Abstract
Toxicologic and epidemiologic studies have linked benzo[a]pyrene (B[a]P) exposure with cardiovascular diseases such as atherosclerosis. The mechanisms of action leading to these diseases have not been fully understood. One key step in the development of atherosclerosis is vascular endothelial dysfunction, which is characterized by increased adhesiveness. To determine if B[a]P could lead to increased endothelial adhesiveness, the effects of B[a]P on human endothelial cell intercellular adhesion molecule-1 (ICAM-1) expression was investigated. B[a]P was able to increase ICAM-1 protein only after pretreatment with the aryl hydrocarbon receptor (AhR) agonist β-naphthoflavone (β-NF). Knockdown of AhR by siRNA or treatment with AhR antagonist α-naphthoflavone (α-NF) eliminated the induction of ICAM-1 from B[a]P, confirming the necessity of AhR in this process. Likewise, B[a]P only increased monocyte adhesion to the vascular endothelium when cells were pretreated with β-NF. Experiments were done to define a signaling mechanism. B[a]P increased phosphorylation of MEK and p38-MAPK, and inhibitors to these proteins blunted the ICAM-1 induction. B[a]P was also able to increase AP-1 DNA binding and phosphorylation of c-Jun. Phosphorylation of c-Jun was disrupted by MEK and p38-MAPK inhibitors linking the signaling cascade. Finally, the importance of membrane microdomains, caveolae, was demonstrated by knockdown of the structural protein caveolin-1. Disruption of caveolae eliminated the B[a]P induced ICAM-1 expression. These data suggest a possible pro-inflammatory mechanism of action of B[a]P involving caveolae, leading to increased vascular endothelial adhesiveness, and this inflammation may be a critical step in the development of B[a]P-induced atherosclerosis.
Keywords: polycyclic aromatic hydrocarbons, atherosclerosis, intercellular adhesion molecule-1, aryl hydrocarbon receptor, caveolae
Introduction
A mounting interest in the correlation between air pollution and increased cardiovascular events has developed over the last few decades. Epidemiological studies have linked increased particulate matter (PM10 or PM2.5) exposure with heightened cardiovascular morbidity (Auchincloss et al., 2008) and mortality (Dockery et al., 1993; Pope et al., 2002; Pope et al., 2004). Also, it has been known for years that coronary artery disease is exacerbated by cigarette smoke (Weintraub, 1990). Both of these complex mixtures contain large amounts of polycyclic aromatic hydrocarbons (PAHs) (Davis, 1968; Vincent et al., 1997; Liu et al., 2007), among which is the probable human carcinogen B[a]P, which are formed as byproducts of incomplete combustion processes. Similar to studies on air pollution and cigarette smoke, a cohort of asphalt workers with occupational exposure to B[a]P was shown to have a positive correlation between cumulative and average B[a]P exposure and risk for ischemic heart disease (Burstyn et al., 2005).
B[a]P is monitored by the US Environmental Protection Agency as part of the group termed polycyclic organic matter. B[a]P augments the severity and progression of atherosclerotic plaques in animal models (Albert et al., 1977; Hough et al., 1993; Curfs et al., 2004), which are not attributed to the mutagenic properties of the compound (Curfs et al., 2005). An alternate hypothesis is an increased inflammatory response, a key step in the development and progression of atherosclerosis. B[a]P treatment has been shown to increase the inflammatory chemokine, monocyte chemoattractant protein-1 (MCP-1) in aortic tissues of hyperlipidemic mice (Knaapen et al., 2007). Interestingly, this response is attenuated by treatment with an AhR antagonist. B[a]P is metabolically activated by the AhR-induced enzymes cytochrome P450 1A1 (CYP1A1) and epoxide hydrolase, resulting in carcinogenic B[a]P-diol epoxides (BPDE), as well as the redox cycling o-quinones. Endothelial cells have the highest induction of CYP1A1 in the vasculature of rat models, and these cells have the ability to metabolize B[a]P (Thirman et al., 1994).
The vascular endothelium is susceptible to physiological insult as it is in constant contact with circulating xenobiotics. B[a]P DNA adducts have been found in human atherosclerotic lesions and to a large part localized to the endothelium, thus supporting this concept (Izzotti et al., 1995; Zhang et al., 1998). Vascular endothelium dysfunction is a key initiating event in numerous cardiovascular diseases such as atherosclerosis. Dysfunction of the endothelium is marked by increased adhesiveness caused by the presentation of cellular adhesion molecules, such as ICAM-1 (CD54) and vascular cell adhesion molecule-1 (VCAM-1, CD106). ICAM-1, a member of the immunoglobulin superfamily that binds to β2 integrins, adheres circulating leukocytes to the vascular wall, leading to diapedesis through the vessel wall and accumulation in the intimal layer. Studies have established the importance of ICAM to the development of cardiovascular disease. Pretreatment of hyperlipidemic mice with antibodies against ICAM-1 reduced macrophage homing to aortas (Patel et al., 1998), and Icam1−/− mice fed a western diet have decreased lesion size compared to controls (Nageh et al., 1997). Studies also demonstrate that air pollution and cigarette exposure increase the levels of ICAM-1 in both humans and animal models (Kalra et al., 1994; Shen et al., 1996; O'Neill et al., 2007; Gowdy et al., 2008; Yatera et al., 2008).
The human ICAM-1 gene was cloned and found to contain binding sites for transcription factors including specificity protein-1 (SP-1), activator protein-1 (AP-1), and nuclear factor-κB (NF-κB) (Stade et al., 1990; Voraberger et al., 1991). These transcription factors are activated by a number of upstream kinases such as the MAP kinase (MAPK) family of proteins, including p38 MAPK, extracellular signal-regulated kinase (ERK), and c-Jun N-terminal kinase (JNK). MAPK are serine/threonine kinases that are activated by phosphorylation by MAPKK, such as MEK. These signaling cascades have been shown to be redox sensitive and elicit a large amount of crosstalk between the MAPK members (Torres and Forman, 2003). More recent literature suggests caveolae play a role in some MAPK signaling cascades (Torres and Forman, 2003; Wang et al., 2006; Siddiqui et al., 2007; Zeidan et al., 2008). Caveolae are plasma membrane microdomains enriched in cholesterol and glycosphingolipids, and function in cellular trafficking as well as act as signaling platforms for a range of cascades. Caveolae are characterized by the presence of caveolins, the structural component of these lipid rafts, and are particularly abundant in endothelial cells (Lisanti et al., 1994; Razani et al., 2002; Frank et al., 2003).
It has been shown that B[a]P induces atherosclerosis in hyperlipidemic mouse models and epidemiological studies support this finding. However, mechanisms involved in these events are not fully understood. We hypothesized that B[a]P augments cardiovascular disease by inducing adhesion molecule expression and that this event is due to metabolism by AhR mediated enzymes, as well as signaling through caveolae.
Materials and Methods
Materials
Antibodies used were anti-ICAM-1 (clone RR1/1, Invitrogen, Carlsbad, CA), anti-caveolin-1 (Affinity Bioreagents, Golden, CO), anti-p38 (Cell Signaling Technology, Danvers, MA), anti-phospho-p38 (Thr180/Tyr182) (Cell Signaling Technology), anti-MEK (Santa Cruz Biotechnology, Santa Cruz, CA), anti-phospho-MEK1/2 (Ser217/221) (Cell Signaling Technology), anti-cJun (Zymed, San Francisco, CA), anti-phospho-cJun (Ser73) (Upstate, Lake Placid, NY), anti-AhR (clone RPT1, Abcam, Cambridge, MA), anti-actin (Sigma Aldrich, St. Louis, MO), and anti-GAPDH (Santa Cruz Biotechnology). Anti-rabbit, anti-goat, and anti-mouse secondary antibodies were purchased from Cell Signaling Technology. AlexaFluor 488 conjugated secondary antibody was purchased from Invitrogen. The inhibitors SB203580 and PD98059 were purchased from Calbiochem (EMD Chemicals, Gibbstown, NJ) and dissolved in endotoxin-free DMSO (Sigma Aldrich, St. Louis, MO). Fluoranthene was purchased from Accustandard (New Haven, CT). Human TNF-α, B[a]P, β-NF, α-NF, and propidium iodide were purchased from Sigma Aldrich.
Cell Culture
Primary human umbilical vein endothelial cells (HUVEC) were used in these experiments as models for inflammatory cardiovascular disease. Cells were isolated from human umbilical cord veins as explained previously (Toborek et al., 2002). Human umbilical cords were obtained from the University of Kentucky Labor and Delivery unit. HUVEC were cultured in M199 media (GIBCO Laboratories, Grant Island, NY) supplemented with 20% FBS (Hyclone Laboratories, Logan, UT) as described previously (Toborek et al., 2002). The experimental media contained 10% FBS. B[a]P, β-NF, α-NF, and inhibitors were dissolved in DMSO. The final concentration of DMSO in the culture media did not exceed 0.03%. All vehicle controls and treated cultures contained the same amount of DMSO.
Cytochrome P4501A1 Activity
HUVEC grown on white-walled 96 well plates were treated with β-NF (1 µM) for 16 h. Both β-NF and α-NF have been shown to alter HUVEC CYP1A1 expression and AhR function at this concentration (Merchant et al., 1993; Annas et al., 2000). Cytrochrome P4501A1 activity was measured by increased luminescence due to the conversion of Luciferin-CEE to Luciferin via P450-Glo Assay (Promega, Madison, WI) in accordance with manufacturer’s instructions. CYP1A1 activity was normalized to cell number. Cell number was determined using the Cell-Titer Glo Assay (Promega) as directed by the manufacturer’s instructions.
Western Blotting
Whole-cell lysates were prepared with a lysis buffer containing 50 mM Tris-HCl (pH 8.0), 200 mM NaCl, 20 mM EDTA, 1% SDS, 0.5% Na-deoxycholic acid, 0.01% NP-40, 200 mM sodium orthovanadate, and 100 mM phenylmethylsulfonyl fluoride. Equal amounts of protein (40 µg) were fractionated by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membrane was blocked at room temperature with 5% nonfat milk in Tris-buffered saline (TBS, pH 7.6) containing 0.05% Tween 20, and then washed with TBS-Tween. Antibodies against ICAM-1, p-38, p-p38, p-MEK, p-pMEK, actin, and GAPDH were incubated at 4 °C overnight at a 1:1000 dilution in 5% bovine serum albumin in TBS-Tween. Caveolin-1 antibody was incubated at 1:10,000 dilutions in the same conditions. Horseradish peroxidase-conjugated secondary antibodies were incubated for 1 h at a 1:3000 dilution. Bands were visualized by using ECL or ECL plus immunoblotting detection reagents (Amersham Biosciences, Buckinghamshire, England). Bands were quantified using UN-SCAN-IT gel Version 5.1 (Silk Scientific, Orem, UT) and normalized to actin or GAPDH protein expression.
Monocyte Adhesion
Human THP-1 monocytes (50,000 cells) were activated with TNF-α dissolved in water (10 min) and loaded with the fluorescent probe calcein (Molecular Probes, Carlsbad, CA). HUVECs were pretreated with DMSO or β-NF for 16 h and then treated for 8 h with DMSO, B[a]P (3 or 10 µM), or TNF-α. Monocytes were added to treated endothelial cell monolayers and incubated (30 min), allowing for monocyte adhesion. Unbound monocytes were washed away, and the monolayer was fixed with 1% glutaraldehyde. Attached fluorescent monocytes were counted using a fluorescent microscope (Olympus IX70, Center Valley, PA).
Flow Cytometry
HUVEC were pretreated with β-NF for 16 h and then pretreated with inhibitors to p38 MAPK (SB203580; 10 µM) or MEK (PD98059; 20 µM) for 1 h. After pretreatment, the cells were treated with DMSO or B[a]P (10 µM) for 24 h. HUVEC were washed with PBS, and then removed with trypsin. Cells were centrifuged, washed, and then incubated in 3% bovine serum albumin containing the primary antibody for ICAM-1 (2µg/ml) for 30 min. Cells were then centrifuged, washed, and then resuspended in AlexaFluor 488 labeled secondary antibody (3µg/ml) for 20 min. HUVECs were then washed and stained with propidium iodide (2 µg/ml) for 5 min in order to gate for live cells. Cells were then analyzed by the University of Kentucky Flow Cytometry Facility using a Becton-Dickinson FacsCalibur cell analyzer.
Electrophoretic Mobility Shift Assay
HUVEC were pretreated with α-NF or β-NF for 16 h and then treated with DMSO or B[a]P (10 µM) for 4 h. To extract nuclear protein, cells were incubated with buffer A (10 mM HEPES pH 7.9, 10 mM KCl, 0.1 mM EDTA, 1 mM DTT, 0.5 mM PMSF) for 15 min on ice and then 10% NP-40 was added. After 90–95% of cells were lysed and centrifuged, nuclei were lysed by incubation and shaking for 5 min in buffer B (20 mM HEPES, 0.4 M NaCl, 1 mM EDTA, 1 mM DTT, 1 mM PMSF). Activator protein-1 (AP-1) DNA binding was measured by the Pierce LightShift Chemicluminescent EMSA Kit (Rockford, IL) as directed by manufacturer’s protocol. Briefly, 3 µg nuclear protein incubated with 50 ng/µl Poly (dI·dC) and 20 fmol biotin end-labeled AP-1 DNA is separated on pre-run native polyacrylamide gel. The binding reaction is then transferred and cross-linked to nylon membrane. Finally, biotin-labeled DNA is detected via a Streptavidin-Horseradish Peroxidase Conjugate and Chemiluminescent substrate. Control reactions were conducted containing no protein extract or 200-fold molar excess of unlabeled DNA. AP-1 specificity was determined by a supershift of the band after addition of c-Jun antibody to the binding reaction.
siRNA Transfection
Double stranded small interfering RNA targeted to caveolin-1 and AhR were synthesized by Dharmacon Research (Lafayette, CO) as duplexed, 2’-unprotected, desalted, and purified siRNA as described previously (Cho et al., 2003; N'Diaye et al., 2006). The sequences were as follows: Caveolin-1 5’-CCAGAAGGGACACACAGdTdT-3’ and 5’-AACAUCUACAAGCCCAACAACdTdT-3’; Caveolin-1 control 5’-AGAGCGACUUUACACACdTdT-3’; AhR 5’-GUCGGUCUCUAUGCCGCdTdT-3’; and AhR mutated control 5’-CUCGGUCUCUAUGCCGC-3’. HUVEC were transfected with control or gene-targeted siRNA for 4 h using the GeneSilencer Reagent (Genlantis, San Diego, CA) and OptiMEM serum-free media (Invitrogen) at a final concentration of 120 nM (AhR) or 80 nM (caveolin). After 24 h, cells were pretreated with α-NF or β-NF for 16 h and then treated with DMSO or B[a]P (10 µM) for 24 h. Whole cell lysate was probed for ICAM-1, GAPDH, and caveolin-1 or AhR by immunoblot analysis.
Statistical Analysis
Values are reported as means ± SE of at least three independent groups. Comparisons between treatments were made by one-way or two-way analysis of variance followed by Tukey multiple comparison tests using GraphPad Prism 4.0 software (GraphPad Software, San Diego, CA). Statistical probability of p<0.05 was considered significant.
Results
B[a]P increases ICAM-1 and monocyte adhesion only after AhR activation
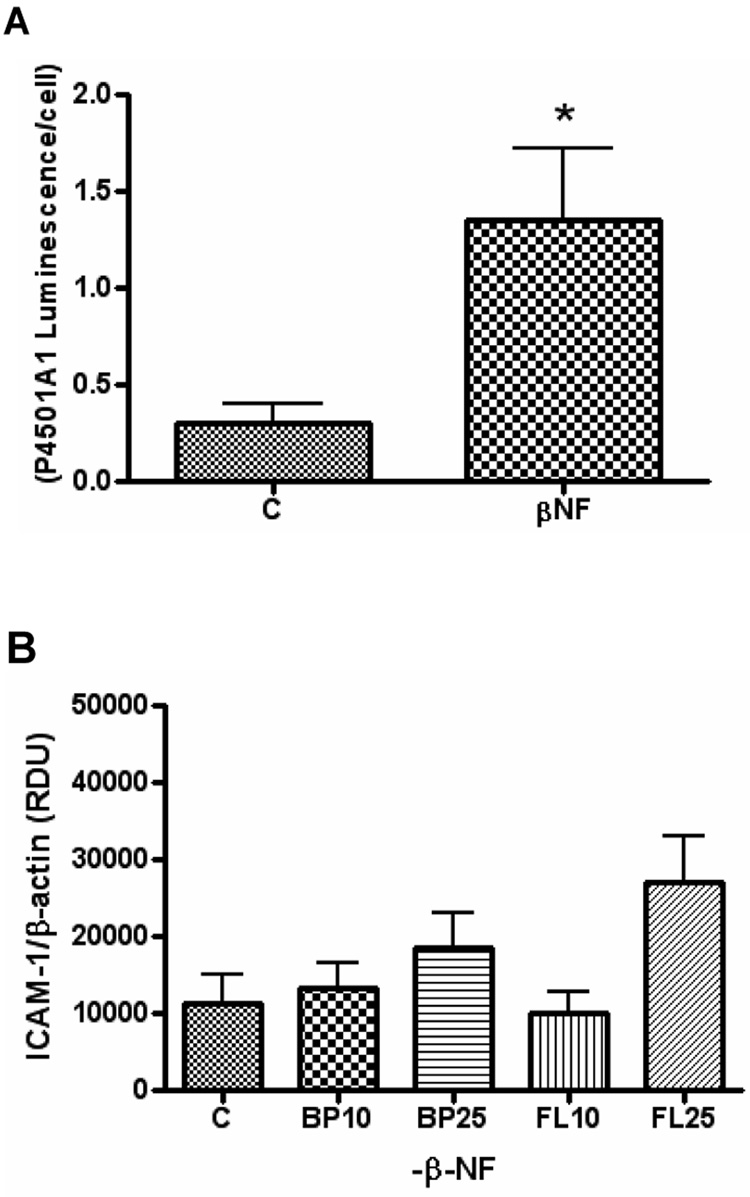
HUVEC were shown to have a responsive AhR by measuring activation of cytochrome P4501A1 after treatment with β-NF (Figure 1A). HUVEC were pre-treated with or without the AhR agonist β-NF and then treated with B[a]P or fluoranthene (FL) at 10–25 µM (Figure 1B and 1C). Exposure to B[a]P increased protein expression of ICAM-1 only after pre-treatment with β-NF (Figure 2B). These effects were specific because FL did not affect ICAM-1 protein levels regardless of pre-treatment with β-NF.
Figure 1. B[a]P induces intercellular adhesion molecule-1 only after aryl hydrocarbon receptor activation.
HUVEC were treated with β–NF (1 µM) for 16 h and then measured for cytochrome P4501A1 activity by P450-Glo Assay. Cell number was measured by CellTiter-Glo Assay (A). Bars represent mean ± SEM of at least three independent experiments measuring P4501A1 Assay luminescence normalized to cell number. HUVEC were pretreated with DMSO (B) or 1 µM β-NF (C) for 16 h and then treated with either DMSO, B[a]P (BP), or fluoranthrene (FL) at 10 and 25 µM for 24 h. Whole cell lysate was probed by immunoblot analysis for ICAM-1 and β-actin and measured by densitometry. Bars represent mean ± SE of at least three independent experiments.
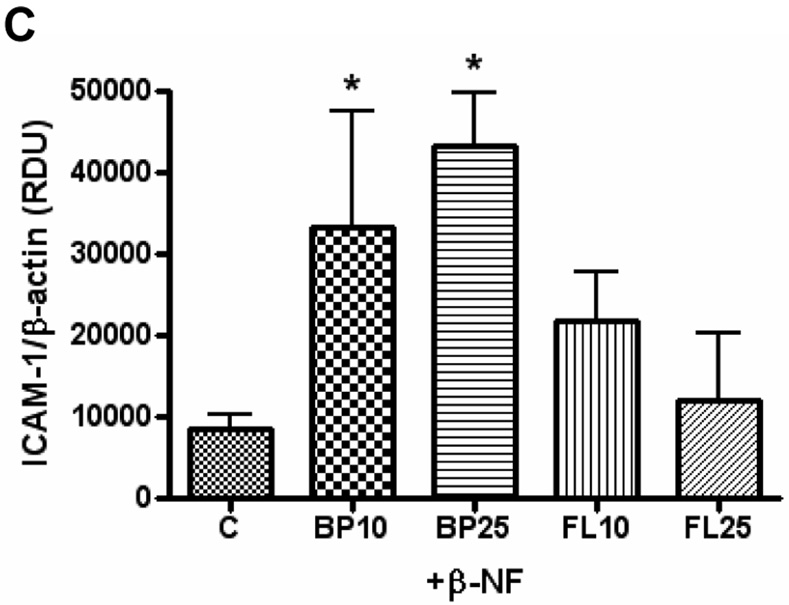
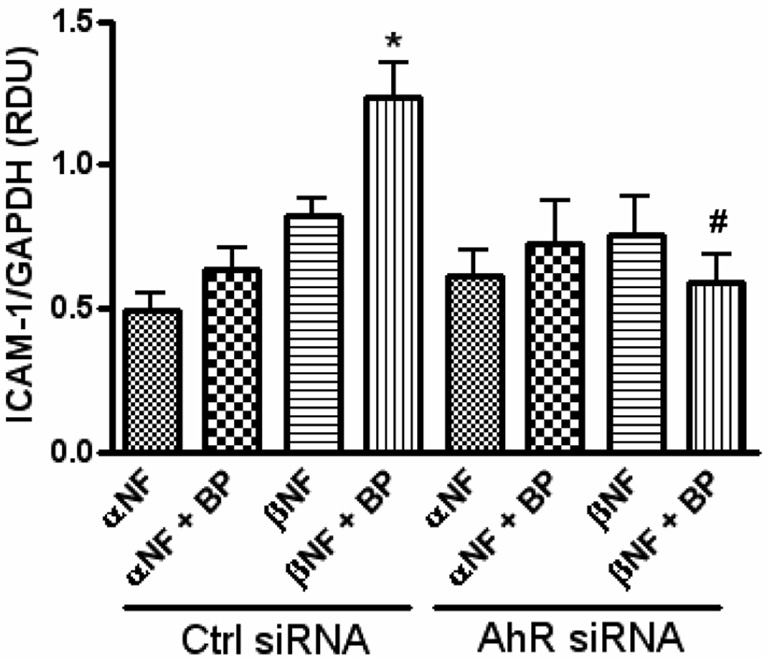
Figure 2. Absence of AhR reduces B[a]P-induced ICAM-1 expression.
HUVEC were transfected with control siRNA or specific AhR siRNA using the GeneSilencer Transfection Kit, which knocked down AhR expression by 92%. After 24 h, cells were pretreated with α-NF or β-NF (1 µM) for 16 h and then treated with DMSO or B[a]P (10 µM) for 24 h. Whole cell lysate was probed for ICAM-1, GAPDH, and AhR by immunoblot analysis and measured by densitometry. Bars represent mean ± SE of at least three independent experiments. * p<0.05 compared to β-NF alone. # p<0.05 compared to same group transfected with AhR siRNA.
To verify the necessity of AhR in β-NF-induced ICAM-1 expression, the receptor was knocked down using gene silencing technology. Transfection with AhR siRNA resulted in over 90% reduction in AhR protein for 72 h (data not shown). These cells were then pre-treated with β-NF or the AhR antagonist, α-NF, and then treated with B[a]P or DMSO (Figure 2). In cells transfected with the control siRNA, exposure to B[a]P increased protein expression of ICAM-1 only after pre-treatment with β-NF, but not when pre-treated with α-NF. This response was eliminated when the cells were transfected with specific AhR siRNA, further signifying the requirement of this nuclear receptor.
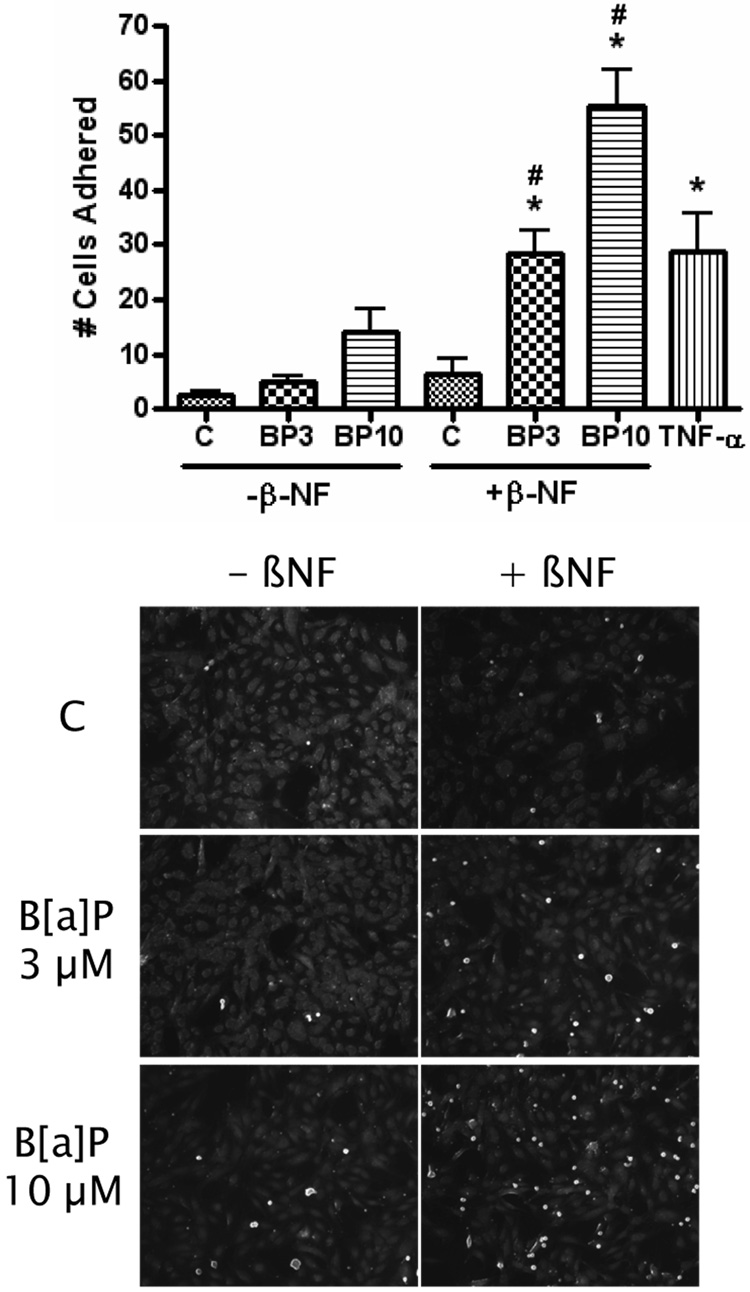
Similar response pattern was seen after measurement of monocyte adhesion. B[a]P increased the adhesion of fluorescently labeled monocytes to the treated endothelium at 3–10 µM only in the groups pre-treated with β-NF (Figure 3). TNF-α was used as a positive control for monocyte adhesion.
Figure 3. B[a]P induces monocyte adhesion only after AhR activation.
HUVEC were pretreated with DMSO or β-NF (1 µM) for 16 h and then treated with DMSO or B[a]P (BP) at 3 and 10 µM for 24 h. THP-1 monocytes were activated by TNF-α treatment and then labeled with calcein. Monocytes were allowed to adhere to the treated HUVEC for 30 min and then unbound cells were washed off. Bound monocytes were visualized and counted by fluorescent microscope. Bars represent mean ± SE of three independent experiments. Images are representative microscopic fields showing bound monocytes in white. * p<0.05 compared to control. # p<0.05 compared to same group treated with β-NF.
B[a]P induced ICAM-1 is mediated by MEK/p38/AP-1
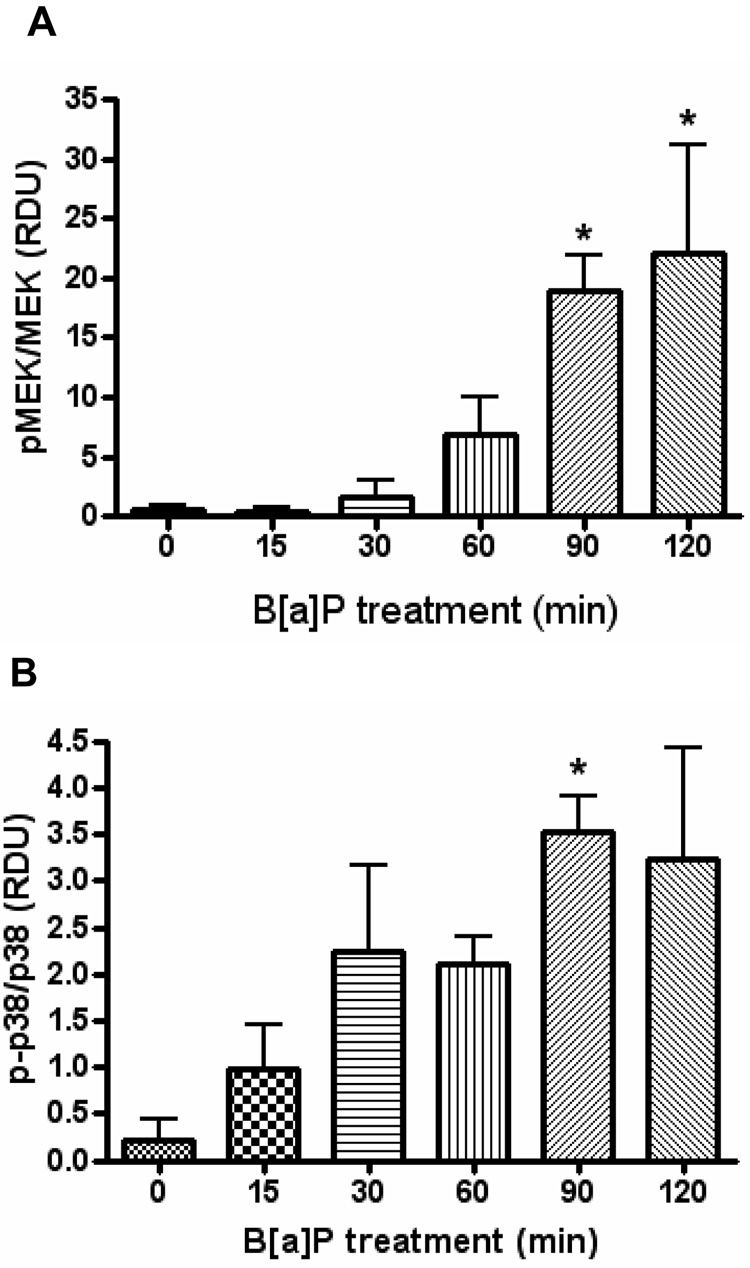
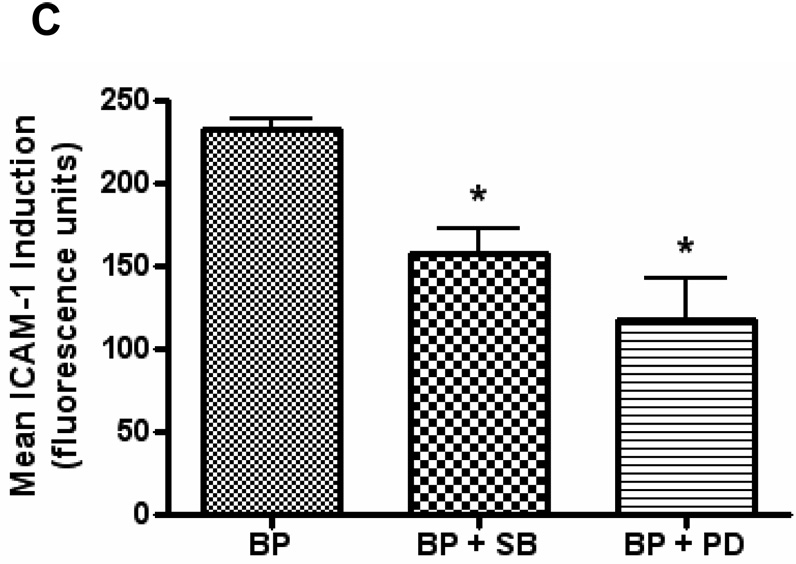
ICAM-1 expression has been shown to be stimulated by a number of signaling pathways including the MAPK cascades, AP-1, NF-κB, as well as a number of other proteins. HUVEC pretreated with β-NF and then treated with B[a]P significantly increased the phosphorylation thus activation of MEK and p38-MAPK, while not changing the total protein levels of these kinases (Figure 4A and 4B). To confirm these findings, HUVEC were pretreated with β-NF and inhibitors to these kinases. Both SB203580 and PD98059 decreased the induction of ICAM-1 protein elicited by β-NF/B[a]P treatment (Figure 4C). Phosphorylation of other MAPK family members, such as ERK1/2, which was unaltered by treatment with β-NF/B[a]P (data not shown).
Figure 4. B[a]P activates MEK and p38.
HUVEC were pretreated with β-NF (1 µM) for 16 h and then treated with DMSO or B[a]P (10 µM) for 15 – 120 min. Whole cell lysate extracts were probed by immunoblot analysis for phosphorylated MEK, total MEK (A), phosphorylated p38, and total p38 (B). Bars represent mean ± SEM of at least three independent experiments. HUVEC were pretreated with β-NF for 16 h and then pretreated with the inhibitors SB203580 (10 µM) or PD98059 (20 µM) for 1 h. Cells were then treated with DMSO or B[a]P (BP, 10 µM) for 24 h. ICAM-1 was measured by flow cytometry (C). Bars represent mean ± SE of the increase in ICAM-1 due to B[a]P treatment. * p<0.05 compared to control.
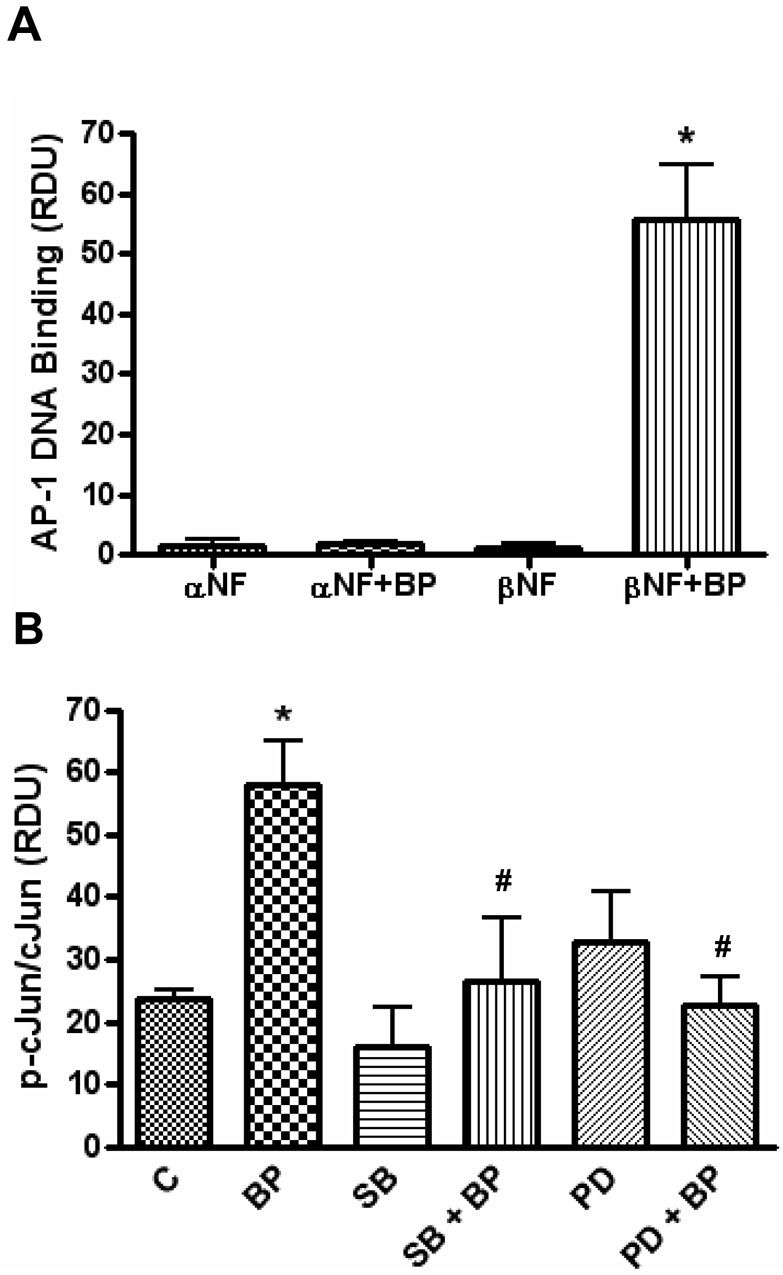
The activation of transcription factors downstream of the MAPK cascade was also investigated. Although treatment with B[a]P increased NF-κB DNA binding, this effect was not dependent upon β-NF pretreatment (data not shown), and thus unlikely to be involved in B[a]P-induced stimulation of ICAM-1. On the other hand, HUVEC pretreated with β-NF but not α-NF (AhR antagonist), and then exposed to B[a]P increased the AP-1 DNA binding (Figure 5A). AP-1 is formed of homo-or heterodimers of Jun and Fos proteins. Phosphorylation of cJun proximal the transactivation domain is required for AP-1 containing cJun to be efficiently activated (Pulverer et al., 1991). Therefore, to connect the activation of AP-1 with the activation of upstream MAPK cascades, cells were pretreated with the inhibitors to MEK (PD98059) and p38 (SB203580) and phosphorylation of cJun was measured. A 2 h exposure to βNF/B[a]P increased the phosphorylation of cJun and p38 and MEK inhibitors markedly blocked this effect (Figure 5B).
Figure 5. B[a]P activates activator protein-1 only after AhR activation and requires MEK and p38.
HUVEC were pretreated with α-NF (AhR antagonist) or β-NF (AhR agonist) for 16 h and then treated with DMSO or B[a]P (10 µM) for 4 h. Nuclear extracts were probed for AP-1 DNA binding (A). HUVEC pretreated with βNF for 16 h were then pretreated with inhibitors to MEK (PD98059, 20 µM) or p38 (SB203580, 10 µM) for 1 h. After pretreatment, cells were treated with DMSO or B[a]P (10 µM) for 120 min. Whole cell lysate was probed by immunoblot analysis for phosphorylated cJun and total cJun and measured by densitometry (B). Bars represent mean ± SE of at least three independent experiments. * p<0.05 compared to control. # p<0.05 compared to BP treated group.
Caveolae are necessary for B[a]P induced ICAM-1
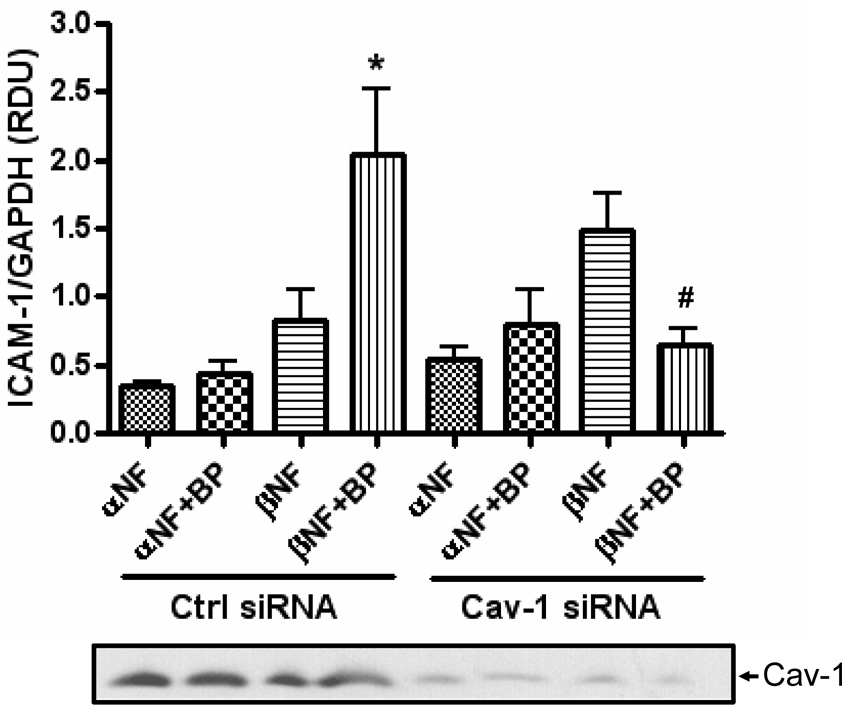
Caveolin-1, the main structural protein of caveolae, is able to concentrate signaling molecules together within caveolae microdomains and has been shown to be necessary for activation of p38-MAPK (Siddiqui et al., 2007; Zeidan et al., 2008). Therefore, the role of caveolin-1 in B[a]P-mediated stimulation of ICAM-1 expression was also explored. HUVEC transfected with caveolin-1 siRNA were found to have 3% of the caveolin-1 protein compared to control transfected cells (Figure 6). Importantly, silencing of caveolin-1 by siRNA in HUVEC eliminated the ability of β-NF/B[a]P to induce ICAM-1 protein expression (Figure 6). Similar to the pattern observed in earlier experiments, ICAM-1 was only induced in cells that were pretreated with β-NF and then treated with B[a]P, whereas this was not the case in cells pretreated with α-NF. Treatment with β-NF/B[a]P did not change the protein levels or phosphorylation status of caveolin-1 (data not shown).
Figure 6. B[a]P does not induce ICAM-1 after caveolin-1 silencing.
HUVEC were treated with control or caveolin-1 siRNA for 4 h using the GeneSilencer Transfection Kit. After 24 h, cells were pretreated with α-NF or β-NF for 16 h and then treated with DMSO or B[a]P (10 µM) for 24 h. Whole cell lysate was probed for ICAM-1, GAPDH, and caveolin-1 by immunoblot analysis and measured by densitometry. Bars represent mean ± SE of at least three independent experiments. The bands below are representative blots of caveolin-1. * p<0.05 compared to β-NF alone. # p<0.05 compared to same group transfected with Cav-1 siRNA.
Discussion
Much attention has been placed on the role of air pollution and the included organics in the development and progression of cardiovascular diseases such as atherosclerosis (Dockery et al., 1993; Pope et al., 2002; Pope et al., 2004; Pope et al., 2006; Auchincloss et al., 2008). B[a]P is a well studied carcinogen that has been shown to lead to atherosclerosis in animal models (Albert et al., 1977; Hough et al., 1993; Curfs et al., 2004) and epidemiological data (Burstyn et al., 2005). We suggest a mechanism by which B[a]P is contributing to atherosclerosis by increasing endothelial dysfunction and enhancing adhesiveness. The data clearly show that B[a]P is able to increase ICAM-1 in primary human endothelial cells and that these events require functional AhR and caveolin-1. The data also illustrate that β-NF/B[a]P induces ICAM-1 by signaling through MEK, p38 MAPK, and AP-1 leading to increased adhesion of monocytes to the activated endothelium.
PAHs are ubiquitous environmental contaminants. Humans are exposed through ingestion of contaminated foods and inhalation of polluted air. Both sources are a consequence of incomplete combustion processes. Animal studies have shown enhanced atherogenesis after PAH exposure for a number of years. More recently, hyperlipidemic mice treated with B[a]P have developed more severe atherosclerotic plaques (Curfs et al., 2004; Curfs et al., 2005). Interestingly, these plaques were characterized as having an increased content of inflammatory leukocytes such as macrophages and lymphocytes. Subsequent literature showed that B[a]P increased expression of MCP-1 in these mice, which was eliminated in vitro by AhR antagonist treatment (Knaapen et al., 2007). MCP-1 plays a critical role in recruitment of leukocytes to the endothelium; however cellular adhesion molecules such as ICAM-1 are responsible for diapedesis of these inflammatory cells into the vessel intima. After adhesion molecules bind circulating leukocytes, they trans-or para-cellularly migrate through the endothelium, after which they can differentiate and take up oxidized lipid particles. The cells then transform into foam cells which create the plaque that is hallmark to atherosclerosis (Glass and Witztum, 2001).
PAHs are a large class of organic compounds that vary by size and structure, for example the number and shape of rings (Ramesh et al., 2004). Two PAHs were examined in this paper, B[a]P and FL. We show that B[a]P can significantly induce ICAM-1 after pretreatment with an AhR inducer, but not after pretreatment with an AhR antagonist or after AhR knockdown. However, FL was not able to increase ICAM-1 either with or without AhR activation. B[a]P is a ligand to AhR, enabling its own metabolism by AhR-controlled enzymes such as CYP1A1, CYP1A2, CYP1B1, glutathione S-transferase, and UDP-glucuronyltransferase. B[a]P will induce the AhR enzymes CYP1A1 and CYP1B1, whereas FL is generally inactive or inhibitory to AhR (Willett et al., 2001; Shimada et al., 2002). As expected, AhR activation did not enhance the ability of FL to induce proinflammatory mediators in HUVEC. These data suggest that β-NF and B[a]P are synergistic in behavior. β-NF enhances the metabolic rate by inducing the AhR-controlled metabolizing enzymes that are necessary for the actions of B[a]P.
It is well understood that the carcinogenic nature of B[a]P is due to activation by P450s and epoxide hydrolases to the diol epoxide, (±)-anti-7β,8α-dihydroxy-9α,10α-epoxy7,8,9,10-tetrahydrobenzo[a]pyrene, however B[a]P can also be metabolically activated to radical cations, as well as reactive and redox active o-quinones (Penning et al., 1999). Our data demonstrate that AhR is necessary for the induction of ICAM-1 protein, suggesting that B[a]P must be metabolically activated by AhR controlled enzymes. The data do not propose a specific metabolite as the active compound. B[a]P is atherogenic irrespective of its mutagenic properties (Curfs et al., 2005), which may suggest that the diol epoxide is not the metabolite causing these effects. ICAM-1 transcription can be controlled by redox sensitive signaling pathways, of which the MAPK and AP-1 are examples. The B[a]P o-quinone can be formed by oxidation from a catechol, thus releasing H2O2 and O2−, and can also undergo reduction to reform the catechol, thus establishing a futile redox cycle in which amplified reactive oxygen species (ROS) are produced (Penning et al., 1999). It is possible that this increased oxidative stress is causing the induction of the inflammatory ICAM-1, making the o-quinone a potential atherogenic metabolite.
ICAM-1 expression has been linked with activation of the MAPK pathways (Tamura et al., 1998; Gao et al., 2002; Yan et al., 2002). In this study, we demonstrated that B[a]P induced ICAM-1 by activating MEK/p38-MAPK/AP-1. In contrast, neither ERK nor NF-κB seemed to contribute substantially to the ICAM-1 induction. B[a]P and its metabolites have been shown to induce MEK and p38 in other cellular systems (Patten Hitt et al., 2002; Chen et al., 2003; Mukherjee and Sikka, 2006; Ouyang et al., 2007). The inhibitors employed in the present study have been widely used as specific blockers for MEK and p38; however it has been suggested that they are somewhat ubiquitous and inhibit activation of other MAPK and enzymes (Birkenkamp et al., 2000). For example, it has even been shown that SB203580 can block CYP1A1 induction by TCDD (Shibazaki et al., 2004a; Shibazaki et al., 2004b).
Caveolae are 50–100 nm nonclathrin-coated plasma membrane microdomains that are enriched in cholesterol and glycosphingolipids and constitute a well studied subset of lipid rafts. They are particularly abundant in endothelial cells (Frank et al., 2003) and play an important role in membrane traffic and cellular signal transduction. Caveolae have been reported to contain a number of proteins including receptors and signaling molecules like G-protein coupled receptors, tyrosine kinases, and serine/threonine kinases (Frank et al., 2003). Disruption of these structures has been shown to lead to interruption of normal cellular signaling cascades, including the MAPK proteins. Disruption of caveolae by methyl-beta-cyclodextrin or by caveolin-1 knockout inhibits p38-MAPK nuclear translocation and activation (Siddiqui et al., 2007; Zeidan et al., 2008). Caveolin-1 is the principal structural component of caveolae (Wolter et al., 2004) and drives caveolae formation by oligimerization with itself and other caveolins and interaction with cholesterol in the membrane (Rothberg et al., 1992; Sargiacomo et al., 1995). Deletion of caveolin-1 results in a complete loss of caveolae, and mice lacking this protein are protected against the development of atherosclerosis (Frank et al., 2004). Our data demonstrate that the deletion of caveolin-1 disrupts the induction of ICAM-1 from B[a]P. Interestingly, it has been proposed that caveolin-1 could play a critical role in the adhesion process either as a signaling platform or as a structural participant in the building of the transcytotic channel (Millan et al., 2006; Bouzin et al., 2007). Adhesion molecules such as ICAM-1 once activated redistribute and cluster into transmigratory cup structures which allow for trans-cellular and trans-endothelial migration of leukocytes from the lumen (Tilghman and Hoover, 2002; Carman and Springer, 2004; Shaw et al., 2004; Yang et al., 2006). It has been recognized that ICAM-1 is translocated to lipid rafts once activated (Amos et al., 2001; Tilghman and Hoover, 2002) and that caveolae play a direct role in the transcytosis process (Millan et al., 2006; Bouzin et al., 2007).
In summary, data from the current experiments demonstrate that B[a]P is able to increase ICAM-1 in human endothelial cells after activation by AhR. In the same manner, B[a]P is able to increase monocyte adhesion to the treated endothelium only after AhR activation. The induction of ICAM-1 is through activation of the MEK/p38-MAPK/AP-1 signaling cascade and requires functional caveolae. These data provide a possible mechanism of increased endothelial adhesiveness to the body of literature showing that exposure to PAHs like B[a]P are a risk factor for cardiovascular diseases such as atherosclerosis.
Acknowledgements
We would like to thank Dr. Thomas Curry and the UK Labor and Delivery staff for help obtaining human umbilical cords. We would also like to thank the UK Flow Cytometry facility for their assistance in analyzing the flow cytometry data. This research was supported by grants from NIEHS/NIH (P42ES07380), AHA Pre-doctoral Fellowship (0613216B), and the University of Kentucky Agricultural Experiment Station.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have no conflict of interest.
References
- Albert RE, Vanderlaan M, Burns FJ, Nishizumi M. Effect of carcinogens on chicken atherosclerosis. Cancer Res. 1977;37:2232–2235. [PubMed] [Google Scholar]
- Amos C, Romero IA, Schultze C, Rousell J, Pearson JD, Greenwood J, Adamson P. Cross-linking of brain endothelial intercellular adhesion molecule (ICAM)-1 induces association of ICAM-1 with detergent-insoluble cytoskeletal fraction. Arterioscler Thromb Vasc Biol. 2001;21:810–816. doi: 10.1161/01.atv.21.5.810. [DOI] [PubMed] [Google Scholar]
- Annas A, Granberg AL, Brittebo EB. Differential response of cultured human umbilical vein and artery endothelial cells to Ah receptor agonist treatment: CYP-dependent activation of food and environmental mutagens. Toxicol Appl Pharmacol. 2000;169:94–101. doi: 10.1006/taap.2000.9054. [DOI] [PubMed] [Google Scholar]
- Auchincloss AH, Roux AV, Dvonch JT, Brown PL, Barr RG, Daviglus ML, Goff DC, Kaufman JD, O'Neill MS. Associations between Recent Exposure to Ambient Fine Particulate Matter and Blood Pressure in the Multi-Ethnic Study of Atherosclerosis (MESA) Environ Health Perspect. 2008;116:486–491. doi: 10.1289/ehp.10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenkamp KU, Tuyt LM, Lummen C, Wierenga AT, Kruijer W, Vellenga E. The p38 MAP kinase inhibitor SB203580 enhances nuclear factor-kappa B transcriptional activity by a non-specific effect upon the ERK pathway. Br J Pharmacol. 2000;131:99–107. doi: 10.1038/sj.bjp.0703534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzin C, Brouet A, De Vriese J, Dewever J, Feron O. Effects of vascular endothelial growth factor on the lymphocyte-endothelium interactions: identification of caveolin-1 and nitric oxide as control points of endothelial cell anergy. J Immunol. 2007;178:1505–1511. doi: 10.4049/jimmunol.178.3.1505. [DOI] [PubMed] [Google Scholar]
- Burstyn I, Kromhout H, Partanen T, Svane O, Langard S, Ahrens W, Kauppinen T, Stucker I, Shaham J, Heederik D, Ferro G, Heikkila P, Hooiveld M, Johansen C, Randem BG, Boffetta P. Polycyclic aromatic hydrocarbons and fatal ischemic heart disease. Epidemiology. 2005;16:744–750. doi: 10.1097/01.ede.0000181310.65043.2f. [DOI] [PubMed] [Google Scholar]
- Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Nguyen N, Tamura K, Karin M, Tukey RH. The role of the Ah receptor and p38 in benzo[a]pyrene-7,8-dihydrodiol and benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide-induced apoptosis. J Biol Chem. 2003;278:19526–19533. doi: 10.1074/jbc.M300780200. [DOI] [PubMed] [Google Scholar]
- Cho KA, Ryu SJ, Park JS, Jang IS, Ahn JS, Kim KT, Park SC. Senescent phenotype can be reversed by reduction of caveolin status. J Biol Chem. 2003;278:27789–27795. doi: 10.1074/jbc.M208105200. [DOI] [PubMed] [Google Scholar]
- Curfs DM, Knaapen AM, Pachen DM, Gijbels MJ, Lutgens E, Smook ML, Kockx MM, Daemen MJ, van Schooten FJ. Polycyclic aromatic hydrocarbons induce an inflammatory atherosclerotic plaque phenotype irrespective of their DNA binding properties. Faseb J. 2005;19:1290–1292. doi: 10.1096/fj.04-2269fje. [DOI] [PubMed] [Google Scholar]
- Curfs DM, Lutgens E, Gijbels MJ, Kockx MM, Daemen MJ, van Schooten FJ. Chronic exposure to the carcinogenic compound benzo[a]pyrene induces larger and phenotypically different atherosclerotic plaques in ApoE-knockout mice. Am J Pathol. 2004;164:101–108. doi: 10.1016/S0002-9440(10)63101-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis HJ. Gas chromatographic determination of benzo(a)pyrene in cigarette smoke. Anal Chem. 1968;40:1583–1585. doi: 10.1021/ac60266a011. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Pope CA, 3rd, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Jr, Speizer FE. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Frank PG, Lee H, Park DS, Tandon NN, Scherer PE, Lisanti MP. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:98–105. doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
- Frank PG, Woodman SE, Park DS, Lisanti MP. Caveolin, caveolae, and endothelial cell function. Arterioscler Thromb Vasc Biol. 2003;23:1161–1168. doi: 10.1161/01.ATV.0000070546.16946.3A. [DOI] [PubMed] [Google Scholar]
- Gao F, Yue TL, Shi DW, Christopher TA, Lopez BL, Ohlstein EH, Barone FC, Ma XL. p38 MAPK inhibition reduces myocardial reperfusion injury via inhibition of endothelial adhesion molecule expression and blockade of PMN accumulation. Cardiovasc Res. 2002;53:414–422. doi: 10.1016/s0008-6363(01)00488-6. [DOI] [PubMed] [Google Scholar]
- Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- Gowdy K, Krantz QT, Daniels M, Linak WP, Jaspers I, Gilmour MI. Modulation of pulmonary inflammatory responses and antimicrobial defenses in mice exposed to diesel exhaust. Toxicol Appl Pharmacol. 2008 doi: 10.1016/j.taap.2008.01.040. [DOI] [PubMed] [Google Scholar]
- Hough JL, Baird MB, Sfeir GT, Pacini CS, Darrow D, Wheelock C. Benzo(a)pyrene enhances atherosclerosis in White Carneau and Show Racer pigeons. Arterioscler Thromb. 1993;13:1721–1727. doi: 10.1161/01.atv.13.12.1721. [DOI] [PubMed] [Google Scholar]
- Izzotti A, De Flora S, Petrilli GL, Gallagher J, Rojas M, Alexandrov K, Bartsch H, Lewtas J. Cancer biomarkers in human atherosclerotic lesions: detection of DNA adducts. Cancer Epidemiol Biomarkers Prev. 1995;4:105–110. [PubMed] [Google Scholar]
- Kalra VK, Ying Y, Deemer K, Natarajan R, Nadler JL, Coates TD. Mechanism of cigarette smoke condensate induced adhesion of human monocytes to cultured endothelial cells. J Cell Physiol. 1994;160:154–162. doi: 10.1002/jcp.1041600118. [DOI] [PubMed] [Google Scholar]
- Knaapen AM, Curfs DM, Pachen DM, Gottschalk RW, de Winther MP, Daemen MJ, Van Schooten FJ. The environmental carcinogen benzo[a]pyrene induces expression of monocyte-chemoattractant protein-1 in vascular tissue: a possible role in atherogenesis. Mutat Res. 2007;621:31–41. doi: 10.1016/j.mrfmmm.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Lisanti MP, Scherer PE, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, Tu YH, Cook RF, Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol. 1994;126:111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LB, Hashi Y, Liu M, Wei Y, Lin JM. Determination of particle-associated polycyclic aromatic hydrocarbons in urban air of Beijing by GC/MS. Anal Sci. 2007;23:667–671. doi: 10.2116/analsci.23.667. [DOI] [PubMed] [Google Scholar]
- Merchant M, Krishnan V, Safe S. Mechanism of action of alpha-naphthoflavone as an Ah receptor antagonist in MCF-7 human breast cancer cells. Toxicol Appl Pharmacol. 1993;120:179–185. doi: 10.1006/taap.1993.1101. [DOI] [PubMed] [Google Scholar]
- Millan J, Hewlett L, Glyn M, Toomre D, Clark P, Ridley AJ. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola-and F-actin-rich domains. Nat Cell Biol. 2006;8:113–123. doi: 10.1038/ncb1356. [DOI] [PubMed] [Google Scholar]
- Mukherjee JJ, Sikka HC. Attenuation of BPDE-induced p53 accumulation by TPA is associated with a decrease in stability and phosphorylation of p53 and downregulation of NFkappaB activation: role of p38 MAP kinase. Carcinogenesis. 2006;27:631–638. doi: 10.1093/carcin/bgi247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N'Diaye M, Le Ferrec E, Lagadic-Gossmann D, Corre S, Gilot D, Lecureur V, Monteiro P, Rauch C, Galibert MD, Fardel O. Aryl hydrocarbon receptor- and calcium-dependent induction of the chemokine CCL1 by the environmental contaminant benzo[a]pyrene. J Biol Chem. 2006;281:19906–19915. doi: 10.1074/jbc.M601192200. [DOI] [PubMed] [Google Scholar]
- Nageh MF, Sandberg ET, Marotti KR, Lin AH, Melchior EP, Bullard DC, Beaudet AL. Deficiency of inflammatory cell adhesion molecules protects against atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1997;17:1517–1520. doi: 10.1161/01.atv.17.8.1517. [DOI] [PubMed] [Google Scholar]
- O'Neill MS, Veves A, Sarnat JA, Zanobetti A, Gold DR, Economides PA, Horton ES, Schwartz J. Air pollution and inflammation in type 2 diabetes: a mechanism for susceptibility. Occup Environ Med. 2007;64:373–379. doi: 10.1136/oem.2006.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W, Ma Q, Li J, Zhang D, Ding J, Huang Y, Xing MM, Huang C. Benzo[a]pyrene diol-epoxide (B[a]PDE) upregulates COX-2 expression through MAPKs/AP-1 and IKKbeta/NF-kappaB in mouse epidermal Cl41 cells. Mol Carcinog. 2007;46:32–41. doi: 10.1002/mc.20260. [DOI] [PubMed] [Google Scholar]
- Patel SS, Thiagarajan R, Willerson JT, Yeh ET. Inhibition of alpha4 integrin and ICAM-1 markedly attenuate macrophage homing to atherosclerotic plaques in ApoE-deficient mice. Circulation. 1998;97:75–81. doi: 10.1161/01.cir.97.1.75. [DOI] [PubMed] [Google Scholar]
- Patten Hitt E, DeLong MJ, Merrill AH., Jr Benzo(a)pyrene activates extracellular signal-related and p38 mitogen-activated protein kinases in HT29 colon adenocarcinoma cells: involvement in NAD(P)H:quinone reductase activity and cell proliferation. Toxicol Appl Pharmacol. 2002;183:160–167. doi: 10.1006/taap.2002.9483. [DOI] [PubMed] [Google Scholar]
- Penning TM, Burczynski ME, Hung CF, McCoull KD, Palackal NT, Tsuruda LS. Dihydrodiol dehydrogenases and polycyclic aromatic hydrocarbon activation: generation of reactive and redox active o-quinones. Chem Res Toxicol. 1999;12:1–18. doi: 10.1021/tx980143n. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. Jama. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Muhlestein JB, May HT, Renlund DG, Anderson JL, Horne BD. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation. 2006;114:2443–2448. doi: 10.1161/CIRCULATIONAHA.106.636977. [DOI] [PubMed] [Google Scholar]
- Pulverer BJ, Kyriakis JM, Avruch J, Nikolakaki E, Woodgett JR. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991;353:670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- Ramesh A, Walker SA, Hood DB, Guillen MD, Schneider K, Weyand EH. Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. Int J Toxicol. 2004;23:301–333. doi: 10.1080/10915810490517063. [DOI] [PubMed] [Google Scholar]
- Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev. 2002;54:431–467. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Sargiacomo M, Scherer PE, Tang Z, Kubler E, Song KS, Sanders MC, Lisanti MP. Oligomeric structure of caveolin: implications for caveolae membrane organization. Proc Natl Acad Sci U S A. 1995;92:9407–9411. doi: 10.1073/pnas.92.20.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SK, Ma S, Kim MB, Rao RM, Hartman CU, Froio RM, Yang L, Jones T, Liu Y, Nusrat A, Parkos CA, Luscinskas FW. Coordinated redistribution of leukocyte LFA-1 and endothelial cell ICAM-1 accompany neutrophil transmigration. J Exp Med. 2004;200:1571–1580. doi: 10.1084/jem.20040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Rattan V, Sultana C, Kalra VK. Cigarette smoke condensate-induced adhesion molecule expression and transendothelial migration of monocytes. Am J Physiol. 1996;270:H1624–H1633. doi: 10.1152/ajpheart.1996.270.5.H1624. [DOI] [PubMed] [Google Scholar]
- Shibazaki M, Takeuchi T, Ahmed S, Kikuchi H. Suppression by p38 MAP kinase inhibitors (pyridinyl imidazole compounds) of Ah receptor target gene activation by 2,3,7,8-tetrachlorodibenzo-p-dioxin and the possible mechanism. J Biol Chem. 2004a;279:3869–3876. doi: 10.1074/jbc.M305880200. [DOI] [PubMed] [Google Scholar]
- Shibazaki M, Takeuchi T, Ahmed S, Kikuchia H. Blockade by SB203580 of Cyp1a1 induction by 2,3,7,8-tetrachlorodibenzo-p-dioxin, and the possible mechanism: possible involvement of the p38 mitogen-activated protein kinase pathway in shuttling of Ah receptor overexpressed in COS-7 cells. Ann N Y Acad Sci. 2004b;1030:275–281. doi: 10.1196/annals.1329.034. [DOI] [PubMed] [Google Scholar]
- Shimada T, Inoue K, Suzuki Y, Kawai T, Azuma E, Nakajima T, Shindo M, Kurose K, Sugie A, Yamagishi Y, Fujii-Kuriyama Y, Hashimoto M. Arylhydrocarbon receptor-dependent induction of liver and lung cytochromes P450 1A1, 1A2, and 1B1 by polycyclic aromatic hydrocarbons and polychlorinated biphenyls in genetically engineered C57BL/6J mice. Carcinogenesis. 2002;23:1199–1207. doi: 10.1093/carcin/23.7.1199. [DOI] [PubMed] [Google Scholar]
- Siddiqui SS, Siddiqui ZK, Uddin S, Minshall RD, Malik AB. p38 MAPK activation coupled to endocytosis is a determinant of endothelial monolayer integrity. Am J Physiol Lung Cell Mol Physiol. 2007;292:L114–L124. doi: 10.1152/ajplung.00257.2005. [DOI] [PubMed] [Google Scholar]
- Stade BG, Messer G, Riethmuller G, Johnson JP. Structural characteristics of the 5′ region of the human ICAM-1 gene. Immunobiology. 1990;182:79–87. doi: 10.1016/S0171-2985(11)80585-1. [DOI] [PubMed] [Google Scholar]
- Tamura DY, Moore EE, Johnson JL, Zallen G, Aiboshi J, Silliman CC. p38 mitogen-activated protein kinase inhibition attenuates intercellular adhesion molecule-1 up-regulation on human pulmonary microvascular endothelial cells. Surgery. 1998;124:403–407. discussion 408. [PubMed] [Google Scholar]
- Thirman MJ, Albrecht JH, Krueger MA, Erickson RR, Cherwitz DL, Park SS, Gelboin HV, Holtzman JL. Induction of cytochrome CYPIA1 and formation of toxic metabolites of benzo[a]pyrene by rat aorta: a possible role in atherogenesis. Proc Natl Acad Sci U S A. 1994;91:5397–5401. doi: 10.1073/pnas.91.12.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman RW, Hoover RL. E-selectin and ICAM-1 are incorporated into detergent-insoluble membrane domains following clustering in endothelial cells. FEBS Lett. 2002;525:83–87. doi: 10.1016/s0014-5793(02)03070-3. [DOI] [PubMed] [Google Scholar]
- Toborek M, Lee YW, Kaiser S, Hennig B. Measurement of inflammatory properties of fatty acids in human endothelial cells. Methods Enzymol. 2002;352:198–219. doi: 10.1016/s0076-6879(02)52020-6. [DOI] [PubMed] [Google Scholar]
- Torres M, Forman HJ. Redox signaling and the MAP kinase pathways. Biofactors. 2003;17:287–296. doi: 10.1002/biof.5520170128. [DOI] [PubMed] [Google Scholar]
- Vincent R, Bjarnason SG, Adamson IY, Hedgecock C, Kumarathasan P, Guenette J, Potvin M, Goegan P, Bouthillier L. Acute pulmonary toxicity of urban particulate matter and ozone. Am J Pathol. 1997;151:1563–1570. [PMC free article] [PubMed] [Google Scholar]
- Voraberger G, Schafer R, Stratowa C. Cloning of the human gene for intercellular adhesion molecule 1 and analysis of its 5′-regulatory region. Induction by cytokines and phorbol ester. J Immunol. 1991;147:2777–2786. [PubMed] [Google Scholar]
- Wang XM, Zhang Y, Kim HP, Zhou Z, Feghali-Bostwick CA, Liu F, Ifedigbo E, Xu X, Oury TD, Kaminski N, Choi AM. Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med. 2006;203:2895–2906. doi: 10.1084/jem.20061536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub WS. Cigarette smoking as a risk factor for coronary artery disease. Adv Exp Med Biol. 1990;273:27–37. doi: 10.1007/978-1-4684-5829-9_4. [DOI] [PubMed] [Google Scholar]
- Willett KL, Wassenberg D, Lienesch L, Reichert W, Di Giulio RT. In vivo and in vitro inhibition of CYP1A-dependent activity in Fundulus heteroclitus by the polynuclear aromatic hydrocarbon fluoranthene. Toxicol Appl Pharmacol. 2001;177:264–271. doi: 10.1006/taap.2001.9296. [DOI] [PubMed] [Google Scholar]
- Wolter DJ, Hanson ND, Lister PD. Insertional inactivation of oprD in clinical isolates of Pseudomonas aeruginosa leading to carbapenem resistance. FEMS Microbiol Lett. 2004;236:137–143. doi: 10.1016/j.femsle.2004.05.039. [DOI] [PubMed] [Google Scholar]
- Yan W, Zhao K, Jiang Y, Huang Q, Wang J, Kan W, Wang S. Role of p38 MAPK in ICAM-1 expression of vascular endothelial cells induced by lipopolysaccharide. Shock. 2002;17:433–438. doi: 10.1097/00024382-200205000-00016. [DOI] [PubMed] [Google Scholar]
- Yang L, Kowalski JR, Yacono P, Bajmoczi M, Shaw SK, Froio RM, Golan DE, Thomas SM, Luscinskas FW. Endothelial cell cortactin coordinates intercellular adhesion molecule-1 clustering and actin cytoskeleton remodeling during polymorphonuclear leukocyte adhesion and transmigration. J Immunol. 2006;177:6440–6449. doi: 10.4049/jimmunol.177.9.6440. [DOI] [PubMed] [Google Scholar]
- Yatera K, Hsieh J, Hogg JC, Tranfield E, Suzuki H, Shih CH, Behzad AR, Vincent R, van Eeden SF. Particulate matter air pollution exposure promotes recruitment of monocytes into atherosclerotic plaques. Am J Physiol Heart Circ Physiol. 2008;294:H944–H953. doi: 10.1152/ajpheart.00406.2007. [DOI] [PubMed] [Google Scholar]
- Zeidan A, Javadov S, Chakrabarti S, Karmazyn M. Leptin-induced cardiomyocyte hypertrophy involves selective caveolae and RhoA/ROCK-dependent p38 MAPK translocation to nuclei. Cardiovasc Res. 2008;77:64–72. doi: 10.1093/cvr/cvm020. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, Weksler BB, Wang L, Schwartz J, Santella RM. Immunohistochemical detection of polycyclic aromatic hydrocarbon-DNA damage in human blood vessels of smokers and non-smokers. Atherosclerosis. 1998;140:325–331. doi: 10.1016/s0021-9150(98)00136-1. [DOI] [PubMed] [Google Scholar]