Abstract
Plants rely on the innate immune system to defend themselves from pathogen attacks. Reactive oxygen species (ROS) and nitric oxide (NO) play key roles in the activation of disease resistance mechanisms in plants. The evolutionarily conserved mitogen-activated protein kinase (MAPK) cascades are universal signal transduction modules in eukaryotes and have been implicated in the plant innate immunity. There have been many disputations about the relationship between the radicals (ROS and NO) and MAPK cascades. Recently, we found that MAPK cascades participate in the regulation of the radical burst. Here, we discuss the regulatory mechanisms of the oxidative and NO bursts in response to pathogen attacks, and crosstalk between MAPK signaling and the radical burst.
Key words: oxidative burst, MAPK, NADPH oxidase, NO burst, plant immunity
Biological Function of ROS and NO
Rapid production of reactive oxygen species (ROS) and nitric oxide (NO), called the oxidative burst and the NO burst, respectively, plays a role in development and diverse physiological processes, such as resistance to biotic or abiotic stress, and hormonal signaling. In plants, apoplastic generation of superoxide (O2−), or its dismutation product hydrogen peroxide (H2O2), has been documented following recognition of pathogens.1 Plasma membrane NADPH oxidase has been implicated in apoplastic ROS production following successful pathogen recognition. The NADPH oxidase was initially described in mammalian neutrophils as a multicomponent complex mediating microbial killing.2 gp91phox is the enzymatic subunit of this oxidase and transfers electrons to molecular oxygen to generate O2−. Plant NADPH oxidases designated as Respiratory Burst Oxidase Homolog (RBOH) have been identified as genes related to mammalian gp91phox and carry an N-terminal extension comprising two EF-hand motifs, suggesting that Ca2+ regulates their activity.2
Recently, NO has also attracted attention as the radical that participates in innate immunity in plants. NO induces activation of the mitogen-activated protein kinase (MAPK),3 and the expression of defense genes, such as Phenylalanine ammonia-lyase and pathogenesis-related proteins.4 In animals, NO is produced by NO synthase (NOS). The sources of NO synthesis in plants include reduction in nitrite by nitrate reductase (NR), oxidation of arginine to citrulline by NOS, and a nonenzymatic NO generation system.5 Although no plant NOS gene has yet been identified, many studies have demonstrated that NO ASSOCIATED1 (NOA1; formerly NOS1) participates in NOS activity and pathogen-triggered NO burst.6–9 Pharmacological and genetic approaches show that NR also plays key roles in NO burst during interaction between pathogens and plants.9–11
ROS and NO play important roles independently or coordinately in plant innate immunity. ROS generated on the plasma membrane can directly cause strengthening of the cell walls via cross-linking of glycoproteins against secondary infection12 and simultaneously activating the Ca2+ channel to increase the level of cytosolic Ca2+.13 Ca2+ may function not only as an inducer of the oxidative burst but also as a signaling molecule downstream of the oxidative burst that causes various cellular responses, including defense. However, NO signaling includes various messenger molecules, such as cGMP, cADP ribose and Ca2+,4 which both directly and indirectly modulate the expression of specific genes.14 NO signaling pathways often include posttranslational modification of target proteins, such as NO-dependent cysteine S-nitrosylation that can modulate the activity and function of different proteins.15–17 NO can also react with O2− to form the reactive molecule peroxynitrite (ONOO−). ONOO− is responsible for tyrosine nitration,18 which is the major toxic reactive nitrogen species in animal cells.19 In plants, NO and O2− are produced simultaneously through a convergent signaling MAPK cascades.9
Crosstalk between MAPK Signaling and the Radical Burst
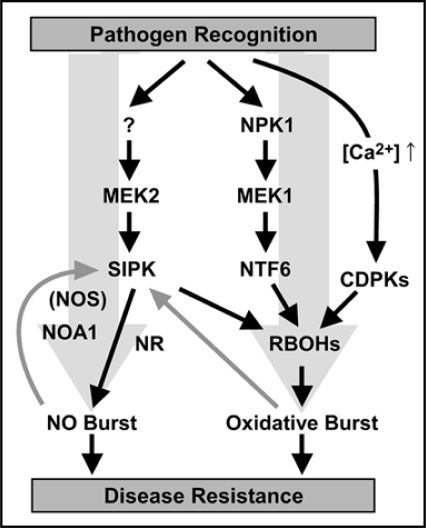
MAPK cascades are major signal transduction modules in eukaryotes and consist of at least three protein kinases, which mediate sequential phosphorylation reactions, as follows: a MAPK kinase kinase (MAPKKK) phosphorylates and activates a MAPK kinase (MAPKK), which, in turn, activates a MAPK by phosphorylation. Many studies have demonstrated that two MAPKs, tobacco salicylic acid-induced protein kinase (SIPK) and wound-induced protein kinase (WIPK), and their orthologs in other plant species play pivotal roles not only in disease responses to several pathogens but also in development and diverse physiological processes.20,21 MAPK cascades and calcium dependent protein kinases (CDPKs) seem to play central roles in the regulation of pathogen-responsive RBOHs, which play a pivotal role in oxidative burst, at the transcriptional and post-translational levels, respectively.9,22,23 MAPK cascades MEK2-SIPK and cytokinesis-related NPK1-MEK1-NTF6 regulate the oxidative burst accompanied by the induction of NbRBOHB expression during defense responses in Nicotiana benthamiana.9 CDPKs activate potato RBOHs by direct phosphorylation of the N-terminal regions.23 In addition, ROS participate in the activation of MAPK. The expression and activation of the Arabidopsis serine/threonine kinase OXIDATIVE SIGNAL-INDUCIBLE1 (OXI1) are induced in response to H2O2.24 OXI1 is required for activation of MAPKs (MPK3 and MPK6, orthologs of WIPK and SIPK, respectively, in Arabidopsis) after treatment with H2O2 or an elicitor. Together, we can assume the existence of a positive feedback circuit between SIPK and RBOHs (Fig. 1).
Figure 1.
Proposed radical burst signaling pathways via MAPK cascades. After pathogen recognition, plants immediately provoke Ca2+ influxes into cytoplasm, activation of MAPK SIPK and NTF6, and oxidative and NO bursts. SIPK and NTF6 activate the RBOHs expression (regulation at the transcriptional level). The increase in Ca2+ concentration in the cytoplasm results in the activation of CDPKs that induce the oxidative burst by direct phosphorylation of RBOHs (regulation at the post-translational level). SIPK also regulates NO burst via unidentified NOS, NOA1 and NR. ROS and NO activate SIPK. Gray arrows indicate positive feedback regulation. The question mark indicates unidentified MAPKKK(s).
NO has also been shown to modulate the activity of MAPK.3 NO donors and recombinant NOS were shown to cause the activation of SIPK.25 Recently, we showed that MAPK cascade MEK2-SIPK regulates NOA1- and NR-mediated NO burst on the basis of pharmacological and genetic analyses.9 Thus, SIPK may give a positive feedback between NO signals as well as ROS signals (Fig. 1).
Involvement of Radical Burst in Defense Responses
Plants have evolved a variety of defense mechanisms to protect themselves against microbial colonization. Plants rely on basal defense by using a much less specific recognition system that identifies pathogen-associated molecular patterns (PAMPs) to prevent the penetration and restrict the growth of pathogens.26 In response to pathogens that avoid, tolerate or suppress the basal defense, plants have evolved resistance proteins, which trigger a battery of inducible defense responses as typified by hypersensitive response (HR) upon pathogen recognition.26 Cell death during the HR is thought to deny nutrients to invading biotrophic pathogens, which can parasitize living plant cells, and be dependent on the balanced production of ROS and NO.27
Genetic proofs for RBOH function in the pathogen-induced oxidative burst were provided by knocking out or down of RBOHs.22,28 Downregulation of RBOH leads to the reduction of disease resistance accompanied by elimination of extracellular ROS formation. For example, a double mutant of the Arabidopsis rbohD and rbohF genes displays reduced HR in response to avirulent bacteria.28 Similarly, NbRBOH-silenced N. benthamiana plants are high susceptible to oomycete Phytophthora infestans, and show less HR phenotype.22 Thus, RBOH proteins are required for ROS production following successful pathogen recognition, and these ROS may serve diverse signaling functions in disease resistance and HR.
The first direct link between NO and disease resistance was provided by the finding that infiltration of the mammalian NOS inhibitors supported increased growth of the incompatible bacterial pathogen.29 The Arabidopsis noa1 knockout mutant shows reduction of NO production and basal defense in response to lipopolysaccharide, a PAMP, and an increase in the susceptibility to virulent bacterial pathogen.8 Similarly, NbNOA1-silenced N. benthamiana plants show high susceptibility to hemi-biotrophic pathogen Colletotrichum orbiculare (syn. C. lagenarium), which is the causal agent of cucumber anthracnose disease.30 Collectively, these results demonstrated that NO has an important signaling function in disease resistance.
Interestingly, recent reports indicate that the effects of the radical burst on the defense response appear to be diverse in plant-pathogen interactions. In N. benthamiana, NbRBOHB silencing shows a strong effect on resistance to P. infestans, but not to C. orbiculare, whereas NbNOA1 silencing induces high susceptibility to C. orbiculare, but not to P. infestans.9 Although ROS usually correlate with successful disease resistance responses, some pathogens may induce production of ROS to their own advantage. For example, necrotrophs appear to stimulate ROS production in the infected tissue to induce cell death that facilitates subsequent infection.31 Interference with the chlorophyll degradation pathway also results in overaccumulation of ROS and an increase in susceptibility to some necrotrophic pathogens.32 Transgenic potato plants expressing a constitutively active form of StMEK2 (StMEK2DD) fused to a pathogen-inducible promoter are resistant to both the biotrophic pathogen P. infestans and the necrotrophic pathogen Alternaria solani.33 However, our preliminary data indicate that transgenic potato plants carrying a constitutively active form of StCDPK5 (StCDPK5VK), which activates StRBOHs, driven by the same pathogen-inducible promoter show high resistance to P. infestans but high susceptibility to A. solani. StMEK2DD induces both ROS and NO production,9 whereas StCDPK5VK induces only ROS production.23 Thus, ROS are produced as part of a complex network of defense signals that respond to pathogen attack, with opposite effects in response to necrotrophic pathogens. However, little is known about the effects of NO on the disease resistance to necrotrophic pathogens, and further investigation is needed to reveal the relationship.
Conclusion Remarks
Many studies have revealed that the radical burst play a pivotal role in the plant immunity. By contrast, ROS and NO seem to diversely function, sometimes with opposite effects. A convergent signaling pathway, that is, MAPK cascades simultaneously regulate ROS and NO production in plants. Thus, plants may have obtained during evolution the signaling pathway regulating both ROS and NO production to adapt to a wide spectrum of pathogens.
Acknowledgements
This work was supported by the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN) and by a Grant-in-Aid for Scientific Research (A) from the Ministry of Education, Science and Culture of Japan.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/6601
References
- 1.Yoshioka H, Bouteau F, Kawano T. Discovery of oxidative burst in the field of plant immunity: Looking back at the early pioneering works and towards the future development. Plant Signal Behavior. 2008;3:153–155. doi: 10.4161/psb.3.3.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008;275:3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- 3.Clarke A, Desikan R, Hurst RD, Hancock JT, Neill SJ. NO way back: Nitric oxide and programmed cell death in Arabidopsis thaliana suspension cultures. Plant J. 2000;24:667–677. doi: 10.1046/j.1365-313x.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- 4.Durner J, Wendehenne D, Klessig DF. Defense gene induction in tobacco by nitric oxide, cyclic GMP and cyclic ADP-ribose. Proc Natl Acad Sci USA. 1998;95:10328–10333. doi: 10.1073/pnas.95.17.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bethke PC, Badger MR, Jones RL. Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell. 2004;16:332–341. doi: 10.1105/tpc.017822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo FQ, Okamoto M, Crawford NM. Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science. 2003;302:100–103. doi: 10.1126/science.1086770. [DOI] [PubMed] [Google Scholar]
- 7.Lombardo MC, Graziano M, Polacco JC, Lamattina L. Nitric oxide functions as a positive regulator of root hair development. Plant Signal Behavior. 2006;1:28–33. doi: 10.4161/psb.1.1.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeidler D, Zahringer U, Gerber I, Dubery I, Hartung T, Bors W, Hutzler P, Durner J. Innate immunity in Arabidopsis thaliana: Lipopolysaccharides activate nitric oxide synthase (NOS) and induce defense genes. Proc Natl Acad Sci USA. 2004;101:15811–15816. doi: 10.1073/pnas.0404536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asai S, Ohta K, Yoshioka H. MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana. Plant Cell. 2008;20:1390–1406. doi: 10.1105/tpc.107.055855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Modolo LV, Augusto O, Almeida IMG, Pinto-Maglio CAF, Oliveira HC, Seligman K, Salgado I. Decreased arginine and nitrite levels in nitrate reductase-deficient Arabidopsis thaliana plants impair nitric oxide synthesis and the hypersensitive response to Pseudomonas syringae. Plant Sci. 2006;171:34–40. [Google Scholar]
- 11.Yamamoto-Katou A, Katou S, Yoshioka H, Doke N, Kawakita K. Nitrate reductase is responsible for elicitin-induced nitric oxide production in Nicotiana benthamiana. Plant Cell Physiol. 2006;47:726–735. doi: 10.1093/pcp/pcj044. [DOI] [PubMed] [Google Scholar]
- 12.Bradley D, Kjellbom P, Lamb C. Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell. 1992;70:21–30. doi: 10.1016/0092-8674(92)90530-p. [DOI] [PubMed] [Google Scholar]
- 13.Lecourieux D, Mazars C, Pauly N, Ranjeva R, Pugin A. Analysis and effects of cytosolic free calcium increases in response to elicitors in Nicotiana plumbaginifolia cells. Plant Cell. 2002;14:2627–2641. doi: 10.1105/tpc.005579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grun S, Lindermayr C, Durner J. Nitric oxide and gene regulation in plants. J Exp Bot. 2006;57:507–516. doi: 10.1093/jxb/erj053. [DOI] [PubMed] [Google Scholar]
- 15.Feechan A, Kwon E, Yun BW, Wang Y, Pallas JA, Loake GJ. A central role for S-nitrosothiols in plant disease resistance. Proc Natl Acad Sci USA. 2005;22:8054–8059. doi: 10.1073/pnas.0501456102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindermayr C, Saalbach G, Durner J. Proteomic identification of S-nitrosylated proteins in Arabidopsis. Plant Physiol. 2005;137:921–930. doi: 10.1104/pp.104.058719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romero-Puertas MC, Campostrini N, Mattí A, Righetti PG, Perazzolli M, Zolla L, Roepstorff P, Delledonne M. Proteomic analysis of S-nitrosylated proteins in Arabidopsis thaliana undergoing hypersensitive response. Proteomics. 2008;8:1459–1469. doi: 10.1002/pmic.200700536. [DOI] [PubMed] [Google Scholar]
- 18.Lamattina L, Garcia-Mata C, Graziano M, Pagnussat G. Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol. 2003;54:109–136. doi: 10.1146/annurev.arplant.54.031902.134752. [DOI] [PubMed] [Google Scholar]
- 19.Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell. 2007;19:63–73. doi: 10.1105/tpc.106.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Liu Y, Bruffett K, Lee J, Hause G, Walker JC, Zhang S. Haplo-insufficiency of MPK3 in MPK6 mutant background uncovers a novel function of these two MAPKs in Arabidopsis ovule development. Plant Cell. 2008;20:602–613. doi: 10.1105/tpc.108.058032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshioka H, Numata N, Nakajima K, Katou S, Kawakita K, Rowland O, Jones JDG, Doke N. Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell. 2003;15:706–718. doi: 10.1105/tpc.008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi M, Ohura I, Kawakita K, Yokota N, Fujiwara M, Shimamoto K, Doke N, Yoshioka H. Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell. 2007;19:1065–1080. doi: 10.1105/tpc.106.048884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rentel MC, Lecourieux D, Ouaked F, Usher S, Petersen L, Okamoto H, Knight H, Peck SC, Grierson CS, Hirt H, Knight MR. OXI1 kinase is necessary for oxidative burstmediated signalling in Arabidopsis. Nature. 2004;427:858–861. doi: 10.1038/nature02353. [DOI] [PubMed] [Google Scholar]
- 25.Kumar D, Klessig DF. Differential induction of tobacco MAP kinases by the defense signals nitric oxide, salicylic acid, ethylene and jasmonic acid. Mol Plant Microbe Interact. 2000;13:347–351. doi: 10.1094/MPMI.2000.13.3.347. [DOI] [PubMed] [Google Scholar]
- 26.Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 27.Delledonne M, Zeier J, Marocco A, Lamb C. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA. 2001;98:13454–13459. doi: 10.1073/pnas.231178298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torres MA, Dangl JL, Jones JDG. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA. 2002;99:517–522. doi: 10.1073/pnas.012452499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delledonne M, Xia Y, Dixon RA, Lamb C. Nitric oxide functions as a signal in plant disease resistance. Nature. 1998;394:585–588. doi: 10.1038/29087. [DOI] [PubMed] [Google Scholar]
- 30.Kato H, Asai S, Yamamoto-Katou A, Yoshioka H, Doke N, Kawakita K. Involvement of NbNOA1 in NO production and defense responses in INF1-treated Nicotiana benthamiana. J Gen Plant Pathol. 2008;74:15–23. [Google Scholar]
- 31.Govrin E, Levine A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol. 2000;10:751–757. doi: 10.1016/s0960-9822(00)00560-1. [DOI] [PubMed] [Google Scholar]
- 32.Kariola T, Brader G, Li J, Palva ET. Chlorophyllase 1, a damage control enzyme, affects the balance between defense pathways in plants. Plant Cell. 2005;17:282–294. doi: 10.1105/tpc.104.025817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamizo C, Kuchimura K, Kobayashi A, Katou S, Kawakita K, Jones JDG, Doke N, Yoshioka H. Rewiring mitogen-activated protein kinase cascade by positive feedback confers potato blight resistance. Plant Physiol. 2006;140:681–692. doi: 10.1104/pp.105.074906. [DOI] [PMC free article] [PubMed] [Google Scholar]