Abstract
Plants have a variety of chemical and anatomical defences, whose strengths depend on biotic and environmental influences. We show here that root inoculation with belowground bacteria, filamentous gram-positive streptomycetes, can induce plant defence responses. Such induced plant responses can occur belowground in the roots, but also aboveground, in the leaves, and include priming (sensitizing) like characters. Streptomycetes have also evolved mechanisms to facilitate plant root symbioses, mycorrhiza and root nodulation. By promoting fungal growth and by decreasing plant defence responses, these bacteria promote mycorrhiza formation. This minireview covers our current knowledge on the complex interactions that take place between streptomycetes, plants and rhizosphere microbes.
Key words: rhizosphere, bacteria, biocontrol, defence response, Streptomyces
Streptomycetes are traditionally considered as soil dwelling organisms.1 The success of these filamentous bacteria in terrestrial environments is attributed to their ability to produce an array of catabolic enzymes that degrade biopolymers. Streptomyces strains also produce mixtures of antimicrobial compounds, allowing them so to defend their substrates.2 Of the scores of soil-borne microorganisms, streptomycetes have been reported to be most prolific producers of a variety of clinically important biochemicals.3 There is now abundant evidence that some Streptomyces species colonise the rhizospheres of plant roots and even plant tissues,4,5 and it has been suggested that antibiotic production by the streptomycete may protect the host plants against phytopathogens.6,7 Streptomycetes causing plant disease were covered recently by Loria et al.8 Here we illustrate novel roles of streptomycetes in plant biology; inducing plant defences and facilitating symbiosis formation.
Ample evidence indicates that actinomycetes are quantitatively and qualitatively important in the rhizospheres of plants, where they may influence plant growth and protect plant roots against invasion by root pathogens.7 Disease suppression by streptomycetes owes partially to their exudation of various antimicrobials, helminticides and enzymes degrading fungal cell walls and insect exoskeletons.9 However, the presence of biocontrol activity determinants other than direct antagonism has been suggested by several studies.
First of all, in vitro inhibitory activities of the antagonistic strains do not always allow an accurate prediction of disease control potential. Schottel et al.,10 used spontaneous mutants of antagonistic streptomycete strains to assess the importance of antagonism in the biocontrol of potato scab. Despite a reduced in vitro pathogen inhibition activity, most of these mutants demonstrated significant biocontrol activity against potato scab. Our second evidence suggests that bacterial influence on plant growth may be an important determinant in biocontrol. Eight antagonistic Streptomyces isolates were tested for their ability to control Phytophthora root rots on alfalfa and soybean.11 The strongest predictor of disease suppression in alfalfa by the antagonist was an increase in alfalfa biomass following inoculation with the bacterial isolate. In this case, direct enhancement of alfalfa growth by Streptomyces may be one of the key mechanisms by which Streptomyces antagonists enhance plant health. In our preliminary experiments we have observed similar dependence between plant growth promotion and increased disease resistance, while screening for streptomycete strains that are able to suppress brassica dark leaf spot development in Arabidopsis thaliana (Herold M, Schrey S, Tarkka M, unpublished).
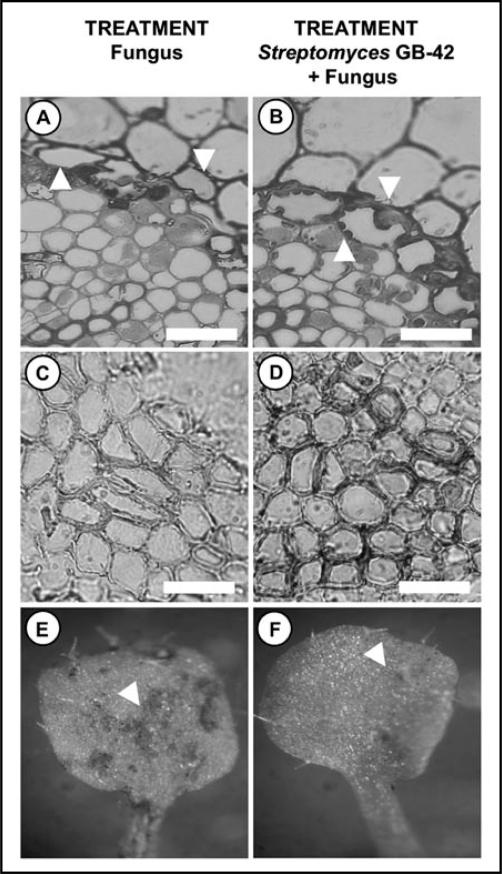
Upon infection by certain rhizobacteria, plants acquire an increased resistance to pathogen attack. This phenomenon has been classified as priming.12 Rhizosphere and endophytic streptomycetes have been recently indicated as such disease resistance inducing species.13,14 We have studied the mechanisms of disease suppression by the streptomycete GB 4-2 against Heterobasidion root and butt rot in Norway spruce seedlings. Curiously, GB 4-2 promoted the physiology of the pathogenic fungus: mycelial growth, germination rate of fungal spores, extension of fungal germ tubes and even early colonisation of outer cortical layer of the plant root were all enhanced by bacterial influence. However, later disease development was blocked by the bacterium, since the port of fungal entry into the vascular tissue, the root cortex, was blocked by the formation of cell wall thickenings in co-inoculated plants (Fig. 1B). In addition, the vascular tissue was rendered inaccessible due to increased xylem formation and strong lignification (Fig. 1D).13 Altogether, these data indicate that the inoculation with GB 4-2 sensitized the plant to respond stronger to the root pathogen.
Figure 1.
Events associated with plant protection by Streptomyces GB 4-2. The stronger capacity to activate defence responses at the sites of secondary infection results in a decrease in disease. (A) Upon 3-wk treatment of Norway spruce roots by the phytopathogen Heterobasidion abietinum, root cortex is colonised by the fungus. (B) Prior treatment with GB 4-2 causes the formation of cell wall thickenings (arrowheads) that block further plant colonisation by the fungus. Whereas the fungus has lysed most of the cells 4 wks after inoculation (C), pre-treatment with GB 4-2 leads to the formation of thick-walled, small diameter tracheids (D). Plant's resistance to secondary infections is also increased in the shoot by GB 4-2. The formation of brassica dark leaf spot (E) in leaves of Arabidopsis thaliana (arrowheads) is suppressed by pre-inoculation of plant roots by GB 4-2 (F). Bars 0.5 mm.
An important finding was associated with this result. The infection of needles by Botrytis cinerea was also reduced in Norway spruce due to pre-treatment by Streptomyces GB 4-2, suggesting increased systemic defences. To further analyse the mechanisms of this, we have examined the accumulation of defence related transcripts in Arabidopsis thaliana during its interaction with GB 4-2 in the roots and/or the phytopathogenic fungus Alternaria brassicicola in the leaves (Fig. 1F; Schrey S, unpublished). The aim of these gene expression analyses is to unravel if GB 4-2 provokes a plant immune response similar to induced systemic resistance (ISR), or systemic acquired resistance (SAR). In both ISR and SAR, prior treatment results in a stronger defence response against subsequent challenge by a pathogen (reviewed in ref. 12). In general, ISR is commonly induced by the challenge of plants by root-colonising bacteria and SAR by phytopathogens. By using a set of Arabidopsis genes related to plant immunity,15 we have observed that the response of Arabidopsis thaliana to GB 4-2 involves changes in expression of genes associated with SAR but also with ISR. Interestingly, ISR-related changes occur in the absence of the challenge by the pathogenic organism, indicating a novel, possibly GB 4-2 specific response pattern in Arabidopsis (Schrey S, unpublished).
Conn et al.,14 investigated the impact of plant protecting actinomycetes on disease resistance related gene expression in Arabidopsis. The bacterial inoculation led to promoted Arabidopsis growth and endophytic colonisation in the plant tissues. Suppression of Erwinia carotovora soft rot as well as of Fusarium oxysporum wilt disease was also observed. Gene expression responses to streptomycetes were specific to the bacterial isolate; e.g., inoculation of Arabidopsis seeds with Streptomyces sp. EN27 resulted in a 19-fold induction of the PR-1 transcript, whereas the closely related strain, Streptomyces sp. EN28, was able to induce PDF1.2 by 23-fold. In dual inoculations the bacteria were able to prime both the SAR and the ISR pathways of Arabidopsis, upregulating genes in either pathway depending on the infecting pathogen. The use of defence compromised mutants of Arabidopsis showed that Streptomyces sp. EN27 induced resistance to E. carotovora by a NPR1-independent and to F. oxysporum by a NPR1-dependent pathway. In conclusion, the gene expression responses to streptomycetes indicate novel patterns of priming by these bacteria, sharing features of the both previously characterized pathways, ISR and SAR.
Plant defence responses can be suppressed by specialised organisms. These organisms can produce physiologically active levels of metabolites such as enzymes that act on plant toxins, or exude their own toxins in planta that interfere with plant metabolism in ways that benefit the attacker.16 They can also produce signals that disrupt the plant's own defence signalling pathways.17 We have gained evidence for attenuation of plant defences by investigating the streptomycete strain Streptomyces AcH 505.
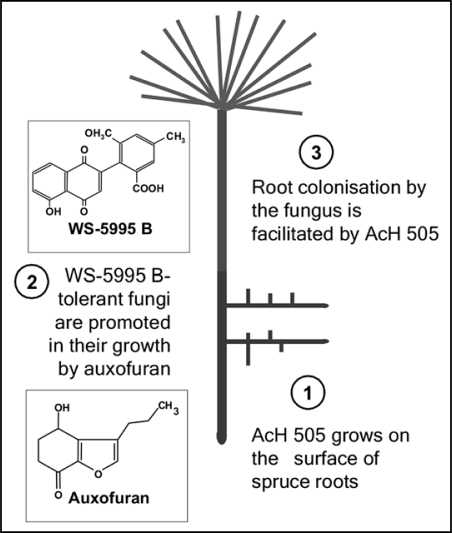
AcH 505 is a so-called mycorrhization helper bacterium, a bacterium that facilitates the formation of mycorrhizal symbiosis.18 According to our current model (Fig. 2) both water soluble as well as volatile bacterial biochemicals are involved in AcH 505-fungus-plant interactions. First of all, fungal mycelial growth is promoted by auxofuran, an auxin related compound. In addition, AcH 505 produces an inhibitor of mycelial growth, the antibiotic WS-5995 B.19 As the influence of WS-5995 B dominates over that of auxofuran, only the WS-5995 B tolerant strains are able to infect the host plant.
Figure 2.
Streptomyces AcH 505 expresses a combination of mechanisms. The metabolite WS 5995 B acts as a selector, since only WS-5995 B sensitive fungi are inhibited by AcH 505. The second dominant metabolite of AcH 505, auxofuran, stimulates mycelial growth of fungi. The presence of AcH 505 promotes colonization of the root by the fungus. This presumably occurs by suppression of the host defence response by AcH 505, reflected by suppressed defence gene activities.
The growth of a WS-5995 B sensitive isolate of an important plant pathogen, Heterobasidion sp., was inhibited by AcH 505, and we envisaged a potential application for AcH 505: simultaneous growth promotion of mycorrhizal as well as growth suppression of pathogenic fungi.20,21 To determine if AcH 505 could serve as a biocontrol agent against Heterobasidion root and butt rot, bacterial influence on mycelial growth of Heterobasidion sp. isolates, on the colonisation of wood discs and Norway spruce roots was determined. It had been previously suggested10 that single pathogen-antagonist strain studies may contribute only limited insight into the dynamics of antagonist-pathogen interactions. Our data agreed well with that suggestion: whereas 11 tested Heterobasidion strains were sensitive against the antibiotic and suppressed by the bacterium, the growth of the 12th strain, Heterobasidion abietinum 331, was unaffected by AcH 505. Hazardous in light of biocontrol applications, root colonisation by Heterobasidion abietinum 331 was promoted by the bacterium due to a decrease in defence related gene expression in Norway spruce.22 Using a culture system where the bacterium was separated from plant roots and fungal mycelium, we have been able to show that increased fungal colonisation is due to volatiles produced by AcH 505 (Störk M, Lehr N and Tarkka M, unpublished). In conclusion, metabolite production by AcH 505 leads to the inhibition of some and to the benefit of other microorganisms. Depending on the species spectrum in the habitat of the host plant, the development of symbiosis and/or disease may be promoted by this streptomycete (Fig. 2). We have recently found an unrelated streptomycete strain that produces the same secondary metabolites as AcH 505 but does not promote the growth of symbiotic fungi (Störk M and Schulz D, unpublished); further work with this strain and mutagenesis experiments may help to unravel the relative importance of each compound in the interactions.
How bacteria influence the establishment of infectious diseases and symbioses in plants is most thoroughly investigated in fluorescent pseudomonads. The role of streptomycetes has gained increased interest, and our aim was to show that the functions first characterized for pseudomonads may also be served by the streptomycetes. There is a great deal of variation in how the streptomycetes influence plant defence responses. The ecological implications of inducing changes in the host plant are striking, especially when we consider bacteria like AcH 505 that promote some and inhibit other plant associated microbes, and simultaneously suppress plant defences. On the other hand, the reports on increased disease resistance after plant treatment with streptomycetes are encouraging in light of biotechnological applications. They suggest that specific streptomycete strains may be able to restrict plant colonization by pathogens that are able to escape their biological warfare. A thorough elucidation of the defence sensitized state on the molecular level will tell if the streptomycetes induce a common pattern of plant responses, and contribute to a better understanding of plant behavior during plant-microbe interactions.
Acknowledgements
Research in our laboratory has been supported by the German Science Foundation. Isolation of Streptomyces AcH 505 secondary metabolites was performed by Julia Riedlinger and Hans-Peter Fiedler. We are indebted to Margret Ecke for excellent technical assistance. We would like to thank Frantisek Baluska for the kind invitation to write this mini-review.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5996
References
- 1.Janssen PH. Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Appl Environ Microbiol. 2006;72:1719–1728. doi: 10.1128/AEM.72.3.1719-1728.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davelos AL, Kinkel LL, Samac DA. Spatial variation in frequency and intensity of antibiotic interactions among Streptomycetes from prairie soil. Appl Environ Microbiol. 2004;70:1051–1058. doi: 10.1128/AEM.70.2.1051-1058.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berdy J. Bioactive microbial metabolites: a personal view. J Antibiot. 2005;58:1–26. doi: 10.1038/ja.2005.1. [DOI] [PubMed] [Google Scholar]
- 4.Sardi P, Saracchi M, Quaroni S, Petrolini B, Borgonovi GE, Merli S. Isolation of endophytic Streptomyces strains from surface-sterilized roots. Appl Environ Microbiol. 1992;58:2691–2693. doi: 10.1128/aem.58.8.2691-2693.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coombs JT, Franco CM. Isolation and identification of actinobacteria from surface-sterilized wheat roots. Appl Environ Microbiol. 2003;69:5603–5608. doi: 10.1128/AEM.69.9.5603-5608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Challis GL, Hopwood DA. Synergy and contingency as driving forces for the evolution of multiple secondary metabolite production by Streptomyces species. Proc Natl Acad Sci USA. 2003;100:14555–14561. doi: 10.1073/pnas.1934677100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarkka MT, Hampp R. Secondary metabolites of soil streptomycetes in biotic interactions. In: Karlovsky P, editor. Secondary Metabolites in Soil Ecology. 14. Berlin, Germany: Springer, Soil Biology Series; 2008. pp. 107–118. [Google Scholar]
- 8.Loria R, Kers J, Joshi M. Evolution of plant pathogenicity in Streptomyces. Annu Rev Phytopathol. 2006;44:469–487. doi: 10.1146/annurev.phyto.44.032905.091147. [DOI] [PubMed] [Google Scholar]
- 9.Weller DM, Raaijmakers JM, Gardener BB, Thomashow LS. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu Rev Phytopathol. 2002;40:309–348. doi: 10.1146/annurev.phyto.40.030402.110010. [DOI] [PubMed] [Google Scholar]
- 10.Schottel JL, Shimizu K, Kinkel LL. Relationships of in vitro pathogen inhibition and soil colonization to potato scab biocontrol by antagonistic Streptomyces spp. Biol Control. 2001;20:102–112. [Google Scholar]
- 11.Xiao K, Samac DA, Kinkel LL. Biological control of Phytophthora root rots on alfalfa and soybean with Streptomyces. Biol Control. 2002;23:285–295. [Google Scholar]
- 12.Conrath U, Pieterse CM, Mauch Mani B. Priming in plant-pathogen interactions. Trends Plant Sci. 2002;7:210–216. doi: 10.1016/s1360-1385(02)02244-6. [DOI] [PubMed] [Google Scholar]
- 13.Lehr NA, Schrey SD, Hampp R, Tarkka MT. Root inoculation with a forest soil streptomycete leads to locally and systemically increased resistance against phytopathogens in Norway spruce. New Phytol. 2008;177:965–976. doi: 10.1111/j.1469-8137.2007.02322.x. [DOI] [PubMed] [Google Scholar]
- 14.Conn VM, Walker AR, Franco CM. Endophytic actinobacteria induce defense pathways in Arabidopsis thaliana. Mol Plant Microbe Interact. 2008;21:208–218. doi: 10.1094/MPMI-21-2-0208. [DOI] [PubMed] [Google Scholar]
- 15.von Rad U, Mueller MJ, Durner J. Evaluation of natural and synthetic stimulants of plant immunity by microarray technology. New Phytol. 2005;165:191–202. doi: 10.1111/j.1469-8137.2004.01211.x. [DOI] [PubMed] [Google Scholar]
- 16.Bruce TJ, Pickett JA. Plant defence signalling induced by biotic attacks. Curr Opin Plant Biol. 2007;10:387–392. doi: 10.1016/j.pbi.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Cui J, Bahrami AK, Pringle EG, Hernandez Guzman G, Bender CL, Pierce NE, Ausubel FM. Pseudomonas syringae manipulates systemic plant defenses against pathogens and herbivores. Proc Natl Acad Sci USA. 2005;102:1791–1796. doi: 10.1073/pnas.0409450102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frey Klett P, Garbaye J, Tarkka M. The mycorrhiza helper bacteria revisited. New Phytol. 2007;176:22–36. doi: 10.1111/j.1469-8137.2007.02191.x. [DOI] [PubMed] [Google Scholar]
- 19.Riedlinger J, Schrey SD, Tarkka MT, Hampp R, Kapur M, Fiedler HP. Auxofuran, a novel metabolite stimulating growth of fly agaric, produced by the mycorrhiza helper bacterium Streptomyces AcH 505. Appl Environ Microbiol. 2006;72:3550–3557. doi: 10.1128/AEM.72.5.3550-3557.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maier A, Riedlinger J, Fiedler HP, Hampp R. Actinomycetales bacteria from a spruce stand: characterization and effects on growth of root symbiotic, and plant parasitic soil fungi in dual culture. Mycol Progr. 2004;3:129–136. [Google Scholar]
- 21.Schrey SD, Schellhammer M, Ecke M, Hampp R, Tarkka MT. Mycorrhiza helper bacterium Streptomyces AcH 505 induces differential gene expression in the ectomycorrhizal fungus Amanita muscaria. New Phytol. 2005;168:205–216. doi: 10.1111/j.1469-8137.2005.01518.x. [DOI] [PubMed] [Google Scholar]
- 22.Lehr NA, Schrey SD, Bauer R, Hampp R, Tarkka MT. Suppression of plant defence response by a mycorrhiza helper bacterium. New Phytol. 2007;174:892–903. doi: 10.1111/j.1469-8137.2007.02021.x. [DOI] [PubMed] [Google Scholar]