/j.plaphy.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Legaz ME, Molina MC, Vicente C. Lichen lectins: Glycoproteins for cell recognition. Current Topics Plant Biol. 2003;3:63–78. [Google Scholar]
- 9.Pérez-Urria E, Vicente C. Purification and some properties of a secreted urease from Evernia prunastri. J Plant Physiol. 1989;133:692–695. [Google Scholar]
- 10.Min W, Dunn AJ, Jones DH. Nonglycosylated pro-concanavalin A is active without polypeptide cleavage. EMBO J. 1992;11:1393–1397. doi: 10.1002/j.1460-2075.1992.tb05174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Legaz ME, Avalos A, de Torres M, Escribano MI, González A, Martín-Falquina A, Pérez-Urria E, Vicente C. Annual variations in arginine metabolism and phenolic content of Evernia prunastri. Environ Exp Bot. 1986;26:385–396. [Google Scholar]
- 12.Vicente C, Legaz ME. Lichen enzymology. In: Galun M, editor. Handbook of Lichenology. Vol. 1. Boca Raton, FL: CRC Press; 1988. pp. 239–284. [Google Scholar]
- 13.Legaz ME, Vicente C, Pedrosa MM. Binding of lichen phenolics to purified secreted arginase from the lichen Evernia prunastri. J Biochem Mol Biol. 2001;34:194–200. [Google Scholar]
- 14.Legaz ME, Vicente C. Two forms of arginase in Evernia prunastri. Biochem Biophys Res Commun. 1982;104:1441–1446. doi: 10.1016/0006-291x(82)91411-5. [DOI] [PubMed] [Google Scholar]
- 15.Conway EJ. Microdiffusion Analysis and Volumetric Error. London: Crosby Loockwood; 1962. pp. 162–165. [Google Scholar]
- 16.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 17.Fontaniella B, Molina MC, Vicente C. An improved method for the separation of lichen symbionts. Phyton (Austria) 2000;40:323–328. [Google Scholar]
- 18.Gomori G. Microscopic Histochemistry. Chicago IL: The University of Chicago Press; 1952. p. 219. [Google Scholar]
- 19.Millanes AM, Fontaniella B, García ML, Solas MT, Vicente C, Legaz ME. Cytochemical location of urease in the cell wall of two different lichen photobionts. Tissue and Cell. 2004;36:373–377. doi: 10.1016/j.tice.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Lison L. Histochemie et Cytochemie Animales. Paris: Gauthier Villard; 1969. p. 6572. (Fre). [Google Scholar]
- 21.Aisaka K, Uwajima T, Terada O. Kinetic properties of galactose oxidase from Gibberella fujikuroi. Agric Biol Chem. 1984;48:1425–1431. [Google Scholar]
- 22.Whittaker JW. The radical chemistry of galactose oxidase. Arch Biochem Biophys. 2005;433:227–239. doi: 10.1016/j.abb.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 23.Caffaro SV, Mateos JL, Vicente C. Changes in the activity of an enzymatic marker bound to plasmalemma during the photoperiodic flowering induction of soybean. Phyton (Austria) 1996;36:9–28. [Google Scholar]
- 24.Pressey R. Tomato α-galactosidases: Conversion of human type B erythrocytes to type O. Phytochemistry. 1984;23:55–58. [Google Scholar]
- 25.Tulsiani DRP, Skudlarek MD, Orgebin-Christ MC. Purification and characterization of two forms of β-galactosidase from rat epididymal luminal fluid: Evidence for their role in the modification of sperm plasma membrane glycoproteins. Biochem. J. 1995;305:41–50. doi: 10.1042/bj3050041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibuya H, Kobayashi H, Yoshida S, Kaneko S, Park GG, Kusakabe I. Purification and characterization of recombinant Mortierella vinacea α-galactosidases I and II expressed in Saccharomyces cerevisiae. Biosci Biotechnol Bioeng. 1999;67:1465–1471. doi: 10.1271/bbb.63.1096. [DOI] [PubMed] [Google Scholar]
- 27.Ahmadjian V. The Lichen Symbiosis. New York, NY: John Wiley and Sons; 1993. pp. 85–87. [Google Scholar]
- 28.Slocum RD, Ahmadjian V, Hildreth KC. Zoosporogenesis in Trebouxia gelatinosa: Potential for zoospore release and implications for the lichen association. Lichenologist. 1980;12:173–187. [Google Scholar]
- 29.Fiechter E, RHonegger R. Seasonal variations in the fine structure of Hypogymnia physodes (lichenized Ascomycetes) and its Trebouxia photobionts. Plant System Evol. 1988;158:249–263. [Google Scholar]
- 30.Czeczuga B, Krukowska KR. Effect of habitat conditions on phycobionts and the content of photosynthesising pigments in five lichen species. J Hattori Bot Lab. 2001;90:293–305. [Google Scholar]