Abstract
Mouse models of cystic fibrosis (CF) display increased sulfotransferase 1E1 (SULT1E1) activity in hepatocytes of cystic fibrosis transmembrane receptor (CFTR)-deficient animals. SULT1E1 is responsible for the sulfation and inactivation of β-estradiol (E2) at physiological concentrations. IGF-1 message levels in CFTR(−/−) mouse livers were positively correlated with body weight and negatively correlated with SULT1E1 activity. Growth hormone (GH) is important in regulation of hepatic IGF-1 expression indicating that E2 levels are involved with GH signaling in hepatocytes. To investigate the effects of E2 and SULT1E1 activity on GH signal transduction in human hepatocytes, SULT1E1 was stably expressed in HepG2 cells. Effects of increased E2 sulfation on the GH signaling pathway and E2-regulated gene expression were examined. Pretreatment of HepG2 cells with 10 nM E2 prior to GH stimulation increased STAT5b phosphorylation and IGF-1 expression. In SULT1E1-transfected HepG2 cells, GH-stimulated STAT5b phosphorylation was significantly decreased. E2 treatment had no effect on STAT5b phosphorylation in the absence of GH stimulation. E2 also had no effect on Jak-2 phosphorylation. E2 has an apparent rapid action on increasing GH-stimulated STAT5b phosphorylation that was not attenuated by the estrogen receptor antagonist, ICI 182,780. Physiological levels of E2 in HepG2 cells increase GH stimulation of IGF-1 production apparently through increased phosphorylated STAT5b levels and transcriptional activation of the IGF-1 gene. The enhanced SULT1E1 activity may have a role in inhibiting GH stimulated STAT5b phosphorylation and IGF-1 synthesis via the sulfation and inactivation of E2.
Keywords: β-Estradiol, sulfotransferase, SULT1E1, growth hormone, insulin-like growth factor 1, STAT5b
INTRODUCTION
Changes in the metabolism of estrogens, particularly β-estradiol (E2), may have a role in alterations in liver function or the development of liver disease. Because sulfotransferase (SULT) 1E1 is the major SULT isoform responsible for the inactivation of β-estradiol (E2) via sulfation at physiological concentrations [6–8], its role in altering liver estrogen levels may be significant in the onset and progression of liver disease. SULT1E1 is a member of the cytosolic SULT gene family involved in Phase II conjugation reactions [1, 2] that catalyze the transfer of the sulfonate group from the obligate cofactor 3′-phosphoadenosine-5′-phosphosulfate (PAPS) to relatively small acceptor molecules. SULT1E1 sulfates and inactivates E2 at picomolar and nanomolar concentrations in cells [3] and can regulate the activation of estrogen receptors (ERs) by E2 in human estrogen-responsive cells and tissues [4, 5]. Therefore, changes in SULT1E1 activity may significantly alter E2 levels and E2-regulated processes in tissues and cells, including those of the liver where it is expressed at significant levels.
The present study was undertaken to further explore the role of SULT1E1 in liver disease via its effects on E2 levels by elucidation of the pathways by which E2 may act in liver. Previous results from our laboratory [6] have demonstrated that increases in SULT1E1 activity in the livers of cystic fibrosis transmembrane receptor (CFTR) - deficient mice result in alterations in the expression of E2-regulated proteins including carbonic anhydrase (CA) III, glutathione-S-transferase (GST) P1 and cytochrome P450 (CYP) 2B9. These changes were consistent with decreased free E2 levels in hepatocytes due to increased SULT1E1 activity. Additionally, mouse models of CF possessing genetic disruption of the CFTR gene (knockout)(KO) or generation of dysfunctional CFTR molecules (ΔF508) frequently display severe growth retardation and low weight gains without showing significant pulmonary disease indicating the involvement of GI problems associated with CF [7]. SULT1E1 expression is significantly increased in livers of CFTR-KO and ΔF508 (−/−) mice with low body weights [6, 8]. Although CFTR is expressed in cholangiocytes or biliary duct cells in mouse and human liver, the loss of CFTR function is associated with the selective induction of SULT1E1 in mouse liver hepatocytes [6]. IGF-1 message levels in CFTR(−/−) mouse livers were positively correlated with body weight and negatively correlated with SULT1E1 activity. Growth hormone (GH) is important in regulation of hepatic IGF-1 expression suggesting that β-estradiol (E2) levels are involved with GH signaling in hepatocytes.
Cystic fibrosis (CF), a major human genetic disease that affects approximately 1 of every 2000 Caucasian infants [9], manifests primarily as pulmonary disease; however, the loss of CFTR function results in physiological and pathological changes in many other tissues including liver [10, 11]. Liver disease occurs in approximately 15–20% of children with CF and is the second rated cause of CF-associated morbidity and mortality [12]. In addition, changes in liver function may occur in CF without the manifestations of liver disease. Understanding the mechanisms by which the loss of CFTR activity alters liver function and its potential role in increasing liver pathologies may provide new avenues for intervention.
In CF patients with growth deficits there is an associated decrease in insulin-like growth factor (IGF) -1 plasma levels [13, 14]. The mechanisms for growth retardation in CF have not been elucidated although there may be multiple causes; one possible factor is the decrease or loss of growth hormone (GH) responsiveness in the liver. GH is a major regulator of IGF-1 synthesis in liver and E2 has also been reported to interact with GH signaling in T47D breast cancer, HEK293, and HuH7 hepatoma cells [15]. Enhanced SULT1E1 activity in CF hepatocytes may decrease the intracellular levels of free active E2. The decrease in E2 may affect aspects of the GH signaling pathway resulting in decreased responsiveness to GH stimulation and decreased production of IGF-1 that is involved in normal bone and tissue growth [13, 14]. E2 and IGF-1 are also involved with stimulating the proliferation and repair of cholangiocytes indicating that the increase in E2 sulfation and inactivation may exacerbate the changes in CFTR-deficient cholangiocytes [16]. E2 also stimulates the expression of CFTR in non-hepatic human tissues [17] suggesting that decreases in estrogenic activity may be detrimental to CFTR levels and function. However, the investigation of the induction of SULT1E1 activity in human CF hepatocytes has been hindered by the lack of availability of human CF liver specimens.
This study investigates the association between increased hepatic SULT1E1 activity with a corresponding inactivation of E2 in the regulation of GH-stimulated STAT5b and Jak-2 phosphorylation and IGF-1 message levels in CFTR (−/−) mice and in human HepG2 hepatocytes stably expressing elevated levels of SULT1E1 activity. Additionally, the effect of increased SULT1E1 activity in HepG2 cells on the expression of E2-regulated proteins was investigated. HepG2 cells stably transformed with SULT1E1 provide a human model for the effects of the induction of SULT1E1 activity that occurs in CFTR (−/−) mouse hepatocytes [6]. GH treatment is beneficial in increasing the growth and body weights of children with CF-associated growth deficits [18]. The GH receptor is a transmembrane protein and belongs to the cytokine receptor family [19]. Upon GH binding, the GH receptor induces activation and phosphorylation of Jak-2 [20] that then phosphorylates GH receptors as well as the signal transducers and activators of transcription (STATs), including STAT5b [21]. STAT5b dimerizes and translocates to the nucleus where it binds to specific DNA motifs within the promoter regions of GH-responsive genes, including IGF-1, to initiate transcription [22]. Physiological concentrations of E2 stimulate STAT5b phosphorylation and IGF-1 expression in response to GH stimulation, a response that is ameliorated by increased SULT1E1 activity. These studies provide the initial evidence that changes in SULT1E1 activity in hepatocytes may be influencing the effect of E2 on intracellular GH signaling pathways as well as E2-regulated gene expression in both human and mouse liver.
METHODS
Materials
Human HepG2 hepatocytes were obtained from the American Type Culture Collection (Rockville, MD). Modified Eagle’s Medium (MEM), geneticin and fetal bovine serum (FBS) were from Life Technologies (Grand Island, NY). Human GH, E2, and DNA polymerase were obtained from Sigma Chemical Co. (St. Louis, MO). ICI 182,780 was purchased from Tocris (Ellisville, MO). [3H]-E2 (56 Ci/mmol) was purchased from DuPont–NEN (Boston, MA). 3′-Phosphoadenosine 5′-phosphosulphate (PAPS) was from Dr. Sanford Singer (University of Dayton, Dayton, OH). STAT-60 was purchased from Tel-Test (Friendswood, TX). Superscript™ Π RNase H-reverse transcriptase was from Invitrogen (Carlsbad, CA). Mouse anti-STAT5 antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-tyrosine phosphorylated (PY)-STAT5b antibody was from Cell Signaling (Danvers, MA). M-PER and the SuperSignal West Pico kit were purchased from Pierce (Rockford, IL). The High-Capacity cDNA Archive kit and TaqMan® Gene Expression Assay kits were obtained from Applied Biosystems (Foster City, CA).
Mice
CFTR-KO mice are the North Carolina strain (B6.129P2-Cftrtm1Unc) in which exon 10 is disrupted and CFTR protein is not synthesized [23]. CFTR-ΔF508 mice (C57BL/6.Cftrtm/Kth, Jackson Laboratory, Bar Harbor, ME) carry a deletion of Phe508 disrupting the proper intracellular transport of the mutant CFTR protein. Mice were housed in the University of Alabama at Birmingham (UAB) Cystic Fibrosis Research Center animal care facility and all experimental protocols were approved by the UAB Institutional Animal Care and Use Committee. The homozygous CFTR-KO and ΔF508 (−/−) mice were obtained by mating heterozygous (+/−) mice. Mice were maintained on a liquid diet after weaning and genotypes were determined by PCR as described previously [24]. After 9–10 weeks of age, the mice were sacrificed, and total RNA was extracted from a portion of the livers using the STAT-60 reagent and stored at −80°C until analysis. To prepare liver cytosol, 100 mg of each mouse liver was homogenized on ice in 5 vol of 10 mM phosphate buffer, pH 7.4, containing 1 mM dithiothreitol and 10% glycerol. Liver homogenates were centrifuged at 100,000 × g for 1 h and cytosol was stored at −80°C until analysis.
Growth and maintenance of stably transformed cell lines
The generation of HepG2 cells stably transformed with either human SULT1E1/pcDNA3.1 or the pcDNA3.1 vector alone was carried out as described previously [25]. The cells were maintained in 10% FBS/MEM/geneticin (600 μg/ml) at 37°C in an incubator with 95% air/5% CO2. Medium was changed every 2–3 days and cells were subcultured at 90% confluency. For experiments, the cells were changed to phenol red-free medium supplemented with 10% charcoal-stripped FBS for 3 days before use. All cultures were changed to phenol red-free serum-free medium 24 h before their use in experiments. All experiments utilizing treated cells were performed in the phenol red-free serum-free medium.
SULT1E1 activity
SULT1E1 activity was determined using 20 nM [3H]-E2 as substrate and 10 μM PAPS as the sulfonate donor as described previously [2]. Protein concentrations were estimated using the Bio-Rad protein assay with gamma globulin as a standard.
Immunoblot analysis
To generate cell lysates, HepG2 cells were rinsed twice with 1 X PBS then lysed in M-PER reagent as per the manufacturer’s instructions. Phosphatase inhibitor cocktails 1 and 2 (Sigma) were immediately added to the lysates. For immunoblot analysis, HepG2 cell lysates (100 μg) were resolved by 10% SDS-polyacrylamide gel electrophoresis and electrophoretically transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat milk then incubated with the different antibodies at the following dilutions: mouse anti-STAT5 (Cell Signaling)(1:500), rabbit anti-phophosphorylated-Tyr-694-STAT5 (PY-STAT5, Cell Signaling)(1:1000) with horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies (1:10,000). Incubation with primary antibodies was at 4°C overnight. All primary antibody incubations were carried out in 0.7% Tween 20 in TBS, pH 8.0, with 0.02% azide. Bound antibodies were detected by chemiluminescence using the SuperSignal West Pico substrate. For detection of total STAT5, blots used to analyze PY-STAT5b expression were stripped by incubation for 30 min at 50°C in stripping buffer (100 mM 2-mercaptoethanol, 2% sodium dodecyl sulfate, 62.5 mM Tris-HCl, pH 6.7) then reprobed with the mouse anti-STAT5 antibody.
IGF-1 mRNA analysis
The mRNA abundance of IGF-1 in CFTR-KO mice and HepG2 cells was quantified by reverse transcription accompanied by real-time PCR. HepG2 cells were treated in triplicate without or with 10 nM E2 for 15 min, then with 500 ng/ml GH for 18 h. At the indicated times, total RNA was extracted using STAT-60 reagent. Reverse transcription was performed using the ABI high-capacity cDNA archive kit as recommended by the manufacturer.
Real-time PCR was performed in an ABI 7500 Real Time thermal cycler using the TaqMan® Universal PCR Master Mix containing AmpliTaq Gold® DNA Polymerase, and TaqMan® Gene Expression Assays (ABI) for human and mouse IGF-1. These include the use of unlabeled PCR primers (900 nM each final concentration) and a FAM™ dye-labeled TaqMan® MGB probe. The control for human was 18s ribosomal RNA and the control for mouse was glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The PCR cycling included 1 cycle each of 50°C × 2 min and 95°C × 10 min then 40 cycles of 95°C × 15 sec and 60°C × 1 min. The am ount of IGF-1 mRNA expression in a calibrator sample which had received no GH or E2 treatment was used to compare the expression in treated samples. Relative expression was determined with the comparative method.
Semi-quantitative RT-PCR mRNA analysis
The expression of several E2-regulated proteins in HepG2 cells was analyzed by semi-quantitative RT-PCR. Total RNA was extracted from control and SULT1E1-over-expresing HepG2 cells using STAT-60. Carbonic anhydrase isoforms II and III (CA II, CA III), glutathione-S-transferase-Pi (GST-Pi), cytochromes P450 (CYP) 2A6 and β-actin messages were amplified via RT-PCR. The CA II primers were; forward 5′-GGACAAGGTTCAGAGCATACTGTGG-3′ and reverse 5′-ACATTCCAGAAGAGGAGGGTG-3′. The CA III primers were; forward 5′-AATTGCCAAGGGGGAAAACC-3′ and reverse 5′-CATCAAGGAAAATCTGGAACTCGC-3′. The GST-Pi primers were; forward 5′-AAGCCTTTTGAGACCCTGCTGTCC-3′ and reverse 5′-GTTTCCCGTTGCCATTGATG-3′. The CYP2A6 primers were; forward 5′-CAAAGGCTATGGCGTGGTATTC-3′ and reverse 5′-TCTTGGCTATGAAGTCCTCCAGC-3′. Specific PCR product formation was analyzed in different cycles and product formation was generally in the linear range of synthesis in cycles 24–25. PCR products in the linear synthesis range were resolved in 1% agarose gels and visualized with ethidium bromide staining. Bands were quantified by scanning densitometry.
Densitometric and statistical analysis
Immunoreactive bands were quantified by densitometric scanning. All experiments were repeated at least twice. Mean ± s.e. represents results from multiple replicates of the same experiment. Data were analyzed by t-test and significance was set at p <0.05 or 0.01.
RESULTS
IGF-1 mRNA expression in CF knockout mice
CFTR-KO and ΔF508 (−/−) mice were generated by breeding (+/−) mice of both strains. The CFTR (−/−) pups generally die at weaning due to meconium ilius unless maintained on a liquid diet [26]. In CFTR (−/−) pups, gastrointestinal pathologies are a major functional aspect of the disease [26] with few pulmonary problems apparent. As reported previously for both CFTR-KO and ΔF508 (−/−) mice, lower body weights were observed in mice that had higher hepatic SULT1E1 activities [6, 8]. Since liver is the primary source for circulating IGF-1 and IGF-1 is an important factor in growth [27], the levels of IGF-1 message were examined in the livers of adult CFTR-KO and ΔF508 (−/−) mice to determine whether increases in SULT1E1 activity were associated with deficits in IGF-1 message expression. Figure 1A shows that in the CFTR (−/−) mice, IGF-1 message levels were decreased in CFTR (−/−) mice whose SULT1E1 activities were elevated. IGF-1 message levels were more than 10-fold lower in many of the CFTR (−/−) mice and the lower IGF-1 message levels in the CFTR (−/−) mice were negatively correlated (coeff. −0.62) with the increased levels of SULT1E1 activity of these animals (Fig. 1A). When IGF-1 message levels were compared to body weights of CFTR (−/−) mice and control (+/+ and +/−) mice, a positive correlation (coeff. 0.70) between IGF-1 message levels and body weights was observed (Fig. 1B).
Figure 1.
IGF-I mRNA levels in livers of mice of different CFTR genotypes. Real-time PCR analysis was carried out using RNA isolated from liver samples obtained from CFTR (−/−), heterozygous (−/+) and normal mice (+/+). IGF-1 mRNA levels were normalized to mouse GAPDH mRNA levels and shown on a relative scale. Panel A shows the association between SULT1E1 activity and IGF-1 message levels. Panel B shows the relationship between IGF-1 message levels and mouse body weight. The coefficients are −0.62 and 0.70 in panels A and B, respectively.
IGF-1 mRNA expression in HepG2 cells
GH therapy is used to increase the growth and weight gain as well as IGF-1 levels in CF patients suggesting a deficit in the response of hepatocytes to GH in CF [18]. Obtaining liver specimens from adult CF patients for the analysis of SULT1E1 expression is an infrequent event. Therefore, to investigate whether increased SULT1E1 activity affected IGF-1 message and E2-regulated gene expression in human hepatocytes, HepG2 cells were stably transformed with a human SULT1E1-pcDNA3.1 expression vector to mimic the increases observed in the CF mouse hepatocytes. The over-expression of SULT1E1 in the transformed HepG2 cells was confirmed by immunoblot analysis (Fig. 2A) and E2 sulfation activity (Fig. 2B). SULT1E1 activity in the SULT1E1-pcDNA3.1 transformed HepG2 cells was increased 7–100 fold as compared to normal HepG2 cells and HepG2 cells transformed with the pcDNA3.1 vector (Fig. 2B). A SULT1E1-transformed HepG2 clone showing an intermediate 30-fold increase of SULT1E1 activity as compared to control HepG2 cells was chosen for further characterization.
Figure 2.
Stable expression of SULT1E1 in human HepG2 hepatocytes. Panel A: immunoblot analysis of SULT1E1 protein expression in HepG2 cells. Lane 1 contains a positive control (human SULT1E1 protein, 35 KDa). Cytosolic proteins from normal HepG2 cells (NL, lane 2), pcDNA-HepG2 cells (P, lanes 3 and 4) and SULT1E1-HepG2 cells (E, lanes 5 and 6) were resolved by SDS-PAGE and immunoblotted with a rabbit anti-SULT1E1 antibody. Panel B: Individual colonies of HepG2 cells transfected with pcDNA or SULT1E1/pcDNA were selected following antibiotic selection. SULT1E1 activity was assayed in the individual clones with 20 nM E2 substrate and expressed as pmol E2 sulfated/min/mg cytosolic protein and compared to the activity in normal HepG2 cells.
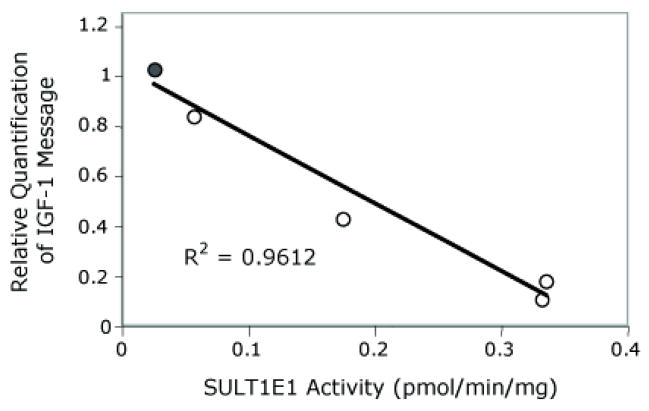
To determine whether increased SULT1E1 activity is associated with changes in IGF-1 expression in transformed HepG2 cells, IGF-1 message levels were determined by quantitative RT-PCR in several of the SULT1E1-HepG2 cell lines. Figure 3 shows the comparison of IGF-1 message levels and SULT1E1 activity in transformed HepG2 cells. In the transformed HepG2 cells, IGF-1 message levels decreased as cytosolic SULT1E1 activity levels increased suggesting that E2 may be involved with regulating IGF-1 expression.
Figure 3.
Association of IGF-1 message levels and SULT1E1 activity in SULT1E1- HepG2 cells. After transfection with SULT1E1-pcDNA, several SULT1E1 over-expressing HepG2 cell lines were generated. SULT1E1 activity in cytosol prepared from the HepG2 cells was determined using 20 nM [3H]-E2 as substrate and 10 μM PAPS as the sulfate donor as described in Methods. Total RNA was extracted and utilized to synthesize cDNA. Human IGF-1 message levels were determined by real-time quantitative PCR using an ABI 7500 thermal cycler and a TaqMan® Gene Expression Assay (ABI) for human IGF-1. 18S Ribosomal RNA was chosen as the endogenous control for total RNA normalization. The relative mRNA expression level of IGF-1 was calculated using the ΔΔCt method. The correlation coefficient is −0.94.
Li et al. [6] have reported that increased SULT1E1 activity in the livers of CFTR-KO and ΔF508 mice is associated with changes in the expression of E2-regulated proteins. Therefore, the effect of increasing SULT1E1 activity on the expression of several E2-regulated proteins was tested in the SULT1E1 transformed HepG2 cells. Figure 4 shows that CA II and GST-Pi message levels were significantly decreased in the SULT1E1-HepG2 cells. In contrast, message levels for CYP2A6 and CA III were not affected by the increase in SULT1E1 activity (data not shown)
Figure 4.
Changes in estrogen-regulated gene expression in HepG2 cells. Total RNA was extracted from HepG2 cells stably expressing SULT1E1. cDNA was reverse transcribed with random primers using SuperScript II reverse transcriptase. CA II, CA III, GST-Pi (GST-Pi) and CYP2A6 messages as well as β-actin were amplified via 30 cycles of polymerase chain reaction (PCR) at 56°C annealing temperature for 1.5 min and 72°C of extension for 1 min using individual gene specific primers. PCR products were resolved in 1% agarose gels and visualized with ethidium bromide staining as shown in panel A. STD refers to DNA standard ladder, “C” indicates control pcDNA HepG2 cells, “E” indicates SULT1E1-pcDNA HepG2 cells. The results of densitometric analysis of PCR product ethidium bromide staining bands were illustrated in panel B. Asterisks indicate a significant difference from control pcDNA samples at p<0.05 using the Student T-test.
Activation of the GH signaling pathway is a major regulatory mechanism for IGF-1 expression in liver [19–22]. IGF-1 mRNA levels are negatively correlated with SULT1E1 activities in the livers of CFTR (−/−) mice suggesting that decreased E2 levels in the liver may be involved in GH signaling (Fig. 1). To investigate whether increases in E2 or decreases in active E2 due to increased E2 sulfation affected GH signaling and IGF-1 message expression, SULT1E1-transformed HepG2 cells were used. The effects of E2 treatment on GH stimulated IGF-1 message expression were investigated in control and SULT1E1-HepG2 cells. Figure 5 shows that treatment of SULT1E1-HepG2 cells and pcDNA-HepG2 cells with GH increased the production of IGF-1 message as compared to untreated cells. GH treatment of control pcDNA-HepG2 cells increased IGF-1 message levels 8.2-fold; however, in the SULT1E1-HepG2 cells IGF-1 message levels increased only 2.6-fold. Pretreatment of the pcDNA-HepG2 and SULT1E1-HepG2 cells with 10 nM E2 prior to addition of GH resulted in a 51% increase in IGF-1 message in the pcDNA-HepG2 cells. In contrast, no significant increase in IGF-1 message was detected in the SULT1E1-HepG2 cells. The data indicate that physiological concentrations of E2 increased GH-stimulated IGF-1 message levels in control HepG2 cells, whereas increased SULT1E1 activity in the SULT1E1-HepG2 cells inhibited the increase in IGF-1 message upon GH stimulation even in the presence of added E2.
Figure 5.
Quantitative RT-PCR analysis of IGF-1 mRNA levels in human HepG2 cells treated with GH or GH and E2. HepG2 cells stably expressing SULT1E1 or control cells transfected with pcDNA were treated with or without 10 nM E2 for 15 min, followed by 500 ng/ml GH for 18 h. Total RNA was isolated from the cells and IGF-1 message levels analyzed as described in Methods. The histogram shows the mean ± S.E. of the relative levels of IGF-1 mRNA normalized to 18s ribosomal RNA in the cells. The symbols (*, &) show the difference between untreated pCDNA or SULT1E1-HepG2 cells and treatment with GH alone or E2 and GH at p<0.01, respectively. The symbol (#) indicates a difference at p<0.001 between pcDNA and SULT1E1-HepG2 cells treated with GH alone or GH and E2. The symbol ($) indicates a difference in pcDNA-HepG2 cells treated with GH or E2 and GH at p<0.01.
Over-expression of SULT1E1 reduces STAT5b phosphorylation
STAT5 is one of the major signal transduction proteins involved in growth and development, and is activated by phosphorylation in the GH signaling pathway [28]. In hepatocytes, STAT5b is the major isoform [29, 30]. In HepG2 cells STAT5b is the major isoform and STAT5a is below the limits of detection. GH is a potent stimulator for the formation of phosphorylated (PY)-STAT5b and subsequently IGF-1 synthesis and secretion in the liver [20, 31]. Activation of the GH receptor results in the phosphorylation of Jak-2 that subsequently stimulates the phosphorylation and activation of STAT5b. PY-STAT5b translocates to the nucleus and stimulates the transcription of several genes including IGF-1 [21]. To investigate whether E2 treatment and increased SULT1E1 activity altered the phosphorylation of STAT5b, GH was used to stimulate STAT5b phosphorylation with or without E2 pretreatment in control pcDNA- and SULT1E1-HepG2 cells.
Figure 6A shows that GH stimulates STAT5b phosphorylation in both control pcDNA- HepG2 and SULT1E1-HepG2 cells in a rapid manner with a maximal level of phosphorylation 30–60 min after treatment. However, the levels of PY-STAT5b were higher in the pcDNA-HepG2 cells than in the SULT1E1-HepG2 cells (Fig. 6A). A similar time course of STAT5b phosphorylation was observed in both pcDNA-HepG2 and SULT1E1-HepG2 cells when pretreated with 10 nM E2 for 15 min prior to the addition of GH (Fig. 6B). STAT5b phosphorylation was also significantly greater in the pcDNA-HepG2 cells than the SULT1E1-HepG2 cells. There was no effect of GH or E2 treatment on total STAT5 levels during these short treatment periods (Fig. 6B).
Figure 6.
Time course of GH stimulation of STAT5b phosphorylation in HepG2 cells. Panel A shows the time course of STAT5b phosphorylation by GH alone in control pcDNA and SULT1E1-HepG2 cells. The cells were treated with 500 ng/ml GH and lysed with M-PER at the appropriate times. HepG2 lysates were resolved by SDS-PAGE then phosphorylated STAT5b (PY-STAT5) was analyzed by immunoblot analysis. Panel B demonstrates the effect of pretreatment of the HepG2 cells with E2 on the time course of GH stimulated STAT5b phosphorylation. The cells were treated with 10 nM E2 for 15 min prior to the addition of 500 ng/ml GH. Cell lysates were prepared at the appropriate times and both PY-STAT5b and total STAT5b levels analyzed by immunoblot analysis.
The effect of increasing concentrations of E2 on STAT5b phosphorylation in the HepG2 cells was examined. pcDNA-HepG2 and SULT1E1-HepG2 cells were treated with E2 prior to the addition of GH. Figure 7 shows that a significant increase in PY-STAT5b was detected using 1 nM E2 and that the response increased as the E2 concentration increased to 50 nM. In contrast, a significant increase in PY-STAT5b was not observed in the SULT1E1-HepG2 cells until the E2 concentration reached 10 nM. At a concentration of 10 nM E2, the response in the SULT1E1 cells was only 18% of that detected in pcDNA-HepG2 cells.
Figure 7.
Dose response curve for the stimulation of STAT5b phosphorylation by E2. HepG2 cells stably transfected with the pCDNA vector or cells stably expressing SULT1E1 were treated with increasing concentrations of E2 for 15 min before the addition of 500 ng/ml GH. STAT5b phosphorylation was analyzed by immunoblotting and densitometric analysis. The graph shows the results of three separate experiments carried out in duplicate. The data are expressed as mean ± S.E. with the level of PY-STAT5b in cells treated with GH alone set at 100%. The symbols (#) and (*) indicate significant differences in the untreated PY-STAT5b levels in the two cell lines at p<0.05 and p<0.01, respectively.
Plasma GH levels show a pulsatile pattern of secretion that is variable with respect to age, sex and menstrual status and are regulated primarily by a combination of GH releasing factor, somatosatin and ghrelin [21, 32]. Therefore, the ability of GH concentrations from 50–1000 ng/ml to stimulate STAT5b phosphorylation in both pcDNA- and SULT1E1-HepG2 cells was investigated. Figure 8 shows the stimulation of STAT5b phosphorylation in both pcDNA- and SULT1E1-HepG2 cells by increasing GH concentrations alone or following treatment with 10 nM E2. Increasing concentrations of GH alone at less than 250 ng/ml had a modest effect on increasing PY-STAT5b levels in both cell lines. At these GH concentrations pretreatment with 10 nM E2 had a significant effect on PY-STAT5b in only the pCDNA-HepG2 cells. There was a sharp increase in STAT5b phosphorylation between GH concentrations of 250 and 500 ng/ml in both cell types.
Figure 8.
Dose response curve for the stimulation of STAT5b phosphorylation by GH. The effect of increasing GH concentrations on PY-STAT5b levels in pcDNA and SULT1E1-HepG2 cells was examined with and without pretreatment with 10 nM E2 for 15 min. PY-STAT5b levels were analyzed by immunoblot analysis and the results expressed as mean ± S.E. of three separate experiments done in duplicate.
Timing of E2 interaction with GH stimulation of STAT5b phosphorylation
The effect of the time of E2 pretreatment in both the pcDNA- and SULT1E1-HepG2 cells was analyzed to better understand the mechanism by which E2 stimulated the increased phosphorylation of STAT5b. Figure 9 shows that a short pretreatment of the HepG2 cells with 10 nM E2 prior to stimulation with GH resulted in the largest increases in PY-STAT5b.
Figure 9.
Effect of timing of E2 treatment on GH stimulation of STAT5b phosphorylation. Control and SULT1E1-HepG2 cells were treated with E2 (10 nM) from 30 min before to 15 min after the addition of 500 ng/ml GH. Cell lysates were prepared 30 min after the addition of GH. The levels of PY-STAT5b were analyzed by immunoblotting and densitometry. The level of PY-STAT5b in cells treated with only GH was set at 100%. The data are expressed as mean ± S.E. of the densitometric analysis of PY-STAT5b in three separate experiments.
To understand the mechanism of the enhancement of GH stimulation by E2, several other aspects of the GH signaling pathway were investigated. Treatment of pcDNA-HepG2 cells with 10 nM E2 in the absence of GH stimulation had no detectable effect on the level of PY-STAT5 (data not shown). In addition, E2 pretreatment had no effect on the levels of GH receptor in either pcDNA or SULT1E1-HepG2 cells. Also, neither short-term nor long-term (24 h) E2 and GH treatment had an effect on total Jak2 or phosphorylated Jak2 levels in either pcDNA- or SULT1E1-HepG2 cells (data not shown).
Effect of estrogen receptor (ER) inhibition on STAT5 phosphorylation
Many of the effects of E2 in HepG2 cells are anticipated to involve binding of the ERs, translocation to the nuclei and increases in gene transcription [33]. However, the rapid response of STAT5b phosphorylation to E2 and GH stimulation may not involve ER interactions. To investigate whether ER activation is involved with the increases in STAT5b phosphorylation, ICI 182,780 was used to inhibit the binding of E2 to the ERs. ICI 182,780 is a derivative of E2 that possesses a long, hydrophobic side chain at the 7α-position and exerts anti-estrogenic properties by blocking ER transactivation, impairing ER dimerization and inducing ER degradation [34–36]. Both types of HepG2 cell were pretreated with 1 μM ICI 182,780 for 5 min to 1 h prior to addition of 10 nM E2 and 500 ng/ml GH. PY-STAT5b levels were not attenuated by the addition of ICI 182,780 in either the pcDNA- or SULT1E1-HepG2 cells regardless of the time of ICI 182,780 treatment suggesting that the ERs are not involved in these rapid effects of E2 on STAT5b phosphorylation (data not shown).
DISCUSSION
Changes in the metabolism of estrogens, particularly E2, may have a role in alterations of liver function or in liver disease. Previous studies in CFTR (−/−) mice have demonstrated that high levels of SULT1E1 expression occur in the livers of both CFTRΔF508 and KO animals [6, 8]. Because SULT1E1 has an important role in the inactivation of E2 within cells at physiological concentrations [4, 25], the hepatic SULT1E1 activity in CFTR (−/−) mice is associated with the alteration of E2-regulated protein expression, supporting the evidence that hepatic function is altered in CF [6]. The induction of SULT1E1 activity apparently results from the loss of CFTR function in cholangiocytes. SULT1E1 is not detectable in human MMNK-1 cholangiocytes or in mouse cholangiocytes that characteristically express CFTR; conversely, SULT1E1 is found mouse hepatocytes or human HepG2 cells [3] where CFTR is not detectable. The mechanism by which the loss of CFTR function in cholangiocytes regulates SULT1E1 expression in hepatocytes has not been elucidated.
This manuscript investigates the effects of elevated SULT1E1 activity in human HepG2 hepatocytes on GH-stimulated signaling and E2-regulated protein expression. Li et al. [6] reported that augmented SULT1E1 activity in the livers of CFTR (−/−) mice affects the expression of estrogen-regulated proteins. In the present study, IGF-1 expression is negatively correlated with SULT1E1 activity levels in both CFTR mouse liver and SULT1E1-transformed HepG2 cells. The levels of SULT1E1 activity in CFTR mouse liver correlate inversely with body weight in the CFTR mice, suggesting that estrogens may have a role in regulating body weight [6, 8]. Low body weight and poor growth are common features of CF in children while serum IGF-1 levels are frequently lower in CF patients as compared to control patients [37–39]. To elucidate potential mechanisms by which SULT1E1 may exert its effects in liver, HepG2 cells were transformed with SULT1E1 as a model to evaluate the effects of E2 sulfation activity in hepatocytes of CFTR (−/−) mice. Due to the lack of human CF liver samples for evaluation, it is not been determined whether SULT1E1 is commonly increased in human hepatocytes in CF.
Increased SULT1E1 activity in human breast and endometrial cancer cells inhibits the effects of E2 by increasing E2 sulfation at picomolar and nanomolar concentrations indicating that sulfation is important in regulating the activity of E2 [3, 4, 25]. Sulfation of E2 functions as an inactivation mechanism because sulfated estrogens are incapable of binding to ERs and initiating response. However, in the SULT1E1-HepG2 model there were also distinct effects on other signal transduction pathways. Higher levels of E2 sulfation affected GH signaling by decreasing STAT5b phosphorylation without affecting GH receptor levels or Jak-2 activation. Since several signal transduction pathways intersect by regulating STAT5b phosphorylation, the effects of E2 sulfation on STAT5b phosphorylation may affect a greater number of regulatory mechanisms than the expression of IGF-1 by GH.
In the HepG2 cell system, it appears that E2 is required for optimal GH stimulation of STAT5b phosphorylation and, correspondingly, IGF-1 message expression. Consistent with previous studies, GH alone stimulated STAT5b phosphorylation in cultured HepG2 cells [40] whereas E2 alone had no direct effect on STAT5b phosphorylation. These results differed from those obtained using GH receptor knockout (GHR-KO) mice. Venken et al. [41] reported that pharmacologic levels of E2 (100 nM) in ovariectomized GHR-KO male mice increased Jak2 and STAT5 levels as well as hepatic IGF-1 synthesis. It is possible that at high concentrations E2 is interacting with other receptors or acting through other signal transduction systems. Leung et al. [42] also reported that E2 is capable of altering GH stimulation of STAT5b phosphorylation via the induction of SOC-2 expression. These results differ from the effects observed in HepG2 cells where E2 apparently elicits a rapid response that does not seem to involve binding to the ERs with transcriptional activation of ER-responsive genes.
The mechanism for the interaction of E2 and GH in the regulation of STAT5b phosphorylation is unknown. The effect of E2 and GH on stimulating STAT5b phosphorylation was optimal 15 to 30 min after the addition of GH. Although the exact pathway of E2 rapid action is not well defined, it is believed that these effects do not involve the classical ERs. The observation that the application of the ER antagonist ICI 182,780 did not affect the phosphorylation of STAT5b is consistent with a rapid and nongenomic effect of E2 in the HepG2 cells. Neither changes in GH receptor levels or Jak-2 phosphorylation were detected with the addition of E2 suggesting that E2 is not binding to or indirectly affecting the activation of the GH receptor. Estrogens have been shown to stimulate phospholipase C and adenylate cyclase activity resulting in increased production of inositol lipids and cAMP [43]. Hsieh et al. [44] have demonstrated that G Protein-coupled receptor (GPR) 30 in rat hepatocytes binds E2 and activates PKA. GPR30 is not inhibited by ICI 182,780 but the compound rather appears to mimic the effects of E2 [45]. E2 has also been reported to stimulate STAT5b phosphorylation in a Jak-independent mechanism in porcine aortic endothelial cells [46]. This process was proposed to involve a nongenomic mechanism that involved the ERs directly interacting with c-Src, MAPK and PI3K.
The ability of E2 to enhance STAT5b phosphorylation stimulated by GH in HepG2 cells is contrary to the effects of E2 and GH therapy in CF patients. It is proposed that E2 decreases IGF-1 synthesis in the liver resulting in an increase in GH release from the pituitary [47, 48]. However, this may be the result of long-term effects of E2 on the expression of enzymes such as Soc-2 that regulate Jak-2 phosphorylation [43]. Increased SULT1E1 expression in the CFTR (−/−) mice is associated with decreased body size and IGF-1 message levels in the liver [6]. Many children with CF have a high incidence of poor growth and delayed puberty [37, 39] as well as low IGF-1 plasma levels. GH treatment normalizes pubertal onset in adolescents with CF and improves growth and accumulation of bone mineralization [49, 50]. However, most CF patients also have diabetes and associated fat malabsorption problems that may significantly affect the signaling pathways normally associated with IGF-1 and GH [51].
In summary, the observation that CFTR (−/−) mice with elevated hepatic SULT1E1 activity have decreased hepatic IGF-1 message levels suggests E2 is involved in the regulation of IGF-1 expression. As a model for the induction of SULT1E1 expression in CFTR (−/−) mouse hepatocytes, SULT1E1 was stably expressed in human HepG2 hepatocytes. In HepG2 cells, E2 was capable of increasing the ability of GH to stimulate STAT5b phosphorylation in a rapid non-genomic mechanism apparently not involving the ERs. STAT5b phosphorylation results in augmented transcription of the IGF-1 gene in hepatocytes. The over-expression of SULT1E1 activity in the HepG2 cells inhibited the ability of E2 to interact with GH to stimulate STAT5b phosphorylation and IGF-1 message expression. This demonstrates that higher levels of SULT1E1 activity in the hepatocytes of CFTR (−/−) mice and in transfected HepG2 cells may affect the ability of E2 to activate both genomic and non-genomic pathways.
Supplementary Material
Acknowledgments
This work was supported by a research grant from Cystic Fibrosis Research, Inc. and NIH grant GM38953 to CNF.
Abbreviations
- E2
17β-estradiol
- SULT
sulfotransferase
- CF
cystic fibrosis
- SULT1E1
sulfotransferase 1E1
- CFTR
cystic fibrosis transmembrane receptor
- IGF-1
insulin-like growth factor-1
- GH
growth hormone
- STAT5b
signal transducers and activators of transcription 5b
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Falany CN. Enzymology of human cytosolic sulfotransferases. FASEB J. 1997;11:206–216. doi: 10.1096/fasebj.11.4.9068609. [DOI] [PubMed] [Google Scholar]
- 2.Falany CN, Krasnykh V, Falany JL. Bacterial expression and characterization of a cDNA for human liver estrogen sulfotransferase. J Steroid Biochem Mol Biol. 1995;52:529–539. doi: 10.1016/0960-0760(95)00015-r. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, Varlamova O, Vargas FM, et al. Sulfuryl transfer: the catalytic mechanism of human estrogen sulfotransferase. J Biol Chem. 1998;273:10888–10892. doi: 10.1074/jbc.273.18.10888. [DOI] [PubMed] [Google Scholar]
- 4.Kotov A, Falany JL, Wang J, Falany CN. Regulation of estrogen activity by sulfation in human Ishikawa endometrial adenocarcinoma cells. J Steroid Biochem Mol Biol. 1999;68:137–144. doi: 10.1016/s0960-0760(99)00022-9. [DOI] [PubMed] [Google Scholar]
- 5.Falany JL, Macrina N, Falany CN. Regulation of MCF-7 breast cancer cell growth by beta-estradiol sulfation. Breast Cancer Res Treat. 2002;74:167–176. doi: 10.1023/a:1016147004188. [DOI] [PubMed] [Google Scholar]
- 6.Li L, Falany CN. Elevated hepatic SULT1E1 activity in mouse models of cystic fibrosis alters the regulation of estrogen responsive proteins. J Cyst Fibrosis. 2007;6:23–30. doi: 10.1016/j.jcf.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg LA, Schluchter MD, Parlow AF, Drumm ML. Mouse as a model of growth retardation in cystic fibrosis. Pediatr Res. 2006;59:191–195. doi: 10.1203/01.pdr.0000196720.25938.be. [DOI] [PubMed] [Google Scholar]
- 8.Falany JL, Greer H, Kovacs T, et al. Elevation of hepatic sulphotransferase activities in mice with resistance to cystic fibrosis. Biochem J. 2002;364:115–120. doi: 10.1042/bj3640115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welsh MJ, Tsui L-C, Boat TF, Beaudet AL. In the metabolic basis of inherited disease. McGraw-Hill; New York: 1995. pp. 3799–3863. [Google Scholar]
- 10.Berschneider HM, Knowles MR, Azizkhan RG, et al. Altered intestinal chloride transport in cystic fibrosis. FASEB J. 1988;2:2625–2629. doi: 10.1096/fasebj.2.10.2838365. [DOI] [PubMed] [Google Scholar]
- 11.Trezise AE, Buchwald M. In vivo cell-specific expression of the cystic fibrosis transmembrane conductance regulator. Nature. 1991;353:434–437. doi: 10.1038/353434a0. [DOI] [PubMed] [Google Scholar]
- 12.Durie PR, Kent G, Phillips MJ, Ackerley CA. Characteristic multiorgan pathology of cystic fibrosis in a long-living cystic fibrosis transmembrane regulator knockout murine model. Am J Pathol. 2004;164:1481–1493. doi: 10.1016/S0002-9440(10)63234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boguszewski MC, Kamoi TO, Bento RR, et al. Insulin-like growth factor-1, leptin, body composition, and clinical status interactions in children with cystic fibrosis. Hormone Res. 2007;67:250–256. doi: 10.1159/000098480. [DOI] [PubMed] [Google Scholar]
- 14.Ozen M, Cokugras H, Ozen N, et al. Relation between serum Insulin-like growth factor-I and insulin-like growth factor-binding protein-3 levels, clinical status and growth parameters in prepubertal cystic fibrosis patients. Pediatr Int. 2004;46:429–435. doi: 10.1111/j.1442-200x.2004.01925.x. [DOI] [PubMed] [Google Scholar]
- 15.Leung KC, Johannsson G, Leong GM, Ho KK. Estrogen regulation of growth hormone action. Endocr Rev. 2004;25:693–721. doi: 10.1210/er.2003-0035. [DOI] [PubMed] [Google Scholar]
- 16.Alvaro D, Mancino MG, Glaser S, et al. Proliferating cholangiocytes: a neuroendocrine compartment in th ediseased liver. Gastroenterol. 2007;132:415–431. doi: 10.1053/j.gastro.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Rochwerger L, Dho S, Parker L, et al. Estrogen –dependent expression of the cystic fibrosis transmembrane regulator gene in a novel uterine epithelial cell line. J Cell Sci. 1994;107:2439–2448. doi: 10.1242/jcs.107.9.2439. [DOI] [PubMed] [Google Scholar]
- 18.Hardin DS, Adams-Huet B, Brown D, et al. Growth hormone treatment improves growth and clinical status in prepubertal children with cystic fibrosis: results of a multicenter randomized controlled trial. J Clin Endocrinol Metab. 2006;91:4925–4929. doi: 10.1210/jc.2006-1101. [DOI] [PubMed] [Google Scholar]
- 19.Bazan JF. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci. 1990;87:6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Argetsinger LS, Campbell GS, Yang X, et al. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell. 1993;74:237–244. doi: 10.1016/0092-8674(93)90415-m. [DOI] [PubMed] [Google Scholar]
- 21.Finidori J. Regulators of growth hormone signaling. Vitamin Horm. 2000;59:71–97. doi: 10.1016/s0083-6729(00)59004-9. [DOI] [PubMed] [Google Scholar]
- 22.Herrington J, Smit LS, Schwartz J, Carter-Su C. The role of STAT proteins in growth hormone signaling. Oncogene. 2000;19:2585–2597. doi: 10.1038/sj.onc.1203526. [DOI] [PubMed] [Google Scholar]
- 23.Koller BH, Kim HS, Latour AM, et al. Toward an animal model of cystic fibrosis: targeted interruption of exon 10 of the cystic fibrosis transmembrane regulator gene in embryonic stem cells. Proc Natl Acad Sci. 1991;88:10730–10734. doi: 10.1073/pnas.88.23.10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du M, Jones JR, Lanier J, et al. Aminoglycoside suppression of a premature stop mutation in a Cftr−/− mouse carrying a human CFTR-G542X transgene. J Mol Med. 2002;80:595–604. doi: 10.1007/s00109-002-0363-1. [DOI] [PubMed] [Google Scholar]
- 25.Falany JL, Falany CN. Regulation of estrogen activity by sulfation in human MCF-7 breast cancer cells. Oncol Res. 1997;9:589–596. [PubMed] [Google Scholar]
- 26.Ratcliff R, Evans MJ, Cuthbert AW, et al. Production of a severe cystic fibrosis mutation in mice by gene targeting. Nat Genet. 1993;4:35–41. doi: 10.1038/ng0593-35. [DOI] [PubMed] [Google Scholar]
- 27.Christoforidis A, Maniadaki I, Stanhope R. Growth hormone/insulin-like growth factor-1 axis during puberty. Pediatr Endocrinol Rev. 2005;3:5–10. [PubMed] [Google Scholar]
- 28.Davey HW, Xie T, McLachlan MJ, et al. STAT5b is required for GH-induced liver IGF-I gene expression. Endocrinology. 2001;142:3836–3841. doi: 10.1210/endo.142.9.8400. [DOI] [PubMed] [Google Scholar]
- 29.Holloway MG, Cui Y, Laz EV, et al. Loss of sexually dimorphic liver gene expression upon hepatocyte-specific deletion of Stat5a-Stat5b locus. Endocrinology. 2007;148:1977–1986. doi: 10.1210/en.2006-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phung-Koskas T, Pilon A, Pous C, et al. STAT5B-mediated growth hormone signaling is organized by highly dynamic microtubules in hepatic cells. J Biol Chem. 2005;280:1123–1131. doi: 10.1074/jbc.M409918200. [DOI] [PubMed] [Google Scholar]
- 31.Carter-Su C, King AP, Argetsinger LS, et al. Signalling pathway of GH. Endocr J. 1996;43:S65–S70. doi: 10.1507/endocrj.43.suppl_s65. [DOI] [PubMed] [Google Scholar]
- 32.Waxman DJ, O’Connor C. Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol. 2006;20:2613–2629. doi: 10.1210/me.2006-0007. [DOI] [PubMed] [Google Scholar]
- 33.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116:561–570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fawell SE, White R, Hoare S, et al. Inhibition of estrogen receptor-DNA binding by the “pure” antiestrogen ICI 164,384 appears to be mediated by impaired receptor dimerization. Proc Natl Acad Sci. 1990;87:6883–6887. doi: 10.1073/pnas.87.17.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;51:3867–3873. [PubMed] [Google Scholar]
- 36.Wakeling AE. Tissue-specific actions of antioestrogens. Mutat Res. 1995;333:45–49. doi: 10.1016/0027-5107(95)00130-1. [DOI] [PubMed] [Google Scholar]
- 37.Byard PJ. The adolescent growth spurt in children with cystic fibrosis. Ann Human Biol. 1994;21:229–240. doi: 10.1080/03014469400003242. [DOI] [PubMed] [Google Scholar]
- 38.Arumugam R, LeBlanc A, Seilheimer DK, Hardin DS. Serum leptin and IGF-I levels in cystic fibrosis. Endocr Res. 1998;24:247–257. doi: 10.1080/07435809809135532. [DOI] [PubMed] [Google Scholar]
- 39.Morison S, Dodge JA, Cole TJ, et al. Height and weight in cystic fibrosis: a cross sectional study. UK Cystic Fibrosis Survey Management Committee. Arch Dis Child. 1997;77:497–500. doi: 10.1136/adc.77.6.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park SH, Wiwi CA, Waxman DJ. Signaling cross-talk between hepatocyte nuclear factor 4alpha and growth-hormone-activated STAT5b. Biochem J. 2006;397:159–168. doi: 10.1042/BJ20060332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venken K, Schuit F, Van LL, et al. Growth without growth hormone receptor: estradiol is a major growth hormone-independent regulator of hepatic IGF-I synthesis. J Bone Miner Res. 2005;20:2138–2149. doi: 10.1359/JBMR.050811. [DOI] [PubMed] [Google Scholar]
- 42.Leung KC, Brce J, Doyle N, et al. Regulation of growth hormone signaling by selective estrogen receptor modulators occurs through suppression of protein tyrosine phosphatases. Endocrinology. 2007;148:2417–2423. doi: 10.1210/en.2006-1305. [DOI] [PubMed] [Google Scholar]
- 43.Chen TL, Liu F, Bates RL, Hintz RL. Further characterization of insulin-like-growth factor binding proteins in rat osteoblast-like cell cultures: modulation by 17 beta-estradiol and human growth hormone. Endocrinology. 1991;128:2489–2496. doi: 10.1210/endo-128-5-2489. [DOI] [PubMed] [Google Scholar]
- 44.Hsieh Y-C, Yu H-P, Frink M, et al. G protein-Coupled Receptor 30-Dependent Protein Kinase A Pathway Is Critical in Nongenomic Efects of Estrogen in Attenuating Liver Injury after Trauma-Hemorrhage. AM J Pathol. 2007;170:1210–1218. doi: 10.2353/ajpath.2007.060883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rae JM, Johnson MD. What Does an Orphan G-Protein-Coupled Receptor Have to do with Estrogen? Breast Cancer Res. 2005;7:243–244. doi: 10.1186/bcr1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bjornstrom L, Sjoberg M. Signal transducers and activators of transcription as downstream targets of nongenomic estrogen receptor actions. Mol Endocrinol. 2002;16:2202–2214. doi: 10.1210/me.2002-0072. [DOI] [PubMed] [Google Scholar]
- 47.Cook DM, Ludlam WH, Cook MB. The adult growth hormone deficiency syndrome. Adv Intern Med. 2000;45:297–315. [PubMed] [Google Scholar]
- 48.Cook DM. Growth hormone and estrogen: a clinician’s approach. J Pediatr Endocrinol Metab. 2004;17:1273–1276. [PubMed] [Google Scholar]
- 49.Hardin DS, Ahn C, Prestidge C, et al. Growth hormone improves bone mineral content in children with cystic fibrosis. J Pediatr Endocrinol Metab. 2005;18:589–595. doi: 10.1515/jpem.2005.18.6.589. [DOI] [PubMed] [Google Scholar]
- 50.Hardin DS, Ms-Huet B, Brown D, et al. Growth hormone treatment improves growth and clinical status in prepubertal children with cystic fibrosis: results of a multicenter randomized controlled trial. J Clin Endocrinol Metab. 2006;91:4925–4929. doi: 10.1210/jc.2006-1101. [DOI] [PubMed] [Google Scholar]
- 51.Colombo C, Battezzati PM, Strazzabosco M, Podda M. Liver and biliary problems in cystic fibrosis. Semin Liver Dis. 1998;18:227–235. doi: 10.1055/s-2007-1007159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.