Abstract
Consuming omega-3 fatty acids (ω-3 FA) during pregnancy and lactation benefits fetal and infant brain development and might reduce the severity of preterm births by prolonging pregnancy. However, diets that are relatively rich in ω-3 FA can adversely affect fetal and infant development and the auditory brainstem response (ABR), a measure of brain development and sensory function. We previously examined the offspring of female rats fed excessive, adequate or deficient amounts of ω-3 FA during pregnancy and lactation. The 24-day-old offspring in the Excess group, compared to the Control group, had postnatal growth retardation and poor hearing acuity and prolonged neural transmission times as evidenced by the ABR. The Deficient group was intermediate. The current study followed these offspring to see if these poor outcomes persisted into young adulthood. Based on prior findings, we hypothesized that the Excess and Deficient offspring would “catch-up” to the Control offspring by young adulthood. Female Wistar rats received one of the three diet conditions from day 1 of pregnancy through lactation. The three diets were the Control ω-3 FA condition (ω-3/ω-6 ratio ~ 0.14), the Excess ω-3 FA condition (ω-3/ω-6 ratio ~ 14.0) and Deficient ω-3 FA condition (ω-3/ω-6 ratio ~ 0% ratio). The Control diet contained 7 % soybean oil; whereas the Deficient and Excess ω-3 FA diets contained 7% safflower oil and 7% fish oil, respectively. One male and female offspring per litter were ABR-tested as young adults using tone pip stimuli of 2, 4, 8 and 16 kHz. The postnatal growth retardation and prolonged neural transmission times in the Excess and Deficient pups had dissipated by young adulthood. In contrast, the Excess group had elevated ABR thresholds (hearing loss) at all tone pip frequencies in comparison to the Control and Deficient groups. The Deficient group had worse ABR thresholds than the Control group in response to the 8 kHz tone pips only. The Excess group also had ABR amplitude-intensity profiles suggestive of hyperacusis. These results are consistent with the Barker hypothesis concerning the fetal and neonatal origins of adult diseases. Thus, consuming diets that are excessively rich or deficient in ω-3 FA during pregnancy and lactation seems inadvisable because of risks for long-lasting adverse effects on brain development and sensory function.
Keywords: auditory brainstem response (ABR), Barker hypothesis, docosahexanoic acid (DHA), eicosapentanoic acid (EPA), fetal programming, fish oil, hearing loss, hyperacusis, lactation, omega-3 fatty acids (ω-3 FA), omega-6 fatty acids (ω-6 FA), over-nutrition, postnatal, pregnancy, prenatal
1. Introduction
Omega-3 fatty acids (ω-3 FA) are “essential fatty acids” because it is necessary to acquire them through food consumption. Docosahexanoic acid (DHA) and eicosapentanoic acid (EPA) are the chief ω-3 FA used as dietary supplements, usually in the form of fish oil. Maternal consumption of ω-3 FA during pregnancy and lactation can influence fetal and infant health and development. Regarding pregnancy, some studies reported that diets rich in ω-3 FA can increase birth weight and prolong pregnancy, thereby reducing the incidence and severity of preterm births and low birth weight infants [49,50,60,65]. Regarding lactation, the ω-3 FA in a mother’s milk or in fortified infant formulas improve neurocognitive and visual development during the first year of life in comparison to infants who receive infant formula without ω-3 FA supplementation [2,9,33]. Thus, increasingly higher ω-3 FA doses are being recommended for pregnant women and nursing babies for advancing the health and development of preterm, low birth weight, and even normal infants [1,36,50].
Even though moderate amounts of ω-3 FAs are beneficial to the developing fetus and infant, there is new evidence that too much can be harmful. For example, several human studies found decreased gestational length and/or fetal growth retardation [25,26,48,51,58,68] and increased infant morbidity [68] from high fish oil consumption by the mother during pregnancy. Adverse effects from high ω-3 FA consumption by infants drinking formulas fortified with ω-3 FA include reduced body growth and head circumference [12,37,41], decreased blood arachidonic acid (AA) levels [8] and decreased verbal skills [40,62].
Animal studies report adverse effects as well. For example, prenatal and/or postnatal dietary supplementation with large amounts of ω-3 FA or a high ω-3/ω-6 FA ratio (due to low amounts of ω-6 FA) can result in reduced birth weight, postnatal growth impairment, increased pre- and postnatal mortality, decreased brain sizes, decreased AA levels, and/or abnormal neurobehavioral function [3,18,30,56,70] and abnormal retinal function and structure [38,74]. Studies using the auditory brainstem response (ABR), a measure of brain development and sensory function, found that high levels of dietary ω-3 FA supplementation in pregnant and lactating rats caused the offspring to have prolonged ABR wave latencies [19,30,59,67], delayed acoustic startle reflexes [30,59,67], reduced auditory acuity [18], and evidence suggesting impaired brain myelination [30] when tested as postweanling pups. Effects from pre- and postnatal diets that are rich in ω-3 FA are similar to those caused by ω-3 FA deficiency or a low ω-3/ω-6 ratio (due to ω-6 FA excess) which include impaired visual function [7,74], learning deficits, decreased brain weight and/or altered nerve FA composition [7,9,33,69,71] and ABRs indicating a faster aging brain in old age [6].
Unfortunately, the caveat that rich ω-3 FA diets can be harmful to the fetus and infant is being ignored [19]. Adverse fetal and neonatal environments can “program” the offspring for adult-onset health disorders [4,22]. Yet no one has investigated the possibility that nutritional toxicity from high levels of dietary ω-3 FA or a high ω-3/ω-6 ratio can cause fetal programming of adult-onset disorders, with the exception of a study which found altered body fat composition in adult rats born to dams receiving diets rich in ω-3 FA during pregnancy and lactation [35]. Thus, further research on the potentially harmful effects of prenatal and postnatal ω-3 FA excess and deficiency is rather important, particularly in terms of the long-term consequences.
With these issues in mind, our study’s primary goal was to investigate the possibility that maternal consumption of diets that are excessively rich or deficient ω-3 FA during pregnancy and lactation cause long-term impairment of the offspring’s nervous system as evidenced by the ABR. In prior studies, we found that our “ω-3 FA excess” condition caused postnatal growth retardation, hearing loss [18] and delayed neurotransmission times [19] in 24-day old rat offspring. The current study followed these offspring longitudinally to see if these poor outcomes persisted into young adulthood. A recent study on ω-3 FA deficiency during pregnancy found that the rat offspring had abnormal ABRs as pups, which became normal in young adulthood, then became abnormal again in old adulthood [6]. Others have found this same age-dependent pattern in neurological outcomes following prenatal or neonatal exposure to various brain damaging substances [5,34,43,44,47,61,73]. Consequently, we hypothesized that our ω-3 FA excess and deficient offspring would show normalization of their ABRs during young adulthood.
2. Methods
2.1. Animals and diets
Wayne State University’s animal investigation committee approved the procedures for this study. Institutional and NIH guidelines were followed.
Details of our procedures are detailed elsewhere [18,19]. Briefly, female Wistar rats, 10 weeks of age, were mated individually with male Wistar rats. The presence of a sperm plug was designated as gestational day one. The females were then placed in separate polycarbonate cages (25 × 45 × 20 cm) and randomly assigned to one of the three diet conditions starting from day 1 of pregnancy through the entire period of pregnancy and lactation. The three diets were the Control ω-3 FA condition (ω-3/ω-6 ratio ~ 0.14), the Deficient ω-3 FA condition (ω-3/ω-6 ratio ~ 0% ratio) and the Excess ω-3 FA condition (ω-3/ω-6 ratio ~ 14.0). The number of pregnant dams/litters in the Control, Deficient and Excess conditions were n = 23, 31 and 22, respectively. The Deficient group had slightly more pregnant dams than the other two groups because of a better pregnancy success rate for unknown reasons. The Control diet contained 7% soybean oil. The Deficient ω-3 FA diet contained 7% safflower oil in place of soybean oil. The Excess ω-3 FA diet contained 7% menhaden oil (a type of fish oil) in place of soybean oil. Our rationale for these dose selections is detailed elsewhere [18,19]. Briefly, we considered this an excess ω-3 FA diet because it had a ω-3/ω-6 ratio that was 100 times what is adequate for pregnant and lactating rats and because our diet of 7% fish oil is consistent with other studies on “excess ω-3 FA” reporting adverse developmental effects (see Introduction). All diets were formulated according to AIN-93G standards which have determined that the ω-3/ω-6 ratio ~ 0.14 and 7% oil composition is ideal for pregnant and lactating female rats [50]. Fish oil was selected for the Excess diet because of its use in clinical studies (see Introduction). The soybean and safflower oils in the Control and Deficient diets were selected because they are commonly consumed by humans and in animal studies. We used the naturally occurring fatty acid profiles of the fish, soybean and safflower oils; nothing was artificially altered. The diets were prepared by Dyets Inc (Bethlehem, PA). All three diets contained tertiary-butylhydroquinone (TBHQ) because this preservative prevents oxidation [23,24,55]. Diets were stored at refrigeration temperatures and fresh diet was provided twice weekly to further protect against oxidation. Each diet provided 3.96 kcal/g. Detailed composition of each diet was recently published in this journal [18,19].
Dams had free access to food and water. Food consumption was assessed twice weekly when the old food was discarded and replaced by fresh diet. Dams were weighed on these days as well. Animals were housed at ~53% relative humidity and at ~22ºC room temperature. Within 24 hours after delivery, designated as postnatal day one (PND 1), litters were counted, weighed and reduced to 8 pups per litter, consisting of 4 male and 4 female pups when possible. The remaining pups were euthanized by CO2 exposure and decapitated to ensure death. The retained pups were weaned on PND 21 and kept on their respective experimental diets until the day of ABR testing on PND 24. After PND 24, all offspring channeled into our ABR study were switched to a standard rodent diet (5001 Rodent Diet, PMI Nutrition International).
2.2. ABR procedure
When possible, one male and one female pup/litter were randomly selected for testing in the current study. The other littermates were channeled into a study that analyzed their fatty acid tissue composition [35]. Using male/female littermate pairs allowed assessment of sex-dependent differences and controlled for within-litter effects by limiting the number of pups tested from any one litter. Of the 23 Control litters, 21 litters had both a male and female offspring that were ABR-tested whereas one litter had just one male and one litter had just one female offspring that were tested for a total of n = 44 offspring. Of the 31 Deficient litters, 30 litters had both a male and female offspring whereas one litter had just one female offspring for a total of n = 61 offspring. Of the 22 Excess litters, 21 litters had both a male and female whereas one litter had just one male offspring for a total of n = 43 offspring. Rat offspring were initially tested on PND 24 and those test results were previously reported [18,19]. These same offspring were subsequently retested as young adults and those results are reported in this article. The animals were fully mature young adults, aged 167–178 days (95% confidence interval) at the time of retesting. The rat ABR is fully mature and stable by approximately 70–100 days of age [20] and age-related hearing loss (presbycusis) usually does not occur in rats until about 17 months [15].
Our ABR procedure is detailed elsewhere [16,18]. Prior to ABR recording, each animal was given 100 mg/kg of the anesthetic ketamine (i.p.). Ketamine influences ABR latencies and amplitudes, but the effects are minor and the ABR quality is excellent [17]. Rectal temperature was monitored because temperature can influence the ABR [57] (Model 43TD, Yellow Springs Instruments Co., Yellow Springs, Ohio 45387, USA). A water-circulating heating pad was used to regulate and maintain normothermia by raising or lowering the temperature of the circulating water (Model TP500, Gaymar Industries, Orchard Park, New York 14127, USA).
The ABR was differentially recorded between two subcutaneous platinum E-2 needle electrodes. The active electrode was inserted at the vertex, the reference electrode below the left ear, and the ground electrode below the right ear. Evoked potentials were collected by a Biologic Navigator and amplified 300,000 times with a digital bandpass of 300–3000 Hz. Electrode impedances ranged from 0–9 kΩ. At least 256 responses were averaged. Recordings were made in an electrically shielded, double-walled sound attenuation chamber (Allotech, Inc., Raleigh, North Carolina 27603, USA). Binaural, ‘open field’ tone pips in the ascending order of 2000 Hz, 4000 Hz, 8000 Hz and 16000 Hz were delivered through a TDH-39P headphone positioned in front of the animal (rise/fall time = 0.5 msec, plateau = 10.0 msec, polarity = alternating, repetition rate = 19.0/sec, stimulus intensity = 15 to100 dB peSPL).
2.2.1. ABR latencies (neural transmission times)
The ABR is a series of action potentials and postsynaptic potentials. The rat ABR is composed of four components (labeled P1 to P4) occurring within 6 msec of stimulus onset [15,17,18,20]. Although the neurogenerators of the rat’s ABRs have not been determined, in the mouse they reflect neural activity chiefly from the auditory nerve (P1), the cochlear nucleus (P2), the superior olivary complex (P3), and the lateral lemniscus and/or inferior colliculus (P4) [31]. The latency of each ABR component was measured as the time from the computer’s triggering of the earphone to a wave’s positive peak, including a 0.3 msec acoustic transit time between the earphone and the animal’s pinnae. Two experimenters, who were ‘blind’ as to each animal’s treatment condition, scored the latencies of ABR waves P1, P2, P3 and P4. When scorers disagree (rarely), the scores are averaged. The primary outcome variable was the P4 latency, a measure of neural transmission time along the auditory nerve and brainstem auditory pathway inclusively, in response to the 100 dB stimuli. The secondary outcomes were the latencies of the individual ABR waves and the P1 to P4 interpeak latency (P1-P4 IPL). The P1-P4 IPL measures the brainstem portion of neural transmission by excluding the auditory nerve transmission time.
2.2.2 ABR thresholds (hearing acuity)
ABR thresholds were determined by the method of limits [16,18]. Here, serial ABRs were gathered to a range of stimulus intensities starting at 100dB, then descending to 80, 60, 50, 40, 35, 30, 25, 20, and 15 dB as the ABR threshold was reached and passed. To establish ABR threshold more precisely, 2 and 3 dB changes in stimulus intensity levels were tested around the ABR’s threshold (as determined by visual detection) and multiple ABR traces (2 to 5) were collected at each near-threshold intensity level. Threshold was defined as the lowest intensity to elicit a reliably scored ABR component. An experimenter, who was ‘blind’ as to each animal’s treatment condition, scored the ABR thresholds. A second experimenter then checked the threshold scoring for reliability purposes.
2.2.3 ABR latency-intensity profiles
An ABR latency-intensity (L-I) profile can help determine if a subject’s hearing loss is a conductive hearing loss (CHL) or a sensorineural hearing loss (SNHL). A subject with a CHL would have an elevated ABR threshold and an L-I profile that is displaced upward and parallel to the normal curve. A subject with a SNHL will also have an elevated ABR threshold, however the P2 latencies will typically be normal or near normal in response to loud stimulus intensities but progressively curve upward from the normal range as the stimulus intensity decreases [16]. In rodents, one typically uses the ABR’s P2 wave to derive an animal’s latency-intensity (L-I) profile because the rodent’s P2 wave is the largest wave and the last wave to disappear as the sound stimulus is decreased [16]. P2 latency was measured at the positive peak of this waveform at each stimulus intensity level.
2.2.4 ABR amplitude-intensity profiles
The amplitude of the P2 waveform was used as an index of the ABR’s maximum amplitude because the rodent’s P2 wave is the largest wave and the last wave to disappear as the sound intensity is decreased. P2 amplitude was measured from the positive peak of wave P2 to the subsequent negative trough (labeled N2). This was done at each stimulus intensity level in order to derive amplitude-intensity (A-I) profiles. Whereas the ABR amplitude is a function of neural synchrony and the number of neural units firing, the ABR amplitude can provide diagnostic information on these neural functions.
2.3. Data analysis
Analyses of variances (ANOVA) were used to assess statistical significance. Because we were interested in gender-dependent differences, male and female littermates were treated as individual units of measure. For the ABR variables, we used a three-way ANOVA to test for the effects of Diet, Sex and Tone Pip Frequency. Because Tone Pip was a within-subjects measure, the Greenhouse-Geisser adjustment was used with this variable’s main effects and interactions. Because we sampled from each litter in a highly balanced manner, using only one male and one female from each litter with only rare exception (see 2.2. ABR procedure), it was unnecessary to control for litter-dependent effects by using litter as a variable in the statistical design. If an ANOVA indicated a significant treatment (Diet) effect (p < 0.05), the Bonferroni test was used to make planned pair-wise comparisons between treatment groups. Two-sided tests were used to compare all groups with each other because we hypothesized that they would not differ.
Simple regression analyses were used to see if the ABR thresholds gathered when the offspring were pups (24 days of age) correlated with (predicted) their ABR thresholds gathered when they were young adults. To simplify data analyses, the ABR thresholds in response to the 2, 4, 8 and 16 kHz tone pips were averaged to derive one threshold value for each offspring when it was a pup. This procedure was repeated for each offspring’s young adult threshold data. Details about the pup threshold data for the current set of offspring are published elsewhere [18].
3. Results
3.1. Maternal and birthing outcomes
Maternal and birthing data were reported previously [18,19,35]. There were no group differences in gestational length, maternal weight gain, food consumption during pregnancy or lactation, the number of pups per litter, litter or pup weights at birth, or age of teeth eruption. There was a trend for increased postnatal mortality between birth and weaning for the Excess offspring and their pinna detachment was delayed. On PND 24, we weighed the male/female littermate pairs that were channeled into the ABR study. The respective weights of these Control, Deficient and Excess male pups were 65.3 ± 1.9, 59.8 ± 2.1 and 51.0 ± 2.4 g (mean ± SEM). The respective weights of these Control, Deficient and Excess female pups were 61.3 ± 1.5, 56.1 ± 1.7 and 48.4 ± 2.1 g. The ANOVA indicated a significant effect for Diet group: F(2, 144) = 21.20, p < 0.001. Post hoc comparisons indicated that the Excess male and female pups weighed significantly less than their Control and Deficient cohorts and that the Deficient female pups weighed less than their Control cohorts. Males weighed more than females: F(1, 144) = 4.36, p = 0.038. There was no Diet-by-Sex interaction: F(2, 144) = 0.06, p = 0.94.
3.2. Adult offspring characteristics
The Control, Deficient and Excess offsprings’ respective ages during the ABR recording session were 170 ± 37, 174 ± 34 and 174 ± 33 g, their body temperatures were 37.6 ± 0.4, 37.5 ± 0.4 and 37.6 ± 0.4 °C, the male weights were 653 ± 76, 641 ± 72 and 614 ± 93 g, and the female weights were 418 ± 51, 400 ± 41 and 396 ± 57 g (mean ± SEM). There were no significant Diet group differences in testing age: F (2, 142) = 0.25, p = 0.78, rectal temperature: F (2, 142) = 0.27, p = 0.77, male body weight: F (2, 71) = 1.44, p = 0.24, or female body weight: F (2, 71) = 1.37, p = 0.26. There were no significant effects for Sex or the Sex-by-Diet, except for males weighing more than females: F (1, 142) = 437.61, p < 0.001.
3.3. ABR latencies (neural transmission times)
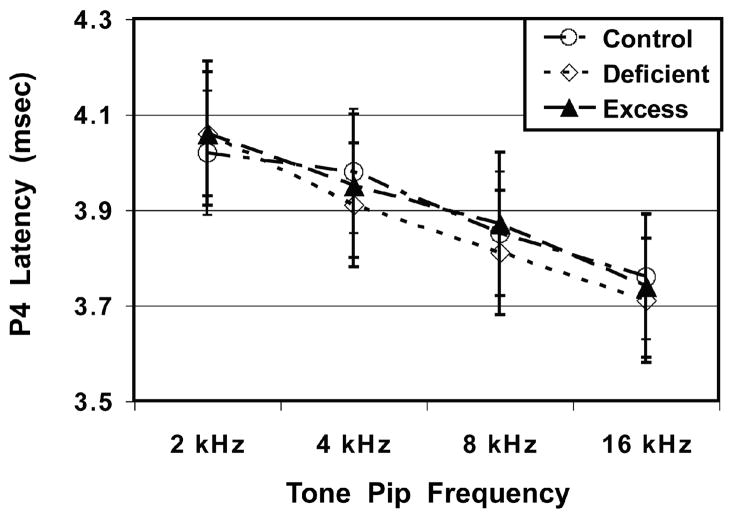
Figure 1 shows neural transmission times (P4 latency) as functions of Diet group and Tone Pip Frequency. There were no significant differences as functions of Diet group: F (2, 142) = 1.20, p = 0.30, Sex: F (1, 142) = 0.25, p = 0.62, or the Diet-by-Sex interaction: F (2, 142) = 0.41, p = 0.66. There was a significant main effect for Tone Pip Frequency, indicating that P4 latency became gradually shorter (faster) as the tone pip frequency progressed from 2 to 16 kHz: F (3, 426) = 261.29, p < 0.001. No significant Diet or Sex effects were found for the secondary outcome variables of P1, P2 or P3 latencies or the P1-P4 IPL.
Fig. 1.
Mean (± SEM) latencies of the ABR’s P4 waveform as functions of Diet group and Tone Pip Frequency. The ω-3 FA Deficiency and Excess diet conditions did not influence P4 latencies in young adult offspring.
3.4. ABR thresholds (hearing acuity)
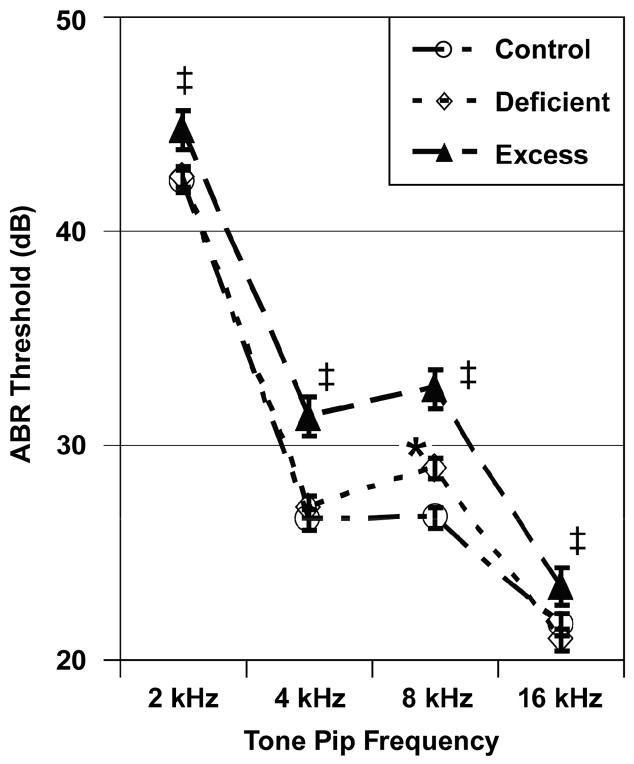
Figure 2 shows ABR thresholds as a function of Diet group and Tone Pip Frequency. The ANOVA indicated a significant effect for Diet group: F (2, 142) = 19.40, p < 0.001. Pairwise comparisons indicated that the Excess group had higher (worse) ABR thresholds than the Control and Deficient groups at all tone pip frequencies. The Deficient group had a significantly higher threshold than the Control group only during the 8 kHz tone pip condition. There were no significant effects for Sex: F (1, 142) = 3.096, p = 0.08 or the Diet-by-Sex interaction: F (2, 142) = 0.25, p = 0.78. There was a significant effect for Tone Pip Frequency, reflecting that rats have progressively better hearing acuity as the tonal frequency progresses from 2 to 16 kHz [16,18]: F (3, 426) = 891.52, p < 0.001. There was a significant interaction between Diet group and Tone Pip Frequency, indicating that Diet group differences were more dramatic in response to the 4 and 8 kHz than to the 2 and 16 kHz tone pip conditions: F (6, 42) = 3.49, p = 0.003.
Fig. 2.
Mean (± SEM) ABR thresholds as functions of Diet group and Tone Pip Frequency. The Excess group had elevated thresholds in comparison to the Control and Deficient groups at all tone pip frequencies. The Deficient group had a significantly elevated threshold in comparison to the Control group only at 8 kHz. Footnotes: ‡ Different from Control and Deficient groups (p < 0.05); * Different from Control group (p < 0.05).
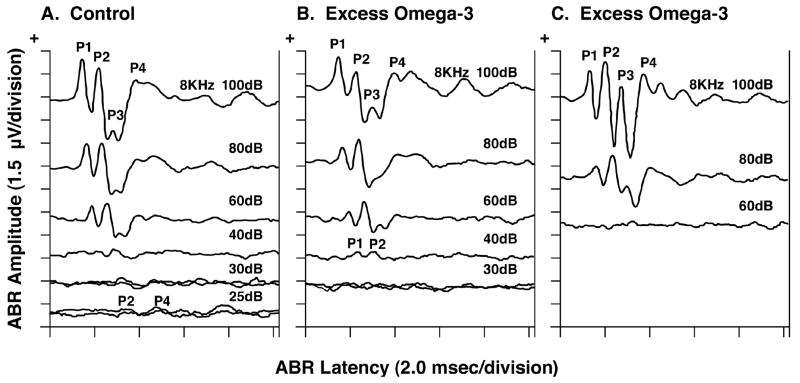
Figure 3 shows serial ABRs in response to 8 kHz tone pips of descending stimulus intensity from representative young adult offspring in the Control and Excess diet groups. The Control animal (panel A) had an ABR still present at 25 dB, whereas the first Excess animal (panel B) had an ABR at 40 dB but not at 30 dB and the second Excess animal (panel C) had an ABR at 80 dB but not at 60 dB. These two Excess animals therefore had elevated ABR thresholds (technically defined as ≥ 2 standard deviations above the Control group’s mean), suggesting respective hearing losses of 15 dB and ≥ 35 dB. Several animals in the Deficient group (not shown) had elevated ABR thresholds very similar to those shown in panel B of Figure 3, but only in response to the 8 kHz tone pips. The Deficient group had normal ABR thresholds at the remaining tone pip frequencies of 2, 4 and 16 kHz.
Fig. 3.
Serial ABRs in response to 8 kHz tone pips of descending stimulus intensity from representative young adult offspring in the Control and the ω-3 FA Excess diet groups. The Control animal (panel A) had an ABR still present at 25 dB, whereas the first Excess animal had an ABR at 40 dB but not at 30 dB (panel B) and the second Excess animal did not have an ABR even at 60 dB (panel C).
3.5. ABR latency-intensity (L-I) profiles
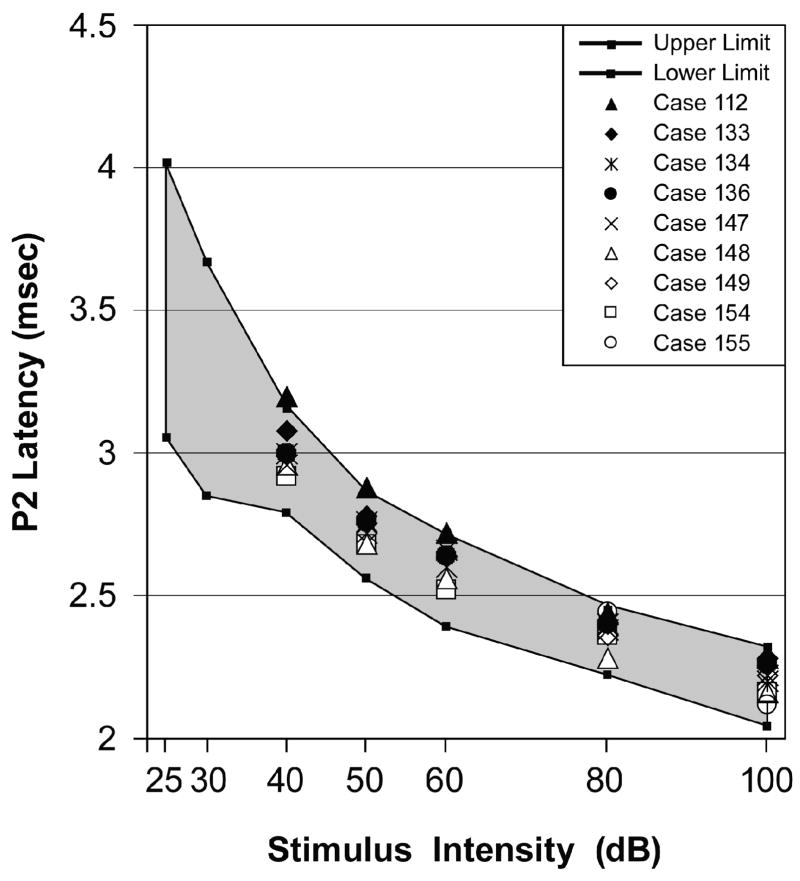
As mentioned in the Methods section, the L-I profile can help diagnose whether a subject’s hearing loss is either CHL or SNHL. Figure 4 shows the L-I profiles in response to the 8 kHz tone pip condition for each animal in the Excess group that had a significantly elevated ABR threshold. A significant elevation in the ABR threshold was defined as being ≥ 2 standard deviations (SD) above the Control group mean [16]. The shaded region in Figure 4 is the range of normalcy derived from Control data. Figure 4 shows that the L-I profiles from all Excess animals with elevated ABR thresholds fell within the range of normalcy, indicating an absence of the typical L-I patterns that are diagnostic of CHL and SNHL. These animals did not have ABRs below 40 dB, however. Results from the 2, 4 and 16 kHz tone pip conditions (not shown) were highly similar to those illustrated in Figure 4.
Fig. 4.
ABR latency-intensity (L-I) profiles for each Excess animal that had an elevated ABR threshold in response to the 8 kHz tone pip condition. The shaded region is the range of normalcy for the P2 wave’s latency (mean ± 2 SD) as derived from the Control group data. Each of these Excess animals had L-I profiles that fell within the range of normalcy with the exception that they did not have ABRs below 40 dB.
3.6. ABR amplitude-intensity (A-I) profiles
The Excess group had elevated ABR thresholds without exhibiting either SNHL or CHL patterns in their L-I profiles. This suggested that the Excess group merely had small amplitude ABRs. To investigate this possibility, we measured the ABR’s P2-N2 amplitude. This waveform was chosen as an index of the ABR’s amplitude for reasons described in the Methods section. We expected the Excess group to have abnormally low ABR amplitudes across the complete range of stimulus intensities under all four tone pip conditions.
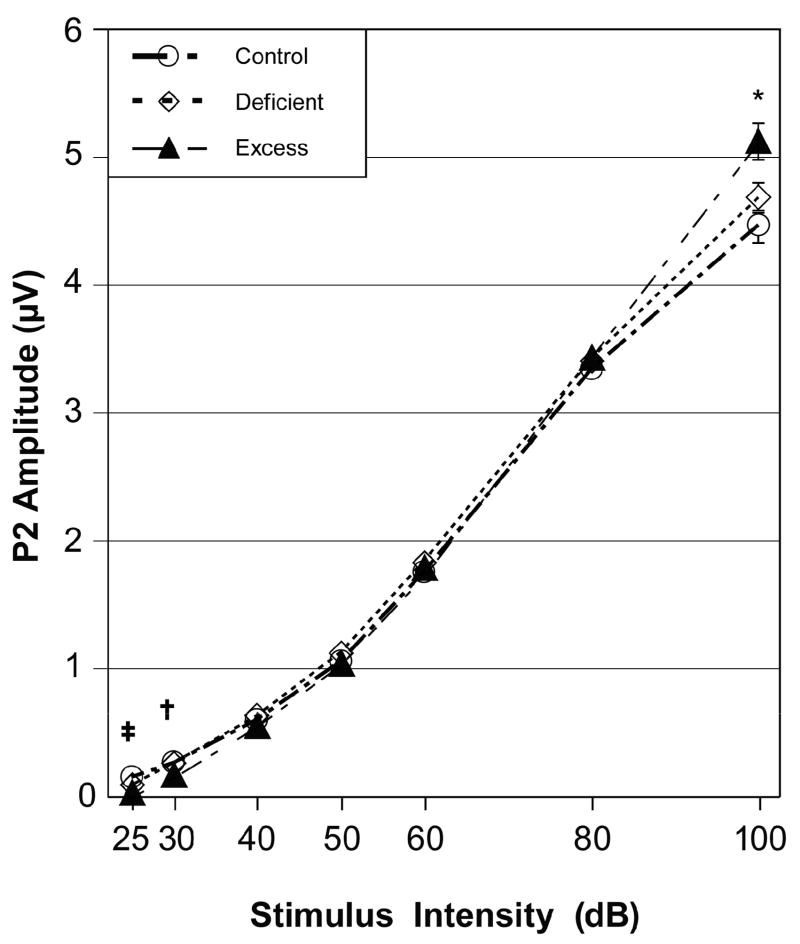
Figure 5 shows the A-I profiles for the P2-N2 wave in response to the 8 kHz tone pip condition for animals in the three diet groups. The ANOVA found a significant effect for Stimulus Intensity, indicating a significant decrease in ABR amplitude as the stimulus intensity decreased: F (6,852) = 2652.59, p < 0.001. There was a significant effect for Sex, reflecting that females had larger P2-N2 amplitudes than males: F (1, 142) = 45.66, p < 0.001. There was no main effect for Diet group: F (2, 142) = 0.70, p = 0.50; and there was no effect for the Diet-by-Sex interaction: F (2, 142) = 0.34, p = 0.71. There was however a significant Diet-by-Stimulus Intensity interaction: F (12, 852) = 4.75, p = 0.002. This interaction is described below.
Fig. 5.
ABR amplitude-intensity (A-I) profiles in response to the 8 kHz tone pip condition for the Control, Deficient and Excess diet groups (mean ± SEM). Footnotes: *Excess greater than Control group (p < 0.05); † Excess smaller than Control and Deficient groups (p < 0.05); ‡ Excess smaller than Control group (p < 0.05).
Consistent with our expectation, univariate analyses of the 8 kHz data showed that the Excess group had lower amplitudes than the Control group for the 25 and 30 dB stimulus intensity conditions. Contrary to our expectation, the Excess group had normal P2-N2 amplitudes for the 40 through 80 dB conditions and significantly larger amplitudes than the Control group for the 100 dB condition. The other three tone pip conditions also showed that the Excess group’s P2-N2 amplitudes were smaller than the Control group’s P2-N2 amplitudes at the lowest stimulus intensities of 25 and 30 dB, were within normal limits for the mid-range stimulus intensities of 40 to 80 dB, and were larger than normal at the highest stimulus intensity of 100 dB (not shown). This abnormal growth pattern in ABR amplitude is also illustrated in Fig. 3C where the Excess offspring had no ABR at 60 dB but a large amplitude ABR at 100 dB. The only exception to this pattern was that the Excess group’s P2-N2 amplitudes were within normal limits at 100 dB for the 2 kHz tone pip condition. The Deficient group’s amplitudes were within normal limits at all stimulus intensities under all four tone pip conditions.
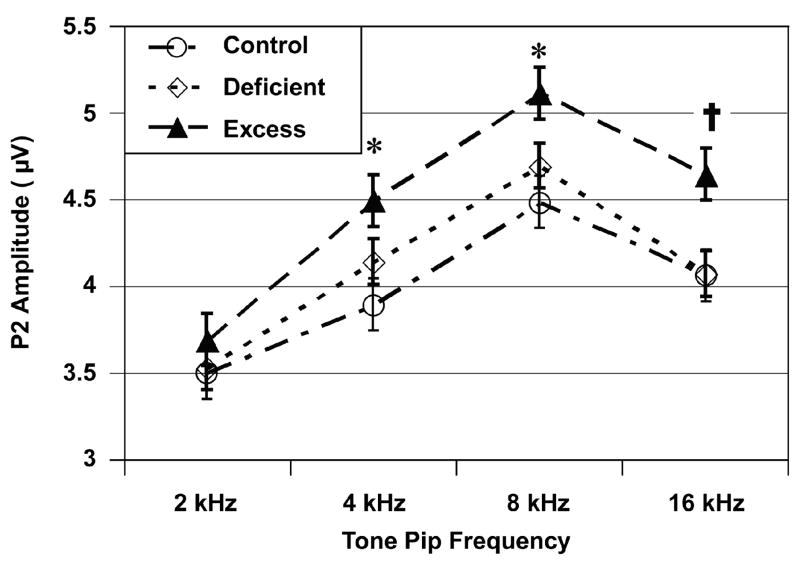
Figure 6 further illustrates the Excess group’s propensity for larger than normal P2-N2 amplitudes in response to loud intensity stimuli. There was a significant effect for Tone Pip Frequency, indicating that the P2-N2 amplitudes differed across the various tone pip frequencies: F (3, 435) = 166.90, p < 0.001. There were also significant effects for Diet group: F (2, 145) = 3.35, p = 0.038 and for the Diet-by-Tone Pip Frequency interaction: F (6, 435) = 3.28, p = 0.006. Subsequent univariate analyses indicated that the Excess group had larger P2-N2 amplitudes than the Control group at 4, 8 and 16 kHz, but not at 2 kHz. The P2-N2 amplitudes of the Deficient group were intermediate to the other two groups but did not differ significantly from either.
Fig. 6.
ABR’s P2-N2 amplitudes as functions of Diet group and Tone Pip Frequency for the 100 dB stimulus intensity condition (mean ± SEM). Footnotes: *Excess greater than Control group (p < 0.05); † Excess greater than Control and Deficient groups (p < 0.05).
3.7. Pup ABR thresholds predicting young adult ABR thresholds
The Control, Deficient and Excess offspring had respective ABR thresholds of 28.3 ± 0.3, 29.1 ± 0.3 and 30.2 ± 0.3 dB as PND 24 pups and ABR thresholds of 29.3 ± 0.5, 30.0 ± 0.4 and 33.0 ± 0.5 dB as young adults (mean ± SEM). At both ages, the Excess offspring had significantly higher ABR thresholds than the other two groups (p ≤ 0.018 or better) With Pearson correlation coefficients (r) of 0.088 and −0.159, the Control and Deficient offspring showed no significant associations between their pup and young adult ABR thresholds. In contrast with r = 0.371 (p = 0.014), the Excess offspring showed a significant association (see 2.3. Data analyses for details). With regards to the 43 Excess offspring: (A) Of the17 offspring with poor thresholds as pups (defined as ≥ 2 SD above the Control group mean), six (35%) had a persistence of poor thresholds into adulthood and 11 had normalization of their thresholds by the time they reached adulthood. (B) Of the 26 offspring with normal thresholds as pups, five (19%) developed poor thresholds by adulthood and 21 retained their normal thresholds into adulthood. An Age-by-Diet ANOVA with the Greenhouse-Geisser adjustments for a within-subject measure was also performed on the ABR data. There was a significant effect for Age group (pup versus adult): F (1, 145) = 35.59, p = 0.001 and for Diet group: F (2, 145) = 25.00, p = 0.001 and for the Age-by-Diet interaction: F (2, 145) = 4.79, p = 0.010. These results indicated that the ABR thresholds were higher when the offspring were adults, that the Excess group had higher ABR thresholds than the Control and Deficient groups, and that the Excess offspring showed a greater age-related increase in their ABR thresholds than their cohorts in the other two diet groups.
4. Discussion
4.1. ABR findings
A prior study on ω-3 FA deficiency during pregnancy found that the rat offspring had abnormal ABRs as pups, which became normal in young adulthood, then became abnormal again in old adulthood [6]. Others have found the same age-dependent pattern in neurological outcomes following prenatal or neonatal exposure to various brain damaging substances [5,34,43,44,47,61,73]. Thus, we hypothesized that our Excess and Deficient offspring would show normalization of their ABRs during young adulthood. Consistent with this hypothesis, we found that the ABR wave P4 latencies (neural transmission times) for the young adult animals in the Excess and Deficient ω-3 FA treatment groups were within normal limits. This contrasts with the prolonged neural transmission times that were seen in these animals when they were 24-day-old pups [19]. Contrary to our hypothesis, we found that the ABR thresholds were elevated (worse hearing acuity) in the young adult Excess animals for the 2, 4, 8 and 16 kHz tone pip conditions and in the young adult Deficient animals for the 8 kHz tone pip condition when compared to the young adult Control animals. These Excess and Deficient animals had elevated ABR thresholds as 24-day-old pups [18]. Thus, they continued this abnormality into adulthood.
The Control and Deficient groups showed no correlations between their respective pup and adult ABR thresholds. In contrast, the Excess group’s pup and adult ABR thresholds were significantly correlated. Of the17 Excess offspring with poor thresholds as pups, six (35%) had a persistence of poor thresholds into young adulthood, suggesting these six offspring had a permanent deficit and that the remaining 11 offspring had temporary deficits as pups that normalized with maturation. Of the 26 Excess offspring with normal thresholds as pups, five (19%) developed poor thresholds by adulthood, suggesting that these offspring experienced early adult-onset deficits and that the remaining 21 pups retained their normalcy into young adulthood.
In addition to the elevated ABR thresholds in the adult Excess offspring, we found differences in their ABR amplitudes. Specifically, the Excess offspring exhibited significantly lower amplitude ABRs in response to low stimulus intensities and significantly higher amplitude ABRs in response to high stimulus intensities compared to Controls. The Deficient offspring showed a similar but non-significant tend. ABR amplitudes reflect the number of neural units firing, their firing rates and/or their neural synchrony [21,53,66]. Thus, the elevated ABR thresholds in the Excess offspring were likely due to such mechanisms in response to low intensity stimuli. In contrast, the Excess offspring also had an over-excitation phenomenon in response to high intensity stimuli that caused the enlarged ABR amplitudes. Such patterns of decreased auditory evoked potential amplitudes at low stimulus intensities and enlarged amplitudes at high stimulus intensities are seen in hearing losses that have accompanying hyperacusis [53,63,76]. An abnormal decrease in neural inhibitory processes is the suspected mechanism underlying such hyperactivity [53,63,76].
This hyperacusis phenomenon in the Excess group was seen in response to the 4, 8 and 16 kHz tone pips, but not in response to the 2 kHz tone pips. This was likely due to differences in perceived stimulus intensity or hearing level (HL). As shown in Figure 2, the ABR thresholds for the 2 kHz condition were about 15–20 dB higher (worse) than the other three conditions. If we had compensated for this poorer hearing acuity at 2 kHz by raising the stimulus an additional 15–20 dB, we likely would have seen the hyperacusis phenomenon.
The ABR threshold and amplitude abnormalities in our young adult Excess and Deficient offspring indicate that their treatments resulted in permanent and/or early adult-onset neurological and sensory deficits. These ABR findings, as well as another study from our laboratory that found increased ω-3 FA levels and increased ω-3/ω-6 ratios in the body fatty acid composition in their male and female littermates [35], are the first studies to show abnormalities in adulthood as a result of exposure to excess amounts of ω-3 FA during pregnancy and lactation. In addition, we have observed a significant decrease in the adult life span of our Excess animals [manuscript in preparation]. Such findings are consistent with Barker’s hypothesis that adverse prenatal and/or postnatal conditions can program the offspring for health disorders in adulthood [4,22,54].
4.2. Adult offspring characteristics
Although there was a tendency for the Excess and Deficient offspring as young adults to weigh less than their Control cohorts, these differences were not significant. This contrasts to the significant postnatal growth retardation seen in these same Excess and Deficient offspring prior to weaning [18,19]. This indicates ability for catch-up growth in these offspring.
4.3. Mechanisms of ω-3 FA nutritional toxicity
There are several mechanisms by which ω-3 FA imbalances can produce adverse effects on offspring development: (A) A balanced ratio of ω-3 and ω-6 FA is required for optimal growth. A relative excess of ω-3 FA will lower AA concentrations in blood, brain and other tissues through competitive displacement. In addition, EPA and DHA are also known to inhibit the synthesis of AA [28]. Lowered concentrations of AA impair fetal and infant growth [11,13]. This is because AA promotes adipose tissue deposition and is a mediator of growth and metabolic hormones [9,29]. (B) Excess ω-3 FA can harm the developing fetus and infant by causing oxidative stress and subsequent cell apoptosis through augmented lipid peroxidation [23–25,72] and reduced antioxidant status [14]. For example, elevated peroxidation levels were found in the mammary glands, skeletal muscle, heart and carcass of female mice given a diet of 19% fish oil + 1% corn oil for four weeks [24]. (C) Various forms of over-nutrition can cause epigenetic and hormonal changes in the fetus or young child which can increase the risks for adult-onset health disorders such as diabetes, hypertension and a shortened life span [52,54,75]. It is possible that fish oil over-nutrition can have similar effects. (D) Offspring growth retardation due to prenatal undernutrition causes reduced cell numbers in rat embryos [39] and in the adrenal glands, kidney, liver and heart of human fetuses [45]. It seems plausible that conditions causing postnatal growth retardation, such as our ω-3 FA Excess and Deficiency conditions, could similarly reduce cell numbers in the nervous system and other vital organs. (E) A diet rich in ω-3 FA can decrease the milk yield of lactating rats, resulting in postnatal growth retardation [32]. (F) Exposure of the oocyte to an environment high in ω-3 FA can result in perturbed mitochondrial distribution, metabolism, and calcium levels, adversely affecting embryo morphology and development [72].
4.4. Conclusions and health implications
In summary, a diet rich in ω-3 FA during pregnancy and lactation caused abnormal ABR thresholds and amplitudes. A diet deficient in ω-3 FA caused similar but less dramatic effects. Our study’s findings have important health implications. Current United State consumption of ω-3 FA is lower than national and international recommendations [1] and large amounts of ω-3 FA are being consumed voluntarily [25,26,48,51,58,68] and being given as treatment for preterm birth [46,49,50,60,65]. Our results and those from other laboratories indicate that both excess and deficient amounts of dietary ω-3 FA during pregnancy and lactation can cause postnatal growth retardation as well as sensory and neurological abnormalities in the offspring.
Thus, consuming or administering large amounts of ω-3 FA to pregnant women or nursing infants seems inadvisable. Others have similarly stressed caution in the over-use of fish oil and other dietary sources of ω-3 FA by pregnant and lactating women [3,10,25,37,68]. In contrast to earlier studies, recent literature reviews have failed to find convincing evidence that prenatal fish oil treatments improve pregnancy outcome [42] and failed to find that postnatal ω-3 FA supplementation during nursing/bottle feeding has lasting effects on infant brain and visual function beyond 1–2 years of age [27,64]. In light of such findings, we conclude that using large amounts of ω-3 FA during pregnancy and lactation has little merit, is potentially harmful to the offspring and should not be advocated in clinical or public practice. Instead, more research is needed to determine: (A) how much perinatal ω-3 FA is too much, too little and just right for the developing human and (B) the long-term consequences in regards to the fetal programming of neurodevelopmental and sensory impairments and the adult-onset diseases of hypertension, diabetes, age-related neural degeneration and a shortened life span [4,22,39].
Acknowledgments
This project was supported by grants from the Gerber Foundation and the National Institute of General Medical Sciences (GM58905), neither of which contributed to the design, analyses or writing of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akabas SR, Deckelbaum RJ. Summary of a workshop on n-3 fatty acids: current status of recommendations and future directions. The American journal of clinical nutrition. 2006;83:1536S–1538S. doi: 10.1093/ajcn/83.6.1536S. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JW, Johnstone BM, Remley DT. Breast-feeding and cognitive development: a meta-analysis. The American journal of clinical nutrition. 1999;70:525–35. doi: 10.1093/ajcn/70.4.525. [DOI] [PubMed] [Google Scholar]
- 3.Arbuckle LD, Rioux FM, Mackinnon MJ, Hrboticky N, Innis SM. Response of (n-3) and (n-6) fatty acids in piglet brain, liver and plasma to increasing, but low, fish oil supplementation of formula. The Journal of nutrition. 1991;121:1536–47. doi: 10.1093/jn/121.10.1536. [DOI] [PubMed] [Google Scholar]
- 4.Barker DJ. The developmental origins of chronic adult disease. Acta Paediatr Suppl. 2004;93:26–33. doi: 10.1111/j.1651-2227.2004.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 5.Barone S, Jr, Stanton ME, Mundy WR. Neurotoxic effects of neonatal triethyltin (TET) exposure are exacerbated with aging. Neurobiol Aging. 1995;16:723–35. doi: 10.1016/0197-4580(95)00089-w. [DOI] [PubMed] [Google Scholar]
- 6.Bourre JM, Durand G, Erre JP, Aran JM. Changes in auditory brainstem responses in alpha-linolenic acid deficiency as a function of age in rats. Audiology. 1999;38:13–8. doi: 10.3109/00206099909072997. [DOI] [PubMed] [Google Scholar]
- 7.Bourre JM, Francois M, Youyou A, Dumont O, Piciotti M, Pascal G, Durand G. The effects of dietary alpha-linolenic acid on the composition of nerve membranes, enzymatic activity, amplitude of electrophysiological parameters, resistance to poisons and performance of learning tasks in rats. The Journal of nutrition. 1989;119:1880–92. doi: 10.1093/jn/119.12.1880. [DOI] [PubMed] [Google Scholar]
- 8.Carlson SE. Arachidonic acid status of human infants: influence of gestational age at birth and diets with very long chain n-3 and n-6 fatty acids. The Journal of nutrition. 1996;126:1092S–8S. doi: 10.1093/jn/126.suppl_4.1092S. [DOI] [PubMed] [Google Scholar]
- 9.Carlson SE. Docosahexaenoic acid and arachidonic acid in infant development. Semin Neonatol. 2001;6:437–49. doi: 10.1053/siny.2001.0093. [DOI] [PubMed] [Google Scholar]
- 10.Carlson SE, Cooke RJ, Rhodes PG, Peeples JM, Werkman SH. Effect of vegetable and marine oils in preterm infant formulas on blood arachidonic and docosahexaenoic acids. The Journal of pediatrics. 1992;120:S159–67. doi: 10.1016/s0022-3476(05)81251-x. [DOI] [PubMed] [Google Scholar]
- 11.Carlson SE, Cooke RJ, Rhodes PG, Peeples JM, Werkman SH, Tolley EA. Long-term feeding of formulas high in linolenic acid and marine oil to very low birth weight infants: phospholipid fatty acids. Pediatric research. 1991;30:404–12. doi: 10.1203/00006450-199111000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Carlson SE, Cooke RJ, Werkman SH, Tolley EA. First year growth of preterm infants fed standard compared to marine oil n-3 supplemented formula. Lipids. 1992;27:901–7. doi: 10.1007/BF02535870. [DOI] [PubMed] [Google Scholar]
- 13.Carlson SE, Werkman SH, Peeples JM, Cooke RJ, Tolley EA. Arachidonic acid status correlates with first year growth in preterm infants. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:1073–7. doi: 10.1073/pnas.90.3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho SH, Choi YS. Lipid peroxidation and antioxidant status is affected by different vitamin E levels when feeding fish oil. Lipids. 1994;29:47–52. doi: 10.1007/BF02537090. [DOI] [PubMed] [Google Scholar]
- 15.Church MW, Abel EL, Kaltenbach JA, Overbeck GW. Effects of prenatal alcohol exposure and aging on auditory function in the rat: preliminary results, Alcoholism. clinical and experimental research. 1996;20:172–9. doi: 10.1111/j.1530-0277.1996.tb01061.x. [DOI] [PubMed] [Google Scholar]
- 16.Church MW, Blakley BW, Burgio DL, Gupta AK. WR-2721 (Amifostine) ameliorates cisplatin-induced hearing loss but causes neurotoxicity in hamsters: dose-dependent effects. J Assoc Res Otolaryngol. 2004;5:227–37. doi: 10.1007/s10162-004-4011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Church MW, Gritzke R. Effects of ketamine anesthesia on the rat brain-stem auditory evoked potential as a function of dose and stimulus intensity. Electroencephalography and clinical neurophysiology. 1987;67:570–83. doi: 10.1016/0013-4694(87)90060-5. [DOI] [PubMed] [Google Scholar]
- 18.Church MW, Jen KLC, Stafferton T, Hotra JW, Adams BR. Reduced auditory acuity in rat pups from excess and deficient omega-3 fatty acid consumption by the mother. Neurotoxicology and teratology. 2007;29:203–10. doi: 10.1016/j.ntt.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Church MW, Jen KL, Dowhan LM, Adams BR, Hotra JW. Excess and deficient omega-3 fatty acid during pregnancy and lactation cause impaired neural transmission in rat pups. Neurotoxicology and teratology. 2008;30:107–17. doi: 10.1016/j.ntt.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Church MW, Williams HL, Holloway JA. Postnatal development of the brainstem auditory evoked potential and far-field cochlear microphonic in non-sedated rat pups. Brain research. 1984;316:23–31. doi: 10.1016/0165-3806(84)90005-1. [DOI] [PubMed] [Google Scholar]
- 21.Davis H. Brain stem and other responses in electric response audiometry. Ann Otol Rhinol Laryngol. 1976;85:3–14. doi: 10.1177/000348947608500103. [DOI] [PubMed] [Google Scholar]
- 22.Fleming TP, Kwong WY, Porter R, Ursell E, Fesenko I, Wilkins A, Miller DJ, Watkins AJ, Eckert JJ. The embryo and its future. Biol Reprod. 2004;71:1046–54. doi: 10.1095/biolreprod.104.030957. [DOI] [PubMed] [Google Scholar]
- 23.Fritsche KL, Johnston PV. Rapid autoxidation of fish oil in diets without added antioxidants. The Journal of nutrition. 1988;118:425–6. doi: 10.1093/jn/118.4.425. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez MJ, Gray JI, Schemmel RA, Dugan L, Jr, Welsch CW. Lipid peroxidation products are elevated in fish oil diets even in the presence of added antioxidants. The Journal of nutrition. 1992;122:2190–5. doi: 10.1093/jn/122.11.2190. [DOI] [PubMed] [Google Scholar]
- 25.Grandjean P, Bjerve KS, Weihe P, Steuerwald U. Birthweight in a fishing community: significance of essential fatty acids and marine food contaminants. International journal of epidemiology. 2001;30:1272–8. doi: 10.1093/ije/30.6.1272. [DOI] [PubMed] [Google Scholar]
- 26.Grandjean P, Weihe P. Neurobehavioral effects of intrauterine mercury exposure: potential sources of bias. Environmental research. 1993;61:176–83. doi: 10.1006/enrs.1993.1062. [DOI] [PubMed] [Google Scholar]
- 27.Hadders-Algra M, Bouwstra H, van Goor SA, Dijck-Brouwer DA, Muskiet FA. Prenatal and early postnatal fatty acid status and neurodevelopmental outcome. Journal of perinatal medicine 35 Suppl. 2007;1:S28–34. doi: 10.1515/JPM.2007.034. [DOI] [PubMed] [Google Scholar]
- 28.Hagve TA, Christensen E, Gronn M, Christophersen BO. Regulation of the metabolism of polyunsaturated fatty acids. Scandinavian journal of clinical and laboratory investigation. 1988;191:33–46. [PubMed] [Google Scholar]
- 29.Hardy SC, Kleinman RE. Fat and cholesterol in the diet of infants and young children: implications for growth, development, and long-term health. The Journal of pediatrics. 1994;125:S69–77. doi: 10.1016/s0022-3476(06)80739-0. [DOI] [PubMed] [Google Scholar]
- 30.Haubner LY, Stockard JE, Saste MD, Benford VJ, Phelps CP, Chen LT, Barness L, Wiener D, Carver JD. Maternal dietary docosahexanoic acid content affects the rat pup auditory system. Brain research bulletin. 2002;58:1–5. doi: 10.1016/s0361-9230(01)00764-x. [DOI] [PubMed] [Google Scholar]
- 31.Henry KR. Auditory brainstem volume-conducted responses: origins in the laboratory mouse. Journal of the American Auditory Society. 1979;4:173–8. [PubMed] [Google Scholar]
- 32.Herrera E, Lopez-Soldado I, Limones M, Amusquivar E, Ramos MP. Lipid metabolism during the perinatal phase, and its implications on postnatal development. Int J Vitam Nutr Res. 2006;76:216–24. doi: 10.1024/0300-9831.76.4.216. [DOI] [PubMed] [Google Scholar]
- 33.Innis SM. The role of dietary n-6 and n-3 fatty acids in the developing brain. Developmental neuroscience. 2000;22:474–80. doi: 10.1159/000017478. [DOI] [PubMed] [Google Scholar]
- 34.Janicke B, Coper H. The effects of prenatal exposure to hypoxia on the behavior of rats during their life span. Pharmacol Biochem Behav. 1994;48:863–73. doi: 10.1016/0091-3057(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 35.Jen K-LC, Church MW, Wang C, Moghaddam M, Dowhan L, Laja F, Sherman J. Perinatal omega-3 fatty acid excess and deficiency affects the fatty acid profiles of rat offspring. submitted (submitted) [Google Scholar]
- 36.Jensen CL. Effects of n-3 fatty acids during pregnancy and lactation. The American journal of clinical nutrition. 2006;83:1452S–1457S. doi: 10.1093/ajcn/83.6.1452S. [DOI] [PubMed] [Google Scholar]
- 37.Jensen CL, Prager TC, Fraley JK, Chen H, Anderson RE, Heird WC. Effect of dietary linoleic/alpha-linolenic acid ratio on growth and visual function of term infants. The Journal of pediatrics. 1997;131:200–9. doi: 10.1016/s0022-3476(97)70154-9. [DOI] [PubMed] [Google Scholar]
- 38.Koutz CA, Wiegand RD, Rapp LM, Anderson RE. Effect of dietary fat on the response of the rat retina to chronic and acute light stress. Exp Eye Res. 1995;60:307–16. doi: 10.1016/s0014-4835(05)80112-5. [DOI] [PubMed] [Google Scholar]
- 39.Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127:4195–202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- 40.Lauritzen L, Jorgensen MH, Olsen SF, Straarup EM, Michaelsen KF. Maternal fish oil supplementation in lactation: effect on developmental outcome in breast-fed infants. Reprod Nutr Dev. 2005;45:535–47. doi: 10.1051/rnd:2005044. [DOI] [PubMed] [Google Scholar]
- 41.Lucia Bergmann R, Bergmann KE, Haschke-Becher E, Richter R, Dudenhausen JW, Barclay D, Haschke F. Does maternal docosahexaenoic acid supplementation during pregnancy and lactation lower BMI in late infancy? Journal of perinatal medicine. 2007;35:295–300. doi: 10.1515/JPM.2007.085. [DOI] [PubMed] [Google Scholar]
- 42.Makrides M, Duley L, Olsen SF. Marine oil, and other prostaglandin precursor, supplementation for pregnancy uncomplicated by pre-eclampsia or intrauterine growth restriction. Cochrane Database Syst Rev. 2006;3:CD003402. doi: 10.1002/14651858.CD003402.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Markel E, Felszeghy K, Luiten PG, Nyakas C. Beneficial effect of chronic nimodipine treatment on behavioral dysfunctions of aged rats exposed to perinatal ethanol treatment. Arch Gerontol Geriatr. 1995;21:75–88. doi: 10.1016/0167-4943(95)00653-3. [DOI] [PubMed] [Google Scholar]
- 44.Martin JC. Irreversible changes in mature and aging animals following intrauterine drug exposure. Neurobehav Toxicol Teratol. 1986;8:335–43. [PubMed] [Google Scholar]
- 45.Naeye RL, Blanc W, Paul C. Effects of maternal nutrition on the human fetus. Pediatrics. 1973;52:494–503. [PubMed] [Google Scholar]
- 46.National Institute of Child Health and Human Development, Omega-3 fatty acid: A randomized trial of omega-3 fatty acid supplementation to prevent preterm birth in pregnancies at high risk, Clinical Trial NCT00135902.
- 47.Nyakas C, Buwalda B, Luiten PG. Hypoxia and brain development. Prog Neurobiol. 1996;49:1–51. doi: 10.1016/0301-0082(96)00007-x. [DOI] [PubMed] [Google Scholar]
- 48.Oken E, Kleinman KP, Olsen SF, Rich-Edwards JW, Gillman MW. Associations of seafood and elongated n-3 fatty acid intake with fetal growth and length of gestation: results from a US pregnancy cohort. Am J Epidemiol. 2004;160:774–83. doi: 10.1093/aje/kwh282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olsen SF, Hansen HS, Sorensen TI, Jensen B, Secher NJ, Sommer S, Knudsen LB. Hypothesis: dietary (N-3)-fatty acids prolong gestation in human beings. Prog Clin Biol Res. 1987;242:51–6. [PubMed] [Google Scholar]
- 50.Olsen SF, Secher NJ. A possible preventive effect of low-dose fish oil on early delivery and pre-eclampsia: indications from a 50-year-old controlled trial. Br J Nutr. 1990;64:599–609. doi: 10.1079/bjn19900063. [DOI] [PubMed] [Google Scholar]
- 51.Olsen SF, Sorensen JD, Secher NJ, Hedegaard M, Henriksen TB, Hansen HS, Grant A. Randomised controlled trial of effect of fish-oil supplementation on pregnancy duration. Lancet. 1992;339:1003–7. doi: 10.1016/0140-6736(92)90533-9. [DOI] [PubMed] [Google Scholar]
- 52.Pembrey ME, Bygren LO, Kaati G, Edvinsson S, Northstone K, Sjostrom M, Golding J. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet. 2006;14:159–66. doi: 10.1038/sj.ejhg.5201538. [DOI] [PubMed] [Google Scholar]
- 53.Phillips DP, Carr MM. Disturbances of loudness perception. J Am Acad Audiol. 1998;9:371–9. quiz 399. [PubMed] [Google Scholar]
- 54.Plagemann A, Heidrich I, Gotz F, Rohde W, Dorner G. Obesity and enhanced diabetes and cardiovascular risk in adult rats due to early postnatal overfeeding. Exp Clin Endocrinol. 1992;99:154–8. doi: 10.1055/s-0029-1211159. [DOI] [PubMed] [Google Scholar]
- 55.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. The Journal of nutrition. 1993;123:1939–51. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 56.Roegge CS, Widholm JJ, Engeseth NJ, Wang X, Brosch KO, Seegal RF, Schantz SL. Delayed spatial alternation impairments in adult rats following dietary n-6 deficiency during development. Neurotoxicology and teratology. 2005;27:485–95. doi: 10.1016/j.ntt.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 57.Rossi GT, Britt RH. Effects of hypothermia on the cat brain-stem auditory evoked response. Electroencephalography and clinical neurophysiology. 1984;57:143–55. doi: 10.1016/0013-4694(84)90173-1. [DOI] [PubMed] [Google Scholar]
- 58.Rump P, Mensink RP, Kester AD, Hornstra G. Essential fatty acid composition of plasma phospholipids and birth weight: a study in term neonates. The American journal of clinical nutrition. 2001;73:797–806. doi: 10.1093/ajcn/73.4.797. [DOI] [PubMed] [Google Scholar]
- 59.Saste MD, Carver JD, Stockard JE, Benford VJ, Chen LT, Phelps CP. Maternal diet fatty acid composition affects neurodevelopment in rat pups. The Journal of nutrition. 1998;128:740–3. doi: 10.1093/jn/128.4.740. [DOI] [PubMed] [Google Scholar]
- 60.Sattar N, Berry C, Greer IA. Essential fatty acids in relation to pregnancy complications and fetal development. Br J Obstet Gynaecol. 1998;105:1248–55. doi: 10.1111/j.1471-0528.1998.tb10002.x. [DOI] [PubMed] [Google Scholar]
- 61.Schallert T. Sensorimotor impairment and recovery of function in brain-damaged rats: reappearance of symptoms during old age. Behav Neurosci. 1983;97:159–64. doi: 10.1037//0735-7044.97.1.159. [DOI] [PubMed] [Google Scholar]
- 62.Scott DT, Janowsky JS, Carroll RE, Taylor JA, Auestad N, Montalto MB. Formula supplementation with long-chain polyunsaturated fatty acids: are there developmental benefits? Pediatrics. 1998;102:E59. doi: 10.1542/peds.102.5.e59. [DOI] [PubMed] [Google Scholar]
- 63.Sendowski I, Braillon-Cros A, Delaunay C. CAP amplitude after impulse noise exposure in guinea pigs. Eur Arch Otorhinolaryngol. 2004;261:77–81. doi: 10.1007/s00405-003-0647-2. [DOI] [PubMed] [Google Scholar]
- 64.Simmer K, Patole S. Longchain polyunsaturated fatty acid supplementation in preterm infants. Cochrane Database Syst Rev. 2004:CD000375. doi: 10.1002/14651858.CD000375.pub2. [DOI] [PubMed] [Google Scholar]
- 65.Smuts CM, Huang M, Mundy D, Plasse T, Major S, Carlson SE. A randomized trial of docosahexaenoic acid supplementation during the third trimester of pregnancy. Obstet Gynecol. 2003;101:469–79. doi: 10.1016/s0029-7844(02)02585-1. [DOI] [PubMed] [Google Scholar]
- 66.Starr A, Sininger Y, Nguyen T, Michalewski HJ, Oba S, Abdala C. Cochlear receptor (microphonic and summating potentials, otoacoustic emissions) and auditory pathway (auditory brain stem potentials) activity in auditory neuropathy. Ear Hear. 2001;22:91–9. doi: 10.1097/00003446-200104000-00002. [DOI] [PubMed] [Google Scholar]
- 67.Stockard JE, Saste MD, Benford VJ, Barness L, Auestad N, Carver JD. Effect of docosahexaenoic acid content of maternal diet on auditory brainstem conduction times in rat pups. Developmental neuroscience. 2000;22:494–9. doi: 10.1159/000017481. [DOI] [PubMed] [Google Scholar]
- 68.Thorsdottir I, Birgisdottir BE, Halldorsdottir S, Geirsson RT. Association of fish and fish liver oil intake in pregnancy with infant size at birth among women of normal weight before pregnancy in a fishing community. Am J Epidemiol. 2004;160:460–5. doi: 10.1093/aje/kwh239. [DOI] [PubMed] [Google Scholar]
- 69.Wainwright PE. Dietary essential fatty acids and brain function: a developmental perspective on mechanisms. Proc Nutr Soc. 2002;61:61–9. doi: 10.1079/pns2001130. [DOI] [PubMed] [Google Scholar]
- 70.Wainwright PE, Jalali E, Mutsaers LM, Bell R, Cvitkovic S. An imbalance of dietary essential fatty acids retards behavioral development in mice. Physiol Behav. 1999;66:833–9. doi: 10.1016/s0031-9384(99)00028-1. [DOI] [PubMed] [Google Scholar]
- 71.Wainwright PE, Xing HC, Mutsaers L, McCutcheon D, Kyle D. Arachidonic acid offsets the effects on mouse brain and behavior of a diet with a low (n-6):(n-3) ratio and very high levels of docosahexaenoic acid. The Journal of nutrition. 1997;127:184–93. doi: 10.1093/jn/127.1.184. [DOI] [PubMed] [Google Scholar]
- 72.Wakefield SL, Lane M, Schulz SJ, Hebart ML, Thompson JG, Mitchell M. Maternal supply of omega-3 polyunsaturated fatty acids alter mechanisms involved in oocyte and early embryo development in the mouse. Am J Physiol Endocrinol Metab. 2008;294:E425–34. doi: 10.1152/ajpendo.00409.2007. [DOI] [PubMed] [Google Scholar]
- 73.Wallace RB, Daniels CE, Altman J. Behavioral effects of neonatal irradiation of the cerebellum. 3. Qualitative observations in aged rats. Dev Psychobiol. 1972;5:35–41. doi: 10.1002/dev.420050105. [DOI] [PubMed] [Google Scholar]
- 74.Weisinger HS, Vingrys AJ, Sinclair AJ. The effect of docosahexaenoic acid on the electroretinogram of the guinea pig. Lipids. 1996;31:65–70. doi: 10.1007/BF02522413. [DOI] [PubMed] [Google Scholar]
- 75.Wu G, Bazer FW, Cudd TA, Meininger CJ, Spencer TE. Maternal nutrition and fetal development. The Journal of nutrition. 2004;134:2169–72. doi: 10.1093/jn/134.9.2169. [DOI] [PubMed] [Google Scholar]
- 76.Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D, Salvi R, Sun W. Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hear Res. 2007;226:244–53. doi: 10.1016/j.heares.2006.06.013. [DOI] [PubMed] [Google Scholar]