Abstract
Neutrophil retention in and release from the bone marrow is a critical process that remains incompletely understood. Previous work has implicated the CXCR4/SDF-1 chemokine axis in the marrow retention of neutrophils, yet the adhesion pathways responsible for this retention are unknown. Since α4β1 integrin (VLA-4) and its ligand VCAM-1 play a central role in the interactions of hematopoietic stem cells, lymphocytes, and developing neutrophils in the marrow, we investigated whether this integrin might be involved in marrow neutrophil retention and release. Here we show that VLA-4 is expressed on murine marrow neutrophils and decreases with maturation, while blockade of this integrin leads to the release of marrow neutrophils. Marrow neutrophils adhere via VLA-4 to VCAM-1, which is expressed on marrow endothelium and stroma, and inhibition of VCAM-1 causes release of marrow neutrophils. Furthermore, SDF-1 (CXCL12) signaling through neutrophil CXCR4 augments VLA-4 adhesion to VCAM-1 in vitro, an effect that is blocked by pre-incubation with pertussis toxin. In vivo blockade of both CXCR4 and α4 causes synergistic release of marrow neutrophils, showing that crosstalk between CXCR4 and VLA-4 modulates marrow retention of these cells. Taken together, these results indicate that the VLA-4/VCAM adhesion pathway is critical in the retention and maturation-controlled release of neutrophils from the marrow, while providing an important link between the CXCR4/SDF-1 signaling axis and the adhesion events that govern this process.
Keywords: Neutrophils, Adhesion Molecules, Chemokines, Hematopoiesis, Rodent
Introduction
Homeostatic control of circulating neutrophil levels is a critical process, as divergence of blood neutrophil content toward either extremely low or high levels may lead to overwhelming infection or inappropriate inflammatory states, respectively. Although the processes governing neutrophil recruitment to sites of inflammation have been extensively studied, comparably little is known of the mechanisms responsible for neutrophil retention within the bone marrow and subsequent release into the circulation, particularly in the homeostatic state (1). We (2) and others (3, 4) have demonstrated that the CXC cytokine Stromal Derived Factor-1 (SDF-1; CXCL12) and its receptor, CXCR4, function to retain neutrophils in the marrow under normal conditions. Modulation of this chemokine/receptor axis by maturation-driven decrease in neutrophil surface CXCR4 expression and signaling in the marrow promotes orderly release of the retained cells to the periphery (2). This work provides insight into the cytokine pathways controlling the retention and release of marrow neutrophils, yet the corollary role of cell surface adhesion molecules in these processes remains unknown.
Several lines of evidence led us to hypothesize that the α4 integrin VLA-4 (α4β1, CD49d/CD29) might participate in the adhesion events governing homeostatic retention and release of bone marrow neutrophils. This integrin has been implicated in the marrow homing and retention of hematopoietic stem cells through its binding of endothelial and stromal cell surface VCAM-1 (CD106) in the marrow (5-10), and both VLA-4 and VCAM appear to be critical for normal lymphopoiesis and myelopoiesis within the marrow (11-14). Furthermore, studies examining GCSF-mediated hematopoietic stem cell mobilization from the marrow, a process that is accompanied by significant blood neutrophilia, have shown that its effects on the marrow, in part, are mediated by downregulation of VCAM, presumably interrupting VLA-4 adhesion (15, 16).
Although VLA-4 expression on human blood neutrophils is controversial (17-20), marrow myeloid precursors have been shown to express high levels of VLA-4, which decrease during cell maturation (21-23), a finding that may suggest VLA-4 involvement in subsequent marrow neutrophil release. Yet, understanding of VLA-4 function in marrow neutrophils is limited. Recent studies have suggested a role for α4 integrins in regulating release of neutrophils from the marrow during inflammatory conditions (24). These authors demonstrated failure of MIP-2-mediated mobilization of neutrophils following blockade of CD49d (the α4 subunit of both VLA-4 [α4β1] and LPAM [α4β7]) in an isolated perfused rat hind limb model of neutrophil release.
In this study we show that VLA-4 and its ligand VCAM are crucial in the homeostatic retention and release bone marrow neutrophils. We further demonstrate that signaling through CXCR4 affects neutrophil release from the bone marrow by modulating VLA-4/VCAM adhesion.
Materials and Methods
Mice
Four to eight week old female C57BL/6 mice were obtained from Harlan (Indianapolis, IN) and housed in the animal facilities of the University of Vermont College of Medicine. All experiments were performed in accordance with the Animal Welfare Act and the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals after review of the protocol by the Animal Care and Use Committee of the University Of Vermont.
Reagents
Rat anti-mouse VCAM-1 (M/K-2; Serotec, Raleigh, NC), CD49d (2B2.32; US Biological, Swampscott, MA), LPAM-1 (DATK32; BD Pharmingen, Burlingame, CA), CD62L (MEL14; Biosource, Camarillo, CA), CD11a (FD441.8; Leinco, St. Louis, MO) and CD54 (YN1/1.7.4 ; eBioscience, San Diego, CA) neutralizing Abs, and appropriate isotype control Abs were obtained commercially. CXCR4 neutralizing polyclonal rabbit anti-mouse Abs (727/268b) (2, 25) were the kind of gift of Dr. Jose-Angel Gonzalo (Millennium Pharmaceuticals, Cambridge, MA). All Abs used in the in vivo experiments were azide-free. For flow cytometry and fluorescent immunohistology, Rat anti-mouse CD16/CD32 (Fc-Block®), FITC-conjugated rat anti-mouse CD49d (clone R1-2), FITC-conjugated rat anti-mouse LPAM-1 (DATK32), PE-conjugated rat anti-mouse Gr-1 (Ly-6C/G; RB6C6.8C5) monoclonal and isotype control Abs were obtained from BD Pharmingen. Mouse anti-human/mouse SDF-1 monoclonal antibody (clone 79018) was obtained from R&D Systems, Minneapolis, MN. Alexa Fluor® 647-conjugated mouse anti-BrdU (PRB-1) and isotype control Abs, as well as Alexa Fluor® 488-conjugated goat anti-rat IgG (H+L) were obtained from Invitrogen, Carlsbad, CA. Recombinant human VCAM-1 (catalog # ADP5) and recombinant mouse CXCL12 (catalog # 460-SD/CF) were obtained from R&D Systems.
Determination of neutrophil surface VLA-4 expression
Marrow and blood neutrophil expression of VLA-4 was determined by flow cytometry, while correlation between levels of VLA-4 expression and the maturational state of the marrow myeloid cells was examined using BrdU pulse labeling in vivo as modified from Basu, et al (26). Briefly, mice were injected with BrdU (50mg/kg in PBS) by tail vein infusion and, 24h later, were euthanized, after which whole blood and femoral marrow were harvested. Marrow plugs were disaggregated and washed twice with PBS, while erythrocyte lysis was performed on whole blood aliquots using Geye's solution, before washing with PBS. 2×106 cells/sample were resuspended in 0.5ml cold 0.25% PFA and fixed on ice for 5min then at RT for 30min. Cells were then pelletted, resuspended in 0.5ml 2N HCl + 0.5% Tween-20, incubated 30 min at 37C before being neutralized with 0.1M sodium borate, and washed with PBS/1%Tween.
The prepared samples were then resuspended in a solution of 1:20 Fc block in PBS/Tween and incubated for 30min on ice, before being pelleted and resuspended in a cocktail of FITC-anti-CD49d (1:100), Alexa Fluor® 647-anti-BrdU (1:250), and PE-anti-Gr-1 (1:500) for 30min on ice. The samples were then washed in PBS/Tween and resuspended in 300μl 0.25% FBS/PBS for flow cytometry. Cells were examined using an LSR II flow cytometer (Becton Dickinson), and data analyzed by FloJo software (Tree Star, Inc., Ashland, OR). Blood and mature marrow neutrophils were identified by gating for Gr-1hi cells, whereas the less mature forms of the myeloid lineage were examined by gating for all Gr-1+ cells (both Gr-1hi and Gr-1lo) when examining marrow neutrophil maturation states with BrdU. These cell populations were then analyzed for CD49d (α4) expression. Results were expressed as both percent positive (using isotype control) and as a ratio of the mean fluorescence intensity of cells stained with CD49d mAb versus isotype control Ab. To determine the relative maturational states of the marrow neutrophils, BrdU content was examined and categorized by flow as negative, dim, or bright, as explained in Results. Three to five separate experiments were performed for each analyzed population of neutrophils. Representative flow histograms are also presented.
Preparation of morphologically mature murine bone marrow neutrophils
In order to obtain marrow neutrophils from the post-mitotic, morphologically mature pool, we used a discontinuous density gradient to separate whole marrow (2, 27). This method exploits the previously noted correlation between marrow neutrophil density and maturity (28, 29). Femurs and tibias of euthanized mice were dissected, the marrow flushed with Hanks buffered saline solution (HBSS), and layered on a 3 step Percoll (Pharmacia) gradient (72%, 64%, and 52%) which was then centrifuged at 1060 g for 30 minutes (27). Cytospin samples of the 72:64% interface revealed >95% morphologically mature appearing neutrophils. Typical yields were approximately 1-2 × 107 neutrophils per mouse, which were >95% viable, with minimal apoptosis (<5%) by TUNEL assay (Boehringer Mannheim). These techniques have previously been shown not cause substantial activation or damage to the isolated cells (2, 27).
Evaluation of the effects of α4 and VCAM-1 neutralizing Abs on neutrophil marrow retention and release in vivo
a) Mobilization of endogenous and labeled neutrophils from marrow
The in vivo effects of Ab-mediated CD49d (α4) blockade were initially assessed by examining the response of circulating neutrophil levels in treated mice. C57Bl/6 mice were injected with either anti-CD49d mAb (2B2.32) or isotype control Abs via tail vein (30μg in 200μl of PBS/BSA). After 4h, 500μl of peripheral blood were obtained by cardiac puncture under pentobarbital anesthesia. Peripheral blood was then analyzed using an Advia 120 hematology analyzer with veterinary software (Siemens/Bayer, Tarrytown, NY). Similar experiments were performed to evaluate the effects of CD106 (VCAM-1) blockade using anti-CD106 (MK/2; 30μg).
The effects of injected blocking Abs on marrow neutrophil release were quantified using methods previously described to monitor the release of labeled cells under homeostatic conditions (2, 27). Briefly, morphologically mature bone marrow neutrophils were isolated and radiolabeled with 111indium-tropolonate as described (27), and aliquots of 5×106 labeled neutrophils in 200μl of PBS with 0.1% bovine serum albumin (PBS/BSA) were infused via tail vein injection into otherwise untreated recipient mice. After infusion, the cells were allowed to localize to marrow for 4h, a time point at which such sequestration has reached a plateau (typically 60-80% of infused cells (27)). CD49d-blocking or isotype control Abs were then administered to the recipient animals via tail vein injection (30μg in 200μL PBS/BSA), and the animals were subsequently bled (10μl) and euthanized 2h later. Mice were dissected and the lungs, spleen, kidneys, gut, liver, and right femur were removed and washed with saline. The remaining mouse carcass was divided into head, tail, thorax and hindquarters. A Wallac 1480 gamma counter, set to count both 173 KeV and 247 KeV peaks of 111indium was used to count radioactivity in all samples. 111Indium content in all tissues was expressed as a percentage of the total counts present in all tissues (the entire mouse). Values for the estimated total blood cpm were based on a predicted total blood volume of approximately 1.5ml for the 4-8 week old C57Bl/6 mouse (30). The marrow content of C57Bl/6 mouse femur was estimated to represent 6% of the total marrow content based on the work of Boggs (31). Neutrophil release was expressed as the percentage change in neutrophil marrow retention in treated animals compared to control animals. Similar experiments were performed to evaluate the effects of Ab-mediated CD106 (VCAM) neutralization (MK/2; 30μg IV) on marrow neutrophil release. To examine the nonspecific effects of antibody binding of neutrophil or marrow cell surface antigens in this model, blocking antibodies against CD62L (L-selectin; MoAb clone MEL14), CD11a (αL; MoAb clone FD441.8) and CD54 (ICAM-1; MoAb clone YN1/1.7.4) were infused in separate similar experiments (30ug) and compared to isotype control Ab infusions.
The synergistic effects of very low dose CD106 (VCAM) and CD184 (CXCR4) Ab blockade on marrow neutrophil release were similarly examined, as we have previously described (2), using Ab doses below the thresholds determined for each individual Ab to show a mobilizing effect. Anti-CD106 (0.01μg IV) and Anti-CXCR4 (0.02μg IV) Abs were administered separately and together to groups of mice as above, and the mice were then bled and dissected for assay 2h later.
In order to further examine the neutrophil mobilization effects of CD49d inhibition, BrdU pulse labeling was performed in mice as described above. 48h after BrdU dosing, a time ∼12-24h prior to normal release of the most mature BrdU-labeled marrow neutrophils (BrdUbright cells), animals were injected with either CD49d antibody or isotype control (30μg in 200μL PBS/BSA). 4h later the mice were euthanized and blood and marrow collected and analyzed by flow cytometry for BrdU incorporation as detailed above. Blood samples were also assayed by hematology analyzer to determine absolute neutrophil content (cells/mL), while marrow samples were analyzed for total nucleated cells (per femur). Absolute blood levels for each neutrophil population (BrdUnegative, BrdUbright, and BrdUdim) were calculated as the percentage of total Gr-1hi cells staining for each BrdU pattern multipled by the absolute blood neutrophil content for each sample. Marrow levels of each neutrophil population were calculated as a percentage of total Gr-1+ cells staining for each BrdU pattern, and normalized to the average total nucleated cell content/femur of isotype control animals.
b) Blockade of neutrophil homing to marrow
The effects of Ab-mediated CD49d blockade were further assessed by measuring marrow homing of intravenously infused labeled marrow neutrophils. Such homing has previously been used to demonstrate the importance of CXCR4 in marrow neutrophil retention (2). Briefly, 5×106 radiolabeled morphologically mature bone marrow neutrophils were infused through the tail vein of each recipient mouse 30min after treatment with anti-CD49d or isotype control Abs (30μg in PBS/BSA). Previous experiments in our laboratory have demonstrated that nonspecific effects on localization mediated through surface-bound Ab (such as due to opsonization) are minimal in this system (2). Neutrophil localization was assayed 4h later, a time point at which marrow localization has reached a stable plateau following infusion (27). The animals were bled (10μl), euthanized, dissected, and assayed as above. The effects of CD106 (VCAM) blockade on neutrophil marrow homing were examined in similar experiments.
In additional experiments, neutralizing Ab specific for the LPAM-1 integrin complex (α4β7) was infused (40μg) in attempts to distinguish which of the known heterodimers of CD49d (α4β1, α4β7) might be critical for neutrophil retention in the marrow, as both are inhibited by CD49d blockade, and both are known to bind similar ligands in the marrow including VCAM-1 (32).
Calcium Flux Assays
In order to examine whether neutrophil binding of CD49d blocking Ab might activate the cells, calcium flux assays were performed as previously described (2). Cells were labeled with the fluorochrome Indo-1/AM (Invitrogen, Carlsbad, CA) and calcium mobilization in response to α4-blocking antibody (2B2.32; 20 ug/ml) or fMLP (1μM; as a positive control) was assayed using an LSR flow cytometer (Becton Dickinson). Results were expressed as the ratio of Indo 1-Violet to Indo 1-Blue against time.
Immunohistology
Femurs were dissected from C57Bl/6 mice and marrow plugs were flushed from the bone with 4% formaldehyde and incubated for 30 minutes. Plugs were then washed with PBS and embedded with OTC Compound (Tissue–Tek) and snap frozen before cryostat sectioning and mounting. Slides were kept at −80°C until stained. For staining, slides were washed in PBS and permeablized with 0.1% Triton X-100 in PBS, before blocking with 1% BSA in PBS followed by 10% goat serum (Invitrogen) in PBS. Slides were then incubated with rat anti-VCAM-1 (1:50; MK/2) for one hour, washed with 1% BSA in PBS, and then incubated with Alexafluor 488 goat anti-rat IgG (H+L; 1:400) for 30 minutes. Slides were then washed with 1% BSA in PBS and counterstained with DAPI (Sigma), before being washed in 1% BSA followed by distilled water. The stained slides were then cover-slipped using Aqua PolyMount (Polyscience, Inc), and imaged at a final magnification of 400X on an Olympus BX50 upright light microscope (Olympus America, Inc., Lake Success, NY) with an attached Optronics MagnaFire digital camera (Optical Analysis Corp., Nashua, NH) and associated MagnaFire software (version 2.0).
Marrow for dual VCAM-1/SDF-1 staining was prepared as above except for the addition of 0.5% cetylpyridinium chloride to the fixation buffer, and the use of mouse on mouse immunodetection technique, as previous described (33) (M.O.M. Kit, Vector Labs, Burlingame, CA). Slides were incubated with mouse anti-SDF-1 (1:200; 79018) and rat anti-VCAM-1 (1:50; MK/2) over night at 4°C, before being washed with PBS, and incubated with biotinylated anti-mouse IgG reagent for 10 minutes followed by streptavidin-Alexafluor 555 and Alexafluor 488 goat anti-rat IgG (H+L; 1:400) for 30 minutes. The slides were then washed with PBS and counterstained with DAPI, before being cover-slipped with Aqua PolyMount, and imaged at a final magnification of 400X on a Zeiss LSM 510 META confocal scanning laser microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY), using LSM Version 3.2 software.
Neutrophil adhesion assays
Neutrophil adhesion assays were performed using indium111 labeled morphologically mature marrow neutrophils, isolated and labeled as described above. Flat bottom 8-well EIA/RIA strips (Corning Life Sciences, Lowell, MA) were coated with recombinant human VCAM (at stated concentrations in 0.1M sodium carbonate, pH 9.0) overnight in a humidified chamber at 4°C. In experiments using co-immobilized SDF-1, wells were next coated with recombinant murine SDF-1 (0.2μg/ml in carbonate buffer) for 30min at RT. Wells were then washed with carbonate buffer and blocked with PBS/20% FBS for 1h at 37°C. Prior to plating cells, wells were washed with running buffer (1× Hanks balanced salt solution with 2mg/ml BSA, 10mM HEPES, 1mM CaCl2, and 1mM MgCl2). 1×105 indium-labeled cells in 50μl of running buffer were added to each well and allowed to adhere for 30min at 37°C. Wells were then washed several times with running buffer and individually counted on a Wallac 1480 automatic gamma counter. All samples are reported as percentages of total loaded cells by normalizing against separately assayed 50μl aliquots of labeled cells. In experiments using Ab blockade, labeled cells were preincubated with CD49d neutralizing, LPAM neutralizing, or isotype control Abs (15μg/ml) at 37°C for 30min, before plating. In experiments using pertussis toxin, isolated neutrophils were incubated overnight at 37°C in DMEM/10% FBS with or without pertussis toxin before being labeled with 111indium and assayed (as above).
Statistical Analysis
Analysis of differences in VLA-4 expression versus maturational state and neutrophil adhesion versus concentration of VCAM were both performed by one-way analysis of variance using Prism 5™ software (GraphPad Software Inc., San Diego, CA). All other comparisons were performed with the student t-test using Prism 5™ software.
Results
VLA-4 expression on murine neutrophils is downregulated during maturation
In order to examine the expression of VLA-4 on murine peripheral blood and morphologically mature, Gr-1hi bone marrow neutrophils, we examined α4 integrin using flow cytometry. As shown in Figure 1A, α4 staining profiles for peripheral blood and bone marrow-derived Gr-1 high neutrophils demonstrate that α4 is highly expressed in both populations (98-99% of cells). Although α4 expressed in the context of the α4β7 integrin complex (LPAM) has also been found at low levels on certain neutrophil populations (34), using Ab specific for the α4β7 heterodimer, we found it to be expressed at very low levels on murine neutrophils (Figure 1B). Thus, the high levels of α4 seen on both blood and marrow neutrophils is presumably expressed in the context of the α4β1 heterodimer (VLA-4). Blood neutrophils show lower levels of α4 expression compared to marrow neutrophils (Figure 1C), suggesting that VLA-4 expression decreases during neutrophil maturation.
Figure 1. Murine neutrophil expression of surface α4 integrin in the context of α4β1 (VLA-4) decreases with maturation.
(A) Murine bone marrow and blood leukocytes were stained with Abs against α4 (CD49d) and Gr-1 (a murine marker of neutrophils) and analyzed by flow cytometry, gating for Gr-1 positive cells. (B) As α4 is expressed as one of two heterodimers (α4β1 and α4β7), murine marrow neutrophils were also stained with Ab specific for LPAM (the α4β7 heterodimer) to rule out significant LPAM expression. (C) Relative α4 expression on marrow and blood neutrophils was compared using relative fluorescence index (RFI) expressed as a ratio of the mean fluorescence intensity of cells stained with α4 Ab versus isotype control Ab. (D) Correlation between marrow neutrophil maturation state and expression of α4 was examined using in vivo pulse labeling with the thymidine analog BrdU (see Methods). In this technique cells that are post-mitotic at the time of pulse (the most mature cells) do not incorporate BrdU (BrdUnegative), whereas less mature, dividing cells incorporate BrdU. These cells are then detected as BrdUbright (more mature) or BrdUdim (least mature) depending on the number of subsequent cell divisions prior to entry into the post-mitotic marrow neutrophil pool. Marrow cells were analyzed 24h after BrdU pulse, and flow cytometry was gated for Gr-1 expression and BrdU staining intensity. α4 staining in the cell populations is expressed as relative fluorescence index to isotype control. Data points from all experiments are the means of 3-5 separate experiments performed for each analyzed population of cells (± SEM). Expression significantly different when compared to each other by *t-test (p<0.001) or **one-way ANOVA (p=0.001).
To further explore the regulation of VLA-4 expression during marrow neutrophil maturation, we examined marrow from mice pulse-labeled with the nucleotide analogue BrdU (26). This technique allows for relative determination of cell maturation state in the marrow using the intensity of BrdU nuclear staining, such that at the time point examined (24h after BrdU pulse) three populations of Gr-1 positive cells are identifiable: 1) cells that were in the post-mitotic compartment of the marrow (metamyelocytes/bands) at the time of pulse and therefore incorporate no BrdU label (BrdU ‘negative,’ the most mature cell population), 2) cells that were in the last phase of the mitotic compartment (myelocytes) and incorporate BrdU just prior to ceasing division and hence have high levels of BrdU incorporation (BrdU ‘bright,’ the second most mature cell population) and, 3) cells still in the mitotic compartment (myeloblasts/myelocytes) that have undergone multiple divisions and thus diluted their nuclear BrdU content (BrdU ‘dim,’ the least mature cell population). We find that α4 expression is highest in the least mature (‘dim’) cells and lowest in the most mature (‘negative’) (Figure 1D), suggesting that marrow neutrophil VLA-4 expression decreases with maturation.
Neutralization of VLA-4 results in neutrophil release from marrow
To determine whether neutrophil retention in the marrow during homeostatic conditions requires VLA-4, neutralizing Ab to CD49d was intravenously infused in mice. Animals injected with anti-CD49d (α4) Ab demonstrate a marked blood neutrophilia 4h after injection compared to isotype control (Figure 2A), suggesting release of marrow neutrophils into the circulation.
Figure 2. VLA-4 integrin blockade results in significant neutrophil release from bone marrow.
(A) Mice were infused with either CD49d (α4) neutralizing or isotype control Abs (30μg) and blood neutrophil levels were measured 4h later. (B) This effect was further examined using neutrophil adoptive transfer in which 111indium-labeled marrow neutrophils were injected into naïve mice and allowed to localize to marrow for 4h before infusion of anti-CD49d or control Ab. Thirty minutes after Ab infusion, blood and tissue 111In-neutrophil content was determined by gamma counting. (C) The role of α4 in neutrophil homing to marrow was investigated in mice injected with blocking Ab or isotype control 30 min prior to labeled cell infusion. Blood and tissue 111In-neutrophil content was determined 4h after cell infusion. (D) To determine whether the effects of α4 blockade reflect disruption of α4β1 (VLA-4) or α4β7 (LPAM), neutralizing Ab specific for LPAM (30μg) was used in similar homing experiments. (E) To examine the nonspecific effects of antibody binding of neutrophil cell surface antigens, blocking antibodies against CD62L (L-selectin) or CD11a (αL) (30 ug) were infused in separate neutrophil adoptive transfer experiments (similar to those reported in panel B) and compared to isotype control Ab infusions. Marrow levels of labeled neutrophils are shown. Data points are the means of 3 to 5 mice/condition (± SEM). Significantly different when compared to control-treated animals, * p<0.005, **p<0.02; ns indicates not significant. (F) To determine whether neutrophil might be activated by binding of the CD49d blocking Ab, calcium flux assays were performed. Cells were labeled with the fluorochrome Indo-1/AM and calcium mobilization in response to α4-blocking antibody (20 ug/ml) or fMLP (1μM; as a positive control) was determined using flow cytometry.
We next sought to confirm that the peripheral neutrophilia observed following systemic α4 blockade reflected accelerated release of post-mitotic marrow neutrophils and not demargination of neutrophils already in the blood granulocyte pool. To examine this possibility, we carried out neutrophil ‘adoptive transfer’ experiments in which labeled morphologically mature marrow neutrophils were infused into untreated animals and allowed to home to marrow. We have employed this technique previously to examine neutrophil kinetics during inflammation and interruption of CXCR4/SDF-1 binding, and it has been found to be both sensitive and specific for marrow release of these cells (2, 27). In the current experiments, after marrow retention was achieved, α4-blocking Ab was infused and the labeled cells released from marrow were quantified 2h later. Neutralizing Ab infusion (but not isotype control) results in decreased numbers of labeled neutrophils found in bone marrow and increased numbers in the liver, spleen, and lung (Figure 2B). To determine whether the effect of α4 blockade in this model was specific and not an artifact of antibody-mediated neutrophil activation or opsonization, the effect of α4 Ab binding on isolated neutrophils was examined by calcium flux, while the effects of blocking Abs to other neutrophil surface receptors not believed to participate in neutrophil marrow retention (L-selectin and integrin αL; CD62L and CD11a respectively) were examined in marrow mobilization experiments as described above. No neutrophil calcium flux was seen in response to α4 Ab binding (Figure 2F), while L-selectin and αL binding-antibodies failed to mobilize labeled neutrophils from the marrow (Figure 2E). Taken together, these results suggest that VLA-4 blockade induces a specific, substantial release of neutrophils from the marrow, with subsequent localization of these cells to other tissues following circulation, as we have seen previously following CXCR4 blockade (2).
To further investigate the importance of VLA-4 in the retention of neutrophils within the marrow, we examined whether α4 blockade might block homing of infused neutrophils to the marrow. Antibody directed against α4 was infused prior to injection of the labeled cells, and the number of labeled neutrophils sequestered in marrow was quantified at 4h. In this model, α4 blockade attenuates homing of neutrophils to marrow (Figure 2C), and induces similar retention in liver, spleen, and lung, as seen in the release studies.
As noted previously, α4 may be expressed in the context of two different integrin heterodimers, α4β1 (VLA-4) and α4β7 (LPAM). Although LPAM appears to be expressed at very low levels on murine neutrophils compared to VLA-4, we wished to exclude its participation in the marrow retention of these cells. We therefore tested the effect of an LPAM-specific neutralizing Ab on neutrophil marrow homing and release. As compared to isotype control Ab, there was no effect of LPAM Ab (Figure 2D). These data suggest that LPAM is not involved in neutrophil interaction with marrow ligands under homeostatic conditions, and that the above demonstrated effects of α4 blockade reflect a critical role for α4β1 (VLA-4) in such interactions.
Marrow neutrophil sensitivity to VLA-4 neutralization increases with cell maturation
In order to examine the relationship between neutrophil maturation state and sensitivity to VLA-4 neutralization, in vivo BrdU pulse labeling experiments were conducted (as described above). Animals were pulsed with BrdU and, 48h later, were then injected with either anti-CD49d Ab or isotype control. Blood and marrow were then examined 4h after Ab injection. Significant decreases in marrow neutrophil content were seen in anti-CD49d treated animals relative to control, with the greatest decrease being seen in the most mature neutrophil population (BrdUnegative) followed by less mature population (BrdUbright), with relatively little change seen in immature (BrdUdim) neutrophil content (Figure 3A). Similarly, significant increases in neutrophil levels were seen in blood following CD49d neutralization, with the largest increases seen in the more mature neutrophil populations (BrdUnegative and BrdUbright), indicating release of these cells from the marrow to the blood. This pattern of release suggests that neutrophil susceptibility to VLA-4 blockade increases with maturation, a finding that is consistent with our finding that marrow neutrophil surface levels of VLA-4 decrease with maturation (Fig 1C), but may also suggest other binding interactions predominate in the retention of myeloid progenitors.
Figure 3. Marrow neutrophil sensitivity to VLA-4 neutralization increases with cell maturation.

Endogenous marrow neutrophil mobilization was examined using in vivo pulse labeling with the thymidine analog BrdU (see Methods) 48h prior to injection of either CD49d neutralizing or isotype control Abs. Mice were then euthanized 4h after Ab injection and blood and marrow analyzed by hematology analyzer and flow cytometry gating for either Gr-1hi (blood) or Gr-1+ (marrow) cells (neutrophils), as well as BrdU staining. In this technique three populations of neutrophils are identified: BrdUnegative (most mature), BrdUbright (less mature), and BrdUdim (least mature) (see Fig Legend 1). (A) Marrow neutrophil levels in anti-CD49d and control Ab-treated animals. Data is expressed as percentage of total Gr-1+ cells, and is normalized for total differences in total marrow cells (per femur). (B) Blood neutrophil levels. Data is expressed as absolute blood levels for each population. Data points are the means of 3 to 5 mice/condition (± SEM). *Significantly different when compared to control-treated animals, p<0.05; ns indicates not significant.
Marrow neutrophils adhere to VCAM in vitro through VLA-4
Given the established role of VCAM as a ligand for VLA-4 (5, 7) (9, 10), we hypothesized that neutrophil adhesion in the marrow might be mediated by VLA-4/VCAM interactions. To initially investigate this hypothesis, we examined marrow neutrophil adhesion to plates coated with varying concentrations of recombinant VCAM. We found high levels of neutrophil adhesion at VCAM concentrations as low as 500ng/mL, with a saturation point at approximately 5ug/mL, and with little nonspecific binding (Figure 4A). This binding is almost completely reversed by α4 blocking Ab (Figure 4B), while LPAM blockade has no effect on binding, indicating that neutrophil adhesion to VCAM is mediated by VLA-4.
Figure 4. Marrow neutrophils adhere to VCAM-1 through VLA-4.

(A) Isolated murine marrow neutrophils were labeled with 111In and incubated for 30min at 37°C in tissue culture wells coated with varying concentrations of VCAM-1, after which the wells were washed, and the cell content of each well was assayed by gamma counting and expressed as a percentage of the total amount plated. (B) Labeled neutrophils were preincubated with α4 or α4β7 (LPAM) neutralizing Abs (15 μg/ml) for 30min prior to VCAM-1 adhesion and analysis. Data points are the means of 3 to 5 separate experiments (± SEM). *Significantly different when compared to isotype control, p<0.0001; ns indicates not significant.
VCAM-1 is expressed widely in murine marrow and VCAM neutralization induces release of neutrophils from the marrow
To examine the distribution of marrow VCAM expression in adult mice, we performed immunofluorescent staining in fixed femoral marrow plugs. VCAM expression in the marrow is widespread (Figure 5A), with strong staining found on both venous sinusoidal endothelium (white arrow), and large interdigitating cells (yellow arrow), morphologically consistent with marrow stromal or ‘nurse’ cells (35). To determine the role of VCAM in the retention of bone marrow neutrophils, we next carried out VCAM-blocking experiments similar to those performed for VLA-4 (above). Infusion of VCAM-neutralizing Ab into mice results in marked blood neutrophilia (Figure 6A) 4h after injection compared to isotype control. That this finding represents release of neutrophils from the marrow was confirmed by measuring the effects of VCAM-blockade in a neutrophil adoptive transfer model, as above. Antibody infusion results in substantial release of cells from marrow, and subsequent localization in liver, spleen, and lung (Figure 6B). Control experiments using blocking antibody against another marrow-expressed integrin (ICAM-1; CD54) showed no evidence of labeled neutrophil mobilization, suggesting that the effects of VCAM blockade are not due to steric or other nonspecific effects of marrow cell binding (Figure 6D). Pretreatment of animals with VCAM neutralizing Ab was found to block marrow homing of subsequently infused labeled neutrophils (Figure 6C) as well, confirming a role for the VLA-4/VCAM adhesion pathway in the marrow retention of neutrophils.
Figure 5. VCAM-1 is expressed on stromal and endothelial cells in murine bone marrow.

Fluorescent immunohistology for VCAM-1 was performed on fixed whole bone marrow plugs from mouse femurs. (A) VCAM-1 expression (green) appears to localize to the venous endothelium (white arrow) and large interdigitating stromal cells (yellow arrow). Blue staining (DAPI) indicates cell nuclei. (B) Secondary-control staining. Magnification 400x.
Figure 6. VCAM-1 blockade releases neutrophils from bone marrow.

(A) Mice were infused with either VCAM-1 (CD106) neutralizing or isotype control Abs (30μg) and blood neutrophil levels were measured 4h later. (B) This effect was further examined using adoptive transfer of 111In-labeled neutrophils 4h before infusion of anti-VCAM-1 or control Ab. Thirty minutes after Ab infusion, blood and tissue 111In-neutrophil content was determined by gamma counting. (C) The role of VCAM-1 in neutrophil homing to marrow was investigated using mice injected with blocking Ab or isotype control Ab 30 min prior to labeled cell infusion. Blood and tissue 111In-neutrophil content was determined 4h after cell infusion. (D) To examine the nonspecific effects of antibody binding of marrow cell surface antigens in this model, blocking antibody against CD54 (ICAM-1) (30 ug) was infused in a separate neutrophil adoptive transfer experiment (similar to those reported in panel B) and compared to isotype control Ab infusion. Marrow levels of labeled neutrophils are shown. Data points are the means of 3 to 5 mice/condition (± SEM). Significantly different when compared to control-treated animals, * p<0.005; ns indicates not significant.
Neutrophil VLA-4/VCAM adhesion is modulated by SDF-1
Given our previous findings that neutrophil surface CXCR4 signaling is critical to marrow retention (2), and reports that CXCR4 signaling in hematopoietic stem cells (36, 37) significantly increases surface VLA-4 binding affinity, we hypothesized that a similar process might modulate neutrophil VLA-4 adhesion in the marrow. We first examined the effects of SDF-1 on neutrophil adhesion in vitro using VCAM-coated plates in the presence or absence of co-plated SDF-1. SDF-1 significantly augments neutrophil binding to VCAM (Figure 7A), and this appears to be specific as SDF-1 does not increase binding to serum coated (blank) wells. The effects of SDF-1 were attenuated when performing the assay at 4°C (data not shown), and were completely abrogated by pre-incubation of the cells with pertussis toxin (Figure 7B), a compound that inhibits Gi protein-coupled signaling and has been shown to block CXCR4 (38). These findings indicate that the observed SDF-1 driven augmentation of VLA-4/VCAM adhesion is dependent on intracellular signaling.
Figure 7. Neutrophil VLA-4/VCAM-1 adhesion is modulated by SDF-1/CXCR4 signaling in vitro.

(A) Neutrophil adhesion to VCAM-1 coated wells was performed as described in the presence or absence of co-immobilized SDF-1. (B) Labeled neutrophils were incubated overnight with the Gi protein-coupled signaling inhibitor pertussis toxin (PTX) prior to VCAM adhesion. Data points are the means of 4-5 separate experiments (± SEM). * Significantly different, p<0.0001; ns indicates not significant.
SDF-1 and VCAM-1 are co-localized in murine marrow and combined CXCR4/VCAM neutralization is synergistic in causing release of neutrophils from the marrow
Based on previous cell culture studies (39), we hypothesized that SDF-1 and VCAM-1 might be closely co-localized in the marrow stroma and examined this question using dual immunofluorescent staining of fixed femoral marrow. SDF-1 and VCAM-1 are found to be widely expressed in the marrow. While rare cells show single expression of either SDF-1 (Fig 8a, white arrows) or VCAM-1 (Fig 8b, light blue arrows), expression of both is predominantly found to co-localize in the marrow (8c, yellow arrows), particularly on the surface of what morphologically appear to be marrow stromal cells. Given this finding and the apparent modulation of VLA-4/VCAM adhesion by CXCR4 simulation (Fig 7), we examined whether the mobilizing effects of VCAM-1 neutralization and CXCR4 signaling inhibition might interact in vivo. We performed neutrophil adoptive transfer experiments, as detailed above, in which we then administered very low doses of both CXCR4 and VCAM-1 blocking Abs (that by themselves had no mobilization effect) simultaneously. Marrow neutrophil release was determined 2h after infusion. Under these circumstances, labeled neutrophil release was greatly augmented compared to either low dose CXCR4 or VCAM-1 Ab infusion alone (Figure 9). Thus, these results suggest a synergistic effect of such blockade, and further support the role of crosstalk between neutrophil CXCR4 and VLA-4 in the regulation of marrow retention.
Figure 8. SDF-1 is closely co-localized with VCAM-1 on the surface of bone marrow stromal cells.

Dual fluorescent immunohistology for SDF-1 and VCAM-1 was performed on fixed whole bone marrow plugs from mouse femurs. Samples were imaged by fluorescent microscopy for each fluorophore individually and together. (A) SDF-1. Red fluorescence denotes SDF-1 staining (white arrowheads indicate cells staining solely for SDF-1). (B) VCAM-1. Green fluorescence indicates VCAM-1 staining (while light blue arrowheads indicate cells staining solely for VCAM-1). (C) Merged image. Yellow fluorescence indicates large areas of co-localization between SDF-1 and VCAM; yellow arrowheads indicate examples of co-localization. (D) Dual secondary-control staining. Blue staining (DAPI) indicates cell nuclei. Magnification 400x.
Figure 9. Neutralization of CXCR4 and VCAM-1 is synergistic in vivo.
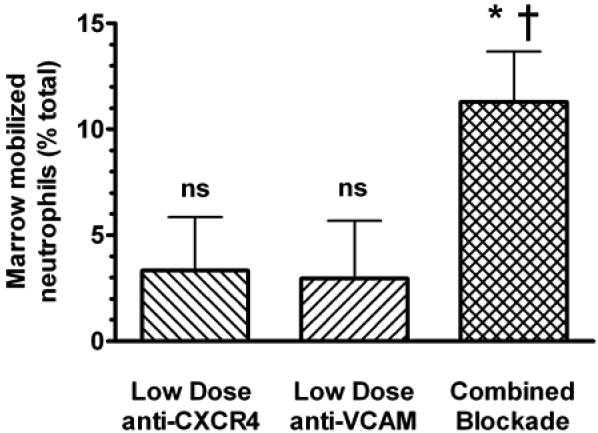
The apparent crosstalk between CXCR4 and VLA-4 was examined in vivo. Mice were treated with low doses of either VCAM blocking or CXCR4 blocking Abs, both Abs simultaneously, or isotype control 4h after labeled marrow neutrophils were sequestered to the marrow, as described. Subsequent marrow content of labeled neutrophils was assayed 2h after antibody infusion. Results are expressed as percent decrease compared to control-treated animals. Data points are the means of 5 separate experiments (± SEM). *Significantly different when compared to control treated animals, p<0.01; †Significantly different when compared to low dose VCAM blocking Ab or low dose CXCR4 blocking Ab alone, p<0.05; ns, not significantly different compared to control treated animals.
Discussion
In the present study we demonstrate a role for integrin α4β1 (VLA-4) and its ligand VCAM-1 in the homeostatic control of neutrophil release from the bone marrow. We further implicate crosstalk between neutrophil surface CXCR4 and VLA-4 in the modulation of VCAM binding, suggesting a direct link between the SDF-1/CXCR4 chemokine axis and the adhesion events retaining neutrophils in the marrow.
Although previous work has shown VLA-4 to be critical in the marrow retention of hematopoietic stem cells and normal hematopoietic development (5, 6, 9-14), its role in subsequent marrow neutrophil retention and release has not been investigated. Although neutrophil expression of VLA-4 has been debated (17-20), particularly as regards mature neutrophils in the circulation, our data suggest that this integrin is expressed at high levels during murine neutrophil development and declines with maturation, a pattern similar to that described with human neutrophil development (21-23). The finding that VLA-4 expression is progressively lost during neutrophil maturation suggests a role for this integrin in the choreography of neutrophil release from the marrow. It may also shed some light on the variable expression of this integrin reported on circulating neutrophils, as changes in the relative maturity of these cells, such as occurs during the accelerated release that accompanies inflammatory states, might be expected to alter measured levels.
In this study, we show that α4 integrin-mediated adhesion events are critical to the retention of neutrophils within the bone marrow. Although α4 may exist in two different heterodimers on the surface of neutrophils (α4β1/VLA-4 and α4β7/LPAM) (34), we find very low levels of LPAM expression on marrow neutrophils, and no effect of specific blockade of this integrin on neutrophil release from the marrow. We therefore believe the effects of α4 blockade are specifically attributable to interruption of VLA-4 adhesion, with minimal contribution from LPAM. This finding, in conjunction with our evidence that marrow neutrophil maturation is associated with increasing sensitivity to α4 blockade, is consistent with our hypothesis that maturing neutrophils are retained within the marrow under the influence of VLA-4, and that such retention wanes with cell maturation and subsequent downregulation of VLA-4 expression leading to controlled release into the circulation.
The best described ligands for VLA-4 are VCAM (CD106) and fibronectin. Previous studies using radioautography of whole marrow (8) have suggested that VCAM is expressed widely in the marrow, and more recent work in newborn mice has shown that the advent of stromal and endothelial VCAM expression coincides with the initiation of granulopoieisis in the marrow (14). Based on these studies and our own work suggesting that post-mitotic neutrophils in the marrow are located in close proximity to the venous sinusoids and the dendritic processes of stromal cells (27), we hypothesized that VCAM might be a critical target for neutrophil adhesion. We show here that VCAM is strongly expressed on both stromal cells and the venous endothelium in murine marrow, and that neutrophil interaction with both marrow stroma and endothelium is likely mediated by the VLA-4/VCAM pathway. It is important, however, to recognize that our studies do not rule out a concomitant role for fibronectin in this process, as has previously been suggested in the marrow-retention of hematopoietic progenitors (14).
VLA-4 exists in low and high avidity states (40), and this is regulated in part by ‘inside out signaling’ in which cytokine receptor activation leads to changes in the binding affinity state of the integrin, modulating cell adhesion (41). One such cytokine receptor is CXCR4, which when activated by the binding of SDF-1 has been shown to significantly increase VCAM binding by VLA-4 in hematopoietic stem cells, lymphocytes, and tumor cell lines (36, 37, 42-46). This process appears to be dependent on close co-expression of SDF-1 and VCAM on cell surfaces (36), a relationship that has been shown to exist on cultured bone marrow stromal cells (39), and is further suggested in vivo in our present study. As a continuation of our previous work implicating CXCR4 signaling in the retention of marrow neutrophils (2), we here show that the neutrophil VLA-4/VCAM adhesion pathway is significantly augmented by SDF-1. This alteration in adhesion appears to require Gi-protein-coupled intracellular signaling, consistent with a dependence on CXCR4 signaling in this process (38). The importance of this interaction in vivo is suggested by the synergistic effects of very low doses of neutralizing Abs against both CXCR4 and VCAM in the mobilization of labeled marrow neutrophils. Thus, the retention and release of marrow neutrophils appears to be governed by both SDF-1/CXCR4 signaling and VLA-4/VCAM adhesion events in the marrow, and these processes may be, to a degree, interdependent.
Interestingly, recent studies have suggested that the neutrophilic growth factor G-CSF may act to drive the release of the marrow neutrophils, in part, through down-regulation of marrow SDF-1 (47, 48), while G-CSF modulation of the combined axis (SDF-1/CXCR4 and VLA-4/VCAM) has been implicated in the release of hematopoietic stem cells (15, 49, 50). In total, this suggests the possibility that maturation related loss of both VLA-4 and CXCR4 may govern the orderly release of neutrophils during the homeostatic setting, while more profound alterations to this complex axis may lead to massive release, as in the case of systemic inflammation.
In summary, we find an important role for VLA-4 and VCAM, in the homeostatic retention and release of neutrophils from the bone marrow. This adhesion interaction appears to be modulated by crosstalk from neutrophil surface CXCR4, and may account, at least in part, for our previous findings that marrow neutrophil retention is dependent on the SDF-1/CXCR4 axis. The orderly release of mature cells from the marrow then may reflect a programmed down-regulation of both neutrophil CXCR4 and VLA-4, while the massive release of often less mature neutrophils that accompanies systemic inflammatory states may reflect direct interruption of these interactions under the influence of mediators such as G-CSF. Further work will be required to delineate these possibilities.
Footnotes
This work was supported by Grants R01 HL084200 and NCRR P20RR15557 from the National Institutes of Health.
References
- 1.Opdenakker G, Fibbe WE, Van Damme J. The molecular basis of leukocytosis. Immunol Today. 1998;19:182–189. doi: 10.1016/s0167-5699(97)01243-7. [DOI] [PubMed] [Google Scholar]
- 2.Suratt BT, Petty JM, Young SK, Malcolm KC, Lieber JG, Nick JA, Gonzalo JA, Henson PM, Worthen GS. Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood. 2004;104:565–571. doi: 10.1182/blood-2003-10-3638. [DOI] [PubMed] [Google Scholar]
- 3.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463–471. doi: 10.1016/s1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- 4.Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–593. doi: 10.1016/s1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 5.Teixido J, Hemler ME, Greenberger JS, Anklesaria P. Role of beta 1 and beta 2 integrins in the adhesion of human CD34hi stem cells to bone marrow stroma. J Clin Invest. 1992;90:358–367. doi: 10.1172/JCI115870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simmons PJ, Masinovsky B, Longenecker BM, Berenson R, Torok-Storb B, Gallatin WM. Vascular cell adhesion molecule-1 expressed by bone marrow stromal cells mediates the binding of hematopoietic progenitor cells. Blood. 1992;80:388–395. [PubMed] [Google Scholar]
- 7.Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV, Wolf NS. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci U S A. 1995;92:9647–9651. doi: 10.1073/pnas.92.21.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobsen K, Kravitz J, Kincade PW, Osmond DG. Adhesion receptors on bone marrow stromal cells: in vivo expression of vascular cell adhesion molecule-1 by reticular cells and sinusoidal endothelium in normal and gamma-irradiated mice. Blood. 1996;87:73–82. [PubMed] [Google Scholar]
- 9.Oostendorp RA, Dormer P. VLA-4-mediated interactions between normal human hematopoietic progenitors and stromal cells. Leuk Lymphoma. 1997;24:423–435. doi: 10.3109/10428199709055581. [DOI] [PubMed] [Google Scholar]
- 10.Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM. Molecular pathways in bone marrow homing: dominant role of alpha(4)beta(1) over beta(2)-integrins and selectins. Blood. 2001;98:2403–2411. doi: 10.1182/blood.v98.8.2403. [DOI] [PubMed] [Google Scholar]
- 11.Ryan DH, Nuccie BL, Abboud CN, Winslow JM. Vascular cell adhesion molecule-1 and the integrin VLA-4 mediate adhesion of human B cell precursors to cultured bone marrow adherent cells. J Clin Invest. 1991;88:995–1004. doi: 10.1172/JCI115403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arroyo AG, Yang JT, Rayburn H, Hynes RO. Differential requirements for alpha4 integrins during fetal and adult hematopoiesis. Cell. 1996;85:997–1008. doi: 10.1016/s0092-8674(00)81301-x. [DOI] [PubMed] [Google Scholar]
- 13.Arroyo AG, Taverna D, Whittaker CA, Strauch UG, Bader BL, Rayburn H, Crowley D, Parker CM, Hynes RO. In vivo roles of integrins during leukocyte development and traffic: insights from the analysis of mice chimeric for alpha 5, alpha v, and alpha 4 integrins. J Immunol. 2000;165:4667–4675. doi: 10.4049/jimmunol.165.8.4667. [DOI] [PubMed] [Google Scholar]
- 14.Tada T, Widayati DT, Fukuta K. Morphological study of the transition of haematopoietic sites in the developing mouse during the peri-natal period. Anat Histol Embryol. 2006;35:235–240. doi: 10.1111/j.1439-0264.2005.00671.x. [DOI] [PubMed] [Google Scholar]
- 15.Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98:1289–1297. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- 16.Levesque JP, Hendy J, Takamatsu Y, Williams B, Winkler IG, Simmons PJ. Mobilization by either cyclophosphamide or granulocyte colony-stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp Hematol. 2002;30:440–449. doi: 10.1016/s0301-472x(02)00788-9. [DOI] [PubMed] [Google Scholar]
- 17.Reinhardt PH, Elliott JF, Kubes P. Neutrophils can adhere via alpha4beta1-integrin under flow conditions. Blood. 1997;89:3837–3846. [PubMed] [Google Scholar]
- 18.Ibbotson GC, Doig C, Kaur J, Gill V, Ostrovsky L, Fairhead T, Kubes P. Functional alpha4-integrin: a newly identified pathway of neutrophil recruitment in critically ill septic patients. Nat Med. 2001;7:465–470. doi: 10.1038/86539. [DOI] [PubMed] [Google Scholar]
- 19.Bochner BS, Luscinskas FW, Gimbrone MA, Jr., Newman W, Sterbinsky SA, Derse-Anthony CP, Klunk D, Schleimer RP. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J Exp Med. 1991;173:1553–1557. doi: 10.1084/jem.173.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirveskari J, Bono P, Granfors K, Leirisalo-Repo M, Jalkanen S, Salmi M. Expression of alpha4-integrins on human neutrophils. J Leukoc Biol. 2000;68:243–250. [PubMed] [Google Scholar]
- 21.Kerst JM, Sanders JB, Slaper-Cortenbach IC, Doorakkers MC, Hooibrink B, van Oers RH, von dem Borne AE, van der Schoot CE. Alpha 4 beta 1 and alpha 5 beta 1 are differentially expressed during myelopoiesis and mediate the adherence of human CD34+ cells to fibronectin in an activation-dependent way. Blood. 1993;81:344–351. [PubMed] [Google Scholar]
- 22.Soligo D, Schiro R, Luksch R, Manara G, Quirici N, Parravicini C, Lambertenghi Deliliers G. Expression of integrins in human bone marrow. Br J Haematol. 1990;76:323–332. doi: 10.1111/j.1365-2141.1990.tb06363.x. [DOI] [PubMed] [Google Scholar]
- 23.Lund-Johansen F, Terstappen LW. Differential surface expression of cell adhesion molecules during granulocyte maturation. J Leukoc Biol. 1993;54:47–55. doi: 10.1002/jlb.54.1.47. [DOI] [PubMed] [Google Scholar]
- 24.Burdon PC, Martin C, Rankin SM. The CXC chemokine MIP-2 stimulates neutrophil mobilization from the rat bone marrow in a CD49d-dependent manner. Blood. 2005;105:2543–2548. doi: 10.1182/blood-2004-08-3193. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalo JA, Lloyd CM, Peled A, Delaney T, Coyle AJ, Gutierrez-Ramos JC. Critical involvement of the chemotactic axis CXCR4/stromal cell-derived factor-1 alpha in the inflammatory component of allergic airway disease. J Immunol. 2000;165:499–508. doi: 10.4049/jimmunol.165.1.499. [DOI] [PubMed] [Google Scholar]
- 26.Basu S, Hodgson G, Katz M, Dunn AR. Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood. 2002;100:854–861. doi: 10.1182/blood.v100.3.854. [DOI] [PubMed] [Google Scholar]
- 27.Suratt BT, Young SK, Lieber J, Nick JA, Henson PM, Worthen GS. Neutrophil maturation and activation determine anatomic site of clearance from circulation. Am J Physiol Lung Cell Mol Physiol. 2001;281:L913–921. doi: 10.1152/ajplung.2001.281.4.L913. [DOI] [PubMed] [Google Scholar]
- 28.Olofsson T, Gartner I, Olsson I. Separation of human bone marrow cells in density gradients of polyvinylpyrrolidone coated silica gel (Percoll) Scand J Haematol. 1980;24:254–262. doi: 10.1111/j.1600-0609.1980.tb01334.x. [DOI] [PubMed] [Google Scholar]
- 29.Berkow RL, Dodson RW. Purification and functional evaluation of mature neutrophils from human bone marrow. Blood. 1986;68:853–860. [PubMed] [Google Scholar]
- 30.Barbee RW, Perry BD, Re RN, Murgo JP. Microsphere and dilution techniques for the determination of blood flows and volumes in conscious mice. Am J Physiol. 1992;263:R728–733. doi: 10.1152/ajpregu.1992.263.3.R728. [DOI] [PubMed] [Google Scholar]
- 31.Boggs DR. Hematopoiesis and aging: IV. Mass and distribution of erythroid marrow in aged mice. Exp Hematol. 1985;13:1044–1047. [PubMed] [Google Scholar]
- 32.Strauch UG, Lifka A, Gosslar U, Kilshaw PJ, Clements J, Holzmann B. Distinct binding specificities of integrins alpha 4 beta 7 (LPAM-1), alpha 4 beta 1 (VLA-4), and alpha IEL beta 7. Int Immunol. 1994;6:263–275. doi: 10.1093/intimm/6.2.263. [DOI] [PubMed] [Google Scholar]
- 33.Petty JM, Sueblinvong V, Lenox CC, Jones CC, Cosgrove GP, Cool CD, Rai PR, Brown KK, Weiss DJ, Poynter ME, Suratt BT. Pulmonary stromal-derived factor-1 expression and effect on neutrophil recruitment during acute lung injury. J Immunol. 2007;178:8148–8157. doi: 10.4049/jimmunol.178.12.8148. [DOI] [PubMed] [Google Scholar]
- 34.Harp JA, Waters TE, Goff JP. Adhesion molecule and homing receptor expression on blood and milk polymorphonuclear leukocytes during the periparturient period of dairy cattle. Vet Immunol Immunopathol. 2005;104:99–103. doi: 10.1016/j.vetimm.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Crocker PR, Gordon S. Isolation and characterization of resident stromal macrophages and hematopoietic cell clusters from mouse bone marrow. J Exp Med. 1985;162:993–1014. doi: 10.1084/jem.162.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peled A, Grabovsky V, Habler L, Sandbank J, Arenzana-Seisdedos F, Petit I, Ben-Hur H, Lapidot T, Alon R. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34(+) cells on vascular endothelium under shear flow. J Clin Invest. 1999;104:1199–1211. doi: 10.1172/JCI7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hidalgo A, Sanz-Rodriguez F, Rodriguez-Fernandez JL, Albella B, Blaya C, Wright N, Cabanas C, Prosper F, Gutierrez-Ramos JC, Teixido J. Chemokine stromal cell-derived factor-1alpha modulates VLA-4 integrin-dependent adhesion to fibronectin and VCAM-1 on bone marrow hematopoietic progenitor cells. Exp Hematol. 2001;29:345–355. doi: 10.1016/s0301-472x(00)00668-8. [DOI] [PubMed] [Google Scholar]
- 38.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. Journal of Experimental Medicine. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaumann DH, Tuischer J, Ebell W, Manz RA, Lauster R. VCAM-1-positive stromal cells from human bone marrow producing cytokines for B lineage progenitors and for plasma cells: SDF-1, flt3L, and BAFF. Mol Immunol. 2007;44:1606–1612. doi: 10.1016/j.molimm.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 40.Masumoto A, Hemler ME. Multiple activation states of VLA-4. Mechanistic differences between adhesion to CS1/fibronectin and to vascular cell adhesion molecule-1. J Biol Chem. 1993;268:228–234. [PubMed] [Google Scholar]
- 41.Chigaev A, Zwartz G, Graves SW, Dwyer DC, Tsuji H, Foutz TD, Edwards BS, Prossnitz ER, Larson RS, Sklar LA. Alpha4beta1 integrin affinity changes govern cell adhesion. J Biol Chem. 2003;278:38174–38182. doi: 10.1074/jbc.M210472200. [DOI] [PubMed] [Google Scholar]
- 42.Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, Slav MM, Nagler A, Lider O, Alon R, Zipori D, Lapidot T. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–3296. [PubMed] [Google Scholar]
- 43.DiVietro JA, Brown DC, Sklar LA, Larson RS, Lawrence MB. Immobilized stromal cell-derived factor-1alpha triggers rapid VLA-4 affinity increases to stabilize lymphocyte tethers on VCAM-1 and subsequently initiate firm adhesion. J Immunol. 2007;178:3903–3911. doi: 10.4049/jimmunol.178.6.3903. [DOI] [PubMed] [Google Scholar]
- 44.Ding Z, Xiong K, Issekutz TB. Chemokines stimulate human T lymphocyte transendothelial migration to utilize VLA-4 in addition to LFA-1. J Leukoc Biol. 2001;69:458–466. [PubMed] [Google Scholar]
- 45.Burger M, Glodek A, Hartmann T, Schmitt-Graff A, Silberstein LE, Fujii N, Kipps TJ, Burger JA. Functional expression of CXCR4 (CD184) on small-cell lung cancer cells mediates migration, integrin activation, and adhesion to stromal cells. Oncogene. 2003;22:8093–8101. doi: 10.1038/sj.onc.1207097. [DOI] [PubMed] [Google Scholar]
- 46.Sanz-Rodriguez F, Hidalgo A, Teixido J. Chemokine stromal cell-derived factor-1alpha modulates VLA-4 integrin-mediated multiple myeloma cell adhesion to CS-1/fibronectin and VCAM-1. Blood. 2001;97:346–351. doi: 10.1182/blood.v97.2.346. [DOI] [PubMed] [Google Scholar]
- 47.Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17:413–423. doi: 10.1016/s1074-7613(02)00424-7. [DOI] [PubMed] [Google Scholar]
- 48.Wengner AM, Pitchford SC, Furze RC, Rankin SM. The coordinated action of G-CSF and ELR + CXC chemokines in neutrophil mobilization during acute inflammation. Blood. 2008;111:42–49. doi: 10.1182/blood-2007-07-099648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, Sandbank J, Zipori D, Lapidot T. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 50.Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111:187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]




