Abstract
Reactive oxygen species (ROS) are continuously produced in several organelles during aerobic metabolism. Furthermore, a wide range of environmental stresses such as chilling, salinity, drought and high light, lead to an elevated production of ROS. ROS can react with biomolecules and cause oxidative damage and even necrosis. Antioxidants and antioxidant-enzymes function to interrupt the cascades of uncontrolled oxidation. On the other hand, ROS influence the expression of genes playing a central role in many signaling pathways. Tools like the exogenous application of oxidative stress-causing agents and the in planta production of ROS in mutants altered in ROS metabolism are increasingly used to assess specific and common responses toward different types of ROS signals. The major challenge is the identification of ROS sensors and signaling components to finally elucidate the molecular mechanisms of oxidative stress response in plants.
Key words: Arabidopsis thaliana, oxidative damage, reactive oxygen species, signaling
Introduction
Reactive oxygen species (ROS) like singlet oxygen, the superoxide anion radical, the hydroxyl radical, and hydrogen peroxide (1O2, O2-., OH· and H2O2) are generated in chloroplasts, mitochondria and peroxisomes as inevitable by-products of aerobic metabolism. Plant cells evolved non-enzymatic and enzymatic mechanisms to tightly control their basal levels and thus limit their action (Fig. 1). Production of ROS is dramatically enhanced in response to high light, cold, drought, wounding or infection. ROS can cause oxidative damage1 but they can also act as signaling molecules that influence expression of nuclear-encoded genes which initiate tissue necrosis or acclimation processes.2–7 Recent studies indicated that the type of ROS or its subcellular production site is critical to provoke specific and selective cellular signals and responses.8,9 This review aims to summarizing available experimental systems used to assess the effects of ROS in plant tissues, with special focus on non-invasive model systems. For a more detailed description of the metabolism of ROS and their participation in oxidative stress and signal transduction, readers are referred to more comprehensive recent reviews.10,11
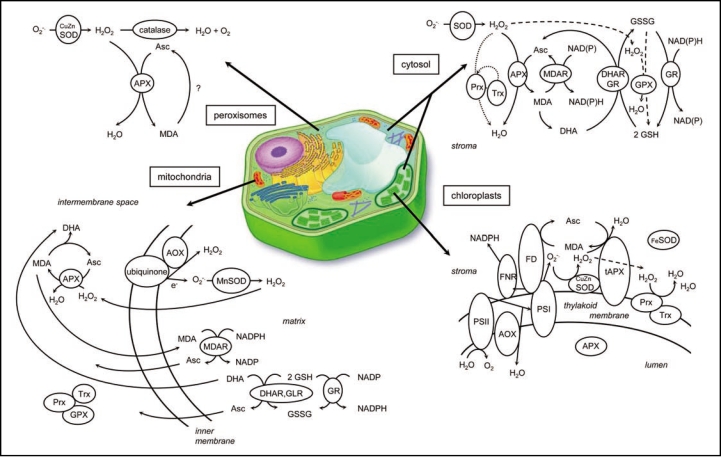
Figure 1.
Cellular location of ROS scavenging pathways in plant cells. The GPX and Prx pathways are indicated in dashed and dotted lines in the cytosol and stroma. Asc, ascorbate; AOX, alternative oxidase; APX: ascorbate peroxidase; CuZnSOD, copper-zinc superoxide dismutase; DHA, dehydroascrobate; DHAR, DHA reductase; FD, ferredoxin; FeSOD; iron superoxide dismutase; FNR, ferredoxin NADPH reductase; GLR, glutaredoxin; GPX, glutathione peroxidase; GR, glutathione reductase; GSH, reduced glutathione; GSSG, oxidized glutathione; MDA, monodehydroascorbate; Prx, Peroxiredoxin; MDAR, MDA reductase; PSI, photosystem I; PSII, photosystem II; tAPX: thylakoid APX; Trx, thioredoxin; Adapted from Mittler et al., 2004.
Invasive Systems: Exogenous Application of Oxidative Stress-Causing Agents
Methylviologen (MV).
The redox-cycling herbicide MV (paraquat) undergoes univalent reduction and transfers electrons to oxygen generating O2-..12 During illumination, photosystem I donates electrons to MV ensuring the steady formation of O2-. in the chloroplasts, thereby inhibiting photosynthesis.13 Arabidopsis leaves floated in the light in 50 µM MV did not accumulate H2O2 and showed unaltered antioxidant machinery by 2 h of treatment. Biochemical and expression profiling analysis of these leaves allowed the identification of O2-. early-response genes, e.g. upregulation of specific members of transcription factor families and heat shock protein genes.14 A common GAAAAGTCAAAC motif containing the W-box consensus sequence of WRKY transcription factors was identified in the promoter of genes highly upregulated by O2-..14 Moreover, the transcription factors WRKY30 and WRKY48 were shown to respond to intracellularly generated ROS.14–16 A short and severe stress imposed by O2-. on Arabidopsis plants indicated a rearrangement of carbon metabolism that reflects a short-term mechanism of survival.17 Many photosynthetic genes and those encoding enzymes of starch and sucrose biosynthetic pathways were downregulated, while those encoding proteins belonging to the mitochondrial electron transport and enzymes of carbohydrate catabolic pathways were induced. A long-term response to MV application was also observed at the transcriptional level.9
Exogenously applied MV requires some time to penetrate the leaves and thus the formation of O2-. is delayed and a retarded response is provoked in comparison to the flu mutant, defective in the regulation of chlorophyll biosynthesis9 (see below).
Alternaria alternata toxin (AAL).
The AAL toxin induces programmed cell death (PCD) through interference with the sphingolipid metabolism.18 Arabidopsis plants infiltrated with nanomolar concentrations of AAL showed an accumulation of H2O2 and induced PCD in the light with features resembling the hypersensitive response.19 Transcription profiling indicated a very early upregulation of transcription factors, proteases and a number of ethylene and ROS-inducible genes potentially involved in transcriptional reprogramming observed at later stages and in the regulation of PCD processes.9,19
3-Aminotriazole (AT).
The herbicide AT is a catalase inhibitor that causes endogenous accumulation of hydrogen peroxide and is thus used as a system for studying H2O2-induced PCD.20 Microarray analysis of Arabidopsis leaves treated with AT revealed a set of new H2O2-responsive genes that were highly regulated in a common fashion during different types of PCD.20,9 These included an oxoglutarate-dependent dioxygenase and various oxidoreductases, the transcription factors Zat11, WRKY75 and NAM, proteosomal components, a heterologous group of genes with diverse functions, and genes encoding proteins with unknown functions. Physiological analysis of knockout lines of the oxoglutarate-dependent dioxygenase confirmed its role in the cell death network.20
Ozone (O3).
O3 is an atmospheric pollutant that is converted in the apoplast into O2-. and H2O2. When plants are exposed to O3, a hypersensitive-like oxidative burst is triggered leading to necrotic lesions.21 It has recently been shown that specific transcription factors, like WRKY70, ERF/AP2, NAM, GT-3a and ERF15 were upregulated in response to exogenously applied O3.9
Harpin.
Harpin is proteinaceous bacterial elicitor secreted by plant pathogens.22 Treatment of Arabidopsis suspension cells with harpin was used as a system to induce ROS formation.23 Harpin was found to initiate two signaling pathways, one leading to an increased generation of H2O2 and the expression of phenalalanine-ammonia lyase and glutathione S-transferase and the other leading to increased glutathione S-transferase and anthranilate synthase expression, independent of H2O2.
H2O2.
Exogenously applied H2O2 confirmed its role as a cell death trigger and showed that high H2O2 concentrations can cause necrosis instead of PCD.23,24 The application of H2O2 to Arabidopsis plants and cultured Arabidopsis cells allowed comprehensive analyses of transcriptional gene networks.25–27 The treatment of Arabidopsis seedlings with H2O2 showed that many non-redundant expressed sequence tags are regulated by H2O2, most of them related to cell rescue, signaling and defense processes, indicating that they have multiple roles in plant responses to stress.26 The treatment of Arabidopsis cells with H2O2 was also used as a model system to investigate the impact of oxidative stress on mitochondrial function.28 This treatment lowered the O2 consumption by mitochondria and the abundance of several key mitochondrial proteins (e.g., aconitase, the E2 subunit of the 2-oxoglutarate dehydrogenase, fumarase, succinyl CoA ligase, subunits of the complex I and the ATP synthase complex). Using 2D-gel electrophoresis coupled to MS/MS, a mitochondrial protease was identified, which is responsible for the degradation of oxidatively damaged proteins.28
Menadione.
Arabidopsis cells incubated with sub-lethal doses of this redox active quinone that generates intracellular O2-. showed a decreased cell growth rate with unaffected cell viability.28 The effect of oxidative stress on mitochondrial function was found to be similar to that caused by the application of H2O2. A peroxiredoxin as part of the thioredoxin system was identified by which mitochondria can detoxify H2O2. A second protein induced by menadione was a mitochondrial disulfide isomerase, which may participate in the redox regulation of the alternative oxidase (AOX). Other mitochondrial proteins identified include a calreticulin, a glyceraldehydehyde-3-phosphate dehydrogenase and a glutathione S-transferase. Moreover, menadione resulted in the degradation of sensitive protein components as also described for H2O2.28
Recently, metabolic changes caused by oxidative stresses were first described.29 Menadione-treated Arabidopsis cells indicated that oxidative stress had profound effects on central metabolic pathways, principally causing an inhibition of the tricarboxylic acid cycle and amino acid metabolism. This was accompanied by a subsequent backing up of glycolysis and an activation of the oxidative pentose phosphate pathway. Microarray analysis showed a switch from anabolic to catabolic metabolism indicative for a metabolic reconfiguration to bypass damaged steps.29
It is worth noting, that this treatment and the external H2O2 application are both non-specific oxidative stresses that act throughout the cell and trigger general responses.
Non-Invasive Model Systems: Use of Mutants Altered in ROS Metabolism
The specific effects of metabolically generated ROS within a particular subcellular compartment can be assessed using transgenic Arabidopsis with altered levels of specific ROS-scavenging enzymes such as the chloroplastic copper zinc-superoxide dismutase (CuZnSOD), cytosolic and thylakoid ascorbate oxidases (APX), peroxisomal catalase (CAT) and the mitochondrial AOX (Fig. 1).16,30–35 Some of these plants were used as model systems to investigate transcriptomic variations triggered by a particular type of ROS.
In the following, different strategies are summarized which were used to modulate ROS levels in planta and to assess corresponding responses.
Thylakoid CuZnSOD.
Studies using knock-down Arabidopsis plants with a reduced expression of the thylakoid-attached CuZnSOD due to a T-DNA insertion in the promoter provided evidence for the importance of the water-water cycle in protecting the photosynthetic apparatus from photooxidative damage.31 Plants grown at moderate light intensities were impaired in growth and showed reduced chloroplast size, chlorophyll content and photosynthetic activity, a phenotype that could be reverted by growth at low light intensities. Microarray analysis of plants grown under controlled conditions revealed changes in transcript levels consistent with an acclimation response to light and indicated that anthocyanin biosynthetic transcripts were up-regulated in these plants.9,31
Cytosolic APX.
Cytosolic apx1 T-DNA mutants showed an accumulation of H2O2, suppression of growth, lower rates of photosynthesis but no alteration of transcripts encoding H2O2-scavenging enzymes under optimal conditions of growth.30 On the contrary, during light stress, the expression of catalase, peroxidases and several transcripts encoding signal transduction proteins (especially heat shock proteins) was elevated pointing at novel components involved in H2O2-sensing. Moreover, the oxidation and degradation of chloroplastic proteins in these plants suggested that the cytosolic APX might be essential for the protection of chloroplasts during light stress.30–34
Thylakoid APX.
Arabidopsis lines overexpressing the thylakoid APX (tAPX) were phenotypically indistinguishable from the wild-type under normal growth conditions. Seedlings did not show an enhanced protection against photoinhibition caused by high light or chilling but an increased resistance to MV-induced photoxidative stress.33 Moreover, after treatment with sodium nitroprusside (SNP, leading to the formation of nitric oxide), these plants accumulated lower levels of H2O2 and were more resistant to nitric oxide-induced cell death.33 On the other hand, tobacco lines overexpressing tAPX showed increased tolerance to chilling and MV-treatment suggesting that tAPX is a limiting factor of antioxidative systems under photooxidative stress in chloroplasts.36 These results suggested that Arabidopsis and tobacco tAPXs might have non-overlapping roles in plant defense mechanisms. Indeed, while tobacco plants with reduced tAPX did not grow to maturity,36 antisense Arabidopsis plants with a reduced level of tAPX expression showed no obvious phenotype under normal conditions but showed enhanced damage upon treatment with SNP.37 The extent of cell death induced by SNP is inversely correlated with APX-scavenging of H2O2. Taken together, these results highlighted the role of Arabidopsis tAPX in the fine modulation of H2O2 levels for signaling.
Catalase.
Antisense tobacco plants with reduced levels of CAT1 (the most abundant isoform in leaves) were used to study several aspects of H2O2 signaling from locally and systemically acquired resistance to the induction of an active cell death program.38,39 These plants exhibited no visible phenotype when grown at low light conditions but showed cell death in clusters of perivenial palisade cells after exposition to high light.38,6 This irreversible cell death process was shown to involve an oxidative burst triggered by the accumulation of photorespiratory H2O2.6 Transcriptional changes monitored during exposition to high light indicated a rapid upregulation of signal transduction components involved in stress resistance and genes associated with cell death, and a downregulation of nuclear-encoded photosynthetic genes.40 Moreover, pretreatment of these plants with high light induced resistance to pathogen infection (Chamnongpol 1996) and cell death caused by an additional exposure to high light,38 clearly indicating the induction of a defense response at lower H2O2 concentrations.
On the other hand, antisense Arabidopsis plants with decreased levels of CAT2 activity (the most prominent leaf isoform, At1g20630) displayed an increased sensitivity towards ozone and photorespiratory H2O2-induced cell-death.32 These plants allowed to assess H2O2-dependent and -independent high light-triggered transcriptional responses during a sustained H2O2-stress over time.16,32 Of major importance was the identification of small heat shock proteins, transcription factors and regulatory genes involved in H2O2 transcriptional gene networks. Moreover, the accumulation of H2O2 was shown to negatively impinge on high light-stress induction of transcripts within the anthocyanin biosynthetic pathway, which correlated with a lack of anthocyanin accumulation in cat2 mutant plants during long-term high light exposure.
AOX.
The mitochondrial AOX transfers electrons from the ubiquinone pool directly to oxygen without energy conservation and may function to prevent the formation of ROS by burning off an excess of energy in form of heat.41,42 AOX antisense cultured tobacco cells produced more ROS than wild-type cells, while AOX-overexpressing cells produced less ROS.43 These studies were extended to Arabidopsis plants overexpressing sense and antisense AOX constructs under the control of the CaMV 35S promoter.35 In intact leaves and roots of antisense AOX lines, enhanced levels of ROS and oxidative damage were observed when the cytochrome pathway was chemically inhibited, whereas an increased AOX activity was sufficient to prevent ROS-related oxidative damage.35 A transcriptome analysis under normal growth conditions provided only limited evidence for oxidative stress being chronically sustained in antisense AOX plants and, if at all, the stress appeared to be restricted to chloroplasts. On the other hand, when grown at low temperature, antisense AOX plants showed a reduced leaf area and smaller rosettes, while AOX overexpressors presented larger leaf areas and rosette sizes.44 These phenotypic differences were not the result of major alterations in the tissue redox state because changes in levels of lipid peroxidation products and the expression of genes encoding antioxidant and electron transfer chain redox enzymes did not correspond with the shoot phenotypes.44 These results demonstrate that AOX plays a role in shoot acclimation to low temperature in Arabidopsis and functions to prevent excess ROS formation in whole tissues in response to environmental stress conditions.
The FLU protein.
FLU is a nuclear-encoded chloroplast protein that plays a key role during the negative feedback control of chlorophyll biosynthesis.45 The inactivation of this protein in the conditional fluorescent Arabidopsis flu mutant leads to an accumulation of protochlorophyllide in the dark. Upon irradiation, protochlorophyllide transfers light energy to ground-state O2 and thus, the flu mutant represents an experimental tool to induce the release of 1O2 within the plastids after a dark-to-light shift. After reillumination, flu mutant plants stopped growing and developed necrotic lesions. In this condition, early stress response genes were activated that were different from those induced by O2-. and H2O2 generated, for example, by treatment with MV.4,9 Specifically induced transcripts in the flu mutant are ethylene-responsive elements-binding proteins, indicative for ethylene signaling.9 Recently, by varying the length of the dark period, conditions were established that either endorse the cytotoxicity of 1O2 and allow non-enzymatic lipid peroxidation or support its signaling role involving oxylipin synthesis.46,47
GDP-mannose pyrophosphorylase.
L-ascorbate is a powerful reducing agent found in millimolar concentrations in plant tissues and playing an important role in scavenging of free radicals. The Arabidopsis mutant vtc1 exhibits reduced levels of ascorbate due to a mutation in the GDP-mannose pyrophosphorylase, involved in the L-ascorbate biosynthetic pathway.48,49 These plants were used to assess the role of ascorbate as antioxidant and to elucidate its role in signal transduction pathways. ROS detoxification is impaired in vtc1 exposed to ozone, sulfur dioxide and ultraviolet B irradiation, indicating that ascorbate is implicated in the defense against these environmental stresses.48 Infection with virulent bacteria (Pseudomonas syringae or Peronospora parasitica) indicated that vtc1 is more resistant to these pathogens and that pathogenesis-related genes are induced in these plants.50,51 Transcript analyses also indicated that growth and development are constrained in vtc1 by a constitutive upregulation of ABA-associated pathways.50,52 In addition, the vtc1 mutant exhibits elevated levels of some senescence-associated gene transcripts as well as enhanced salicylic acid levels.51 These results suggested that low ascorbate is causing vtc1 to enter prematurely at least some stages of senescence.
Tocopherol cyclase and homogentisate phytyl transferase.
Vitamine E is the collective term for tocopherols and tocotrienols, which have the ability to terminate chain reactions of polyunsaturated fatty acids free radicals generated by lipid oxidation. The Arabidopsis mutants vte1 and vte2, deficient in tocopherol cyclase and homogentisate phytyl transferase, respectively, showed a complete loss of tocopherols.53,54 These mutants were used to demonstrate that the antioxidant function of tocopherols in vivo are masked by compensatory protective mechanisms. By crossing the vte1 mutant with the zeaxanthin-deficient npq1 or the PsbS-deficient npq4 mutants55,56 it was shown that tocopherols fulfill two different functions in chloroplasts at the two major sites of 1O2 production, protection of photosystem II against photoinactivation as well as of thylakoids membranes against lipid peroxidation.54 The vte-npq mutants showed high levels of 1O2-mediated lipid peroxidation and was recently used as a genetic model system to demonstrate that 1O2 plays a major destructive role in photo-oxidative damage.47
Glycolate oxidase.
To assess the effects of metabolically generated H2O2 in chloroplasts, transgenic Arabidopsis plants were generated in which the peroxisomal GO was targeted to chloroplasts.15 GO overexpressing plants (GO plants) accumulated H2O2, showed retarded development, yellowish rosettes and impaired photosynthetic performance at moderate light intensities, while this phenotype disappeared at low light. A response to oxidative stress was installed in GO plants, with increased expression of the small heat shock protein (Hsp17.6B-CI or sHSP), the transcription factor WRKY30, the chloroplast-localized ferritin and enhanced foliar APX and CuZnSOD activities. GO plants developed oxidative stress lesions and showed repression of anthocyanin biosynthetic genes under photorespiratory conditions. These plants are unique in that H2O2 is generated inside the chloroplast even in the absence of biotic or abiotic stress or oxidants. Thus, GO plants represent a novel tool to study the action of H2O2 as a signaling molecule in chloroplasts as well as the consequences of oxidative stress from a holistic approach.15
Concluding Remarks
To date, it is not known to which extent the chemical specificity of the ROS species and the cellular compartment of their release may contribute the multiplicity of responses that occur in plants. Stress treatments and external application of oxidative stress-causing agents may activate additional signal transduction pathways that may complicate the analysis of ROS-signal transduction pathways. Some responses of plants to elevated in planta levels of ROS in the absence of abiotic or biotic stresses were investigated by using non-invasive model systems like mutants altered in the ROS scavenging machinery or the flu mutant. Moreover, plants expressing GO in the chloroplasts were recently proposed to represent an ideal system to study the effects of H2O2 directly in chloroplasts because H2O2 accumulation in this organelle can be induced by exposing the plants to different ambient conditions.
A common feature of plants that accumulate ROS is the inhibition of growth and flowering time.15,30,31 This response resembles stress tolerance strategies used by plants exposed to adverse environmental conditions,57 where plants may pass into a state of conservative metabolic activity, similar to hibernation in mammals. Until now, no direct link between ROS production and the inhibition of growth has been elucidated opening a challenging area for future studies.
Acknowledgements
Work in the author's laboratory was founded by grants of the Deutsche Forschungsgemeinschaft to V.G.M.
Abbreviations
- APX
ascorbate peroxidase
- CAT
catalase
- CuZnSOD
copper zinc superoxide dismutase
- H2O2
hydrogen peroxide, OH·, hydroxyl radical
- ROS
reactive oxygen species
- 1O2
singlet oxygen
- O2-.
superoxide anion
- PCD
programmed cell death
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/7036
References
- 1.Halliwell B. Reactive species and antioxidants: redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141:312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen J, Mullineaux PC. Systemic signaling and acclimatation in response to excess excitation energy in Arabidopsis. Science. 1999;284:654–657. doi: 10.1126/science.284.5414.654. [DOI] [PubMed] [Google Scholar]
- 3.Neill S, Desikan R, Hancock J. Hydrogen peroxide signalling. Curr Opin Plant Biol. 2002;5:388–395. doi: 10.1016/s1369-5266(02)00282-0. [DOI] [PubMed] [Google Scholar]
- 4.op dem Camp RG, Przybyla D, Ochsenbein C, Laloi C, Kim C, Danon A, Wagner D, Hideg E, Gobel C, Feussner I, Nater M, Apel K. Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell. 2003;15:2320–2332. doi: 10.1105/tpc.014662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dat J, Vandenabeele S, Vranová E, Van Montagu M, Inzé D, Van Breusegem F. Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci. 2000;57:779–795. doi: 10.1007/s000180050041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dat JF, Pellinen R, Beeckman T, Kangasjärvi J, Langebartels C, Inzé D, Van Breusegem F. Changes in hydrogen peroxide homeostasis trigger an active cell death process in tobacco. Plant J. 2003;33:621–632. doi: 10.1046/j.1365-313x.2003.01655.x. [DOI] [PubMed] [Google Scholar]
- 7.Laloi C, Apel K, Danon A. Reactive oxygen signalling: the latest news. Curr Opin Plant Biol. 2004;7:323–328. doi: 10.1016/j.pbi.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Mullineaux PM, Karpinski S, Baker NR. Spatial dependence of hydrogen peroxide-directed signalling in light-stressed plants. Plant Physiol. 2006;141:346–350. doi: 10.1104/pp.106.078162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gadjev I, Vanderauwera S, Gechev TS, Laloi C, Minkov IN, Shulaev V, Apel K, Inzé D, Mittler R, Van Breusegem F. Transcriptomic footprinting disclose specificity of reactive oxygen species signalling in Arabidopsis. Plant Physiol. 2006;141:436–445. doi: 10.1104/pp.106.078717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 11.Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their function. Plant Physiol. 2006;141:391–396. doi: 10.1104/pp.106.082040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehler AH. Studies on reactions of illuminated chloroplasts. I. Mechanism of reduction of oxygen and other Hill reagents. Arch Biochem. 1951;195; 33:65–77. doi: 10.1016/0003-9861(51)90082-3. [DOI] [PubMed] [Google Scholar]
- 13.Asada K, Takahashi M. Production and scavenging of active oxygen in photosynthesis. In: Kyle DJ, Osmond CB, Arntzen DJ, editors. Photoinhibition. Amsterdam: Elsevier/North Holland Biomedic Press; 1987. pp. 227–280. [Google Scholar]
- 14.Scarpeci TE, Zanor MI, Carrillo N, Mueller-Roeber B, Valle EM. Generation of superoxide anion in chloroplasts of Arabidopsis thaliana during active photosynthesis: a focus on rapidly induced genes. Plant Mol Biol. 2008;66:361–378. doi: 10.1007/s11103-007-9274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fahnenstich H, Scarpeci TE, Valle EM, Flügge U-I, Maurino VG. Generation of H2O2 in chloroplasts of Arabidopsis thaliana overexpressing glycolate oxidase as an inducible system to study oxidative stress. Plant Physiol. 2008 doi: 10.1104/pp.108.126789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanderauwera S, Zimmermann P, Rombauts S, Vandenabeele S, Langebartels C, Gruissem W, Inze D, Van Breusegem F. Genome-wide analysis of hydrogen peroxide-regulated gene expression in Arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiol. 2005;139:806–821. doi: 10.1104/pp.105.065896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scarpeci TE, Valle EM. Rearrangement of carbon metabolism in Arabidopsis thaliana subjected to oxidative stress condition: an emergency survival strategy. Plant Growth Regul. 2008;54:133–142. [Google Scholar]
- 18.Spassieva SD, Markham JE, Hille J. The plant disease resistance gene Asc-1 prevents disruption of sphingolipid metabolism during AAL toxin-induced programmed cell death. Plant J. 2002;32:561–572. doi: 10.1046/j.1365-313x.2002.01444.x. [DOI] [PubMed] [Google Scholar]
- 19.Gechev TS, Gadjev IZ, Hille J. An extensive microarray analysis of AAL-toxin-induced cell death in Arabidopsis thaliana brings new insights into the complexity of programmed cell death in plants. Cell Mol Life Sci. 2004;61:1185–1197. doi: 10.1007/s00018-004-4067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gechev TS, Minkow IN, Hille J. Hydrogen peroxide-induced cell death in Arabidopsis transcriptional and mutant analysis reveals a role of an oxoglutarate-dependent dioxygenase gene in the cell death process. IUBMB Life. 2005;5:181–188. doi: 10.1080/15216540500090793. [DOI] [PubMed] [Google Scholar]
- 21.Sandermann H., Jr Molecular ecology of plants. Trend Plant Sci. 2004;9:406–413. doi: 10.1016/j.tplants.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 22.He SH. Elicitation of plant hypersensitive response by bacteria. Plant Physiol. 1996;112:865–869. doi: 10.1104/pp.112.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desikan R, Reynolds A, Hancock T, Neill SJ. Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defence gene expression in Arabidopsis suspension cultures. Biochem J. 1998;330:115–120. doi: 10.1042/bj3300115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao N, Tada Y, Park P, Nakayashiki H, Tosa Y, Mayama S. Novel evidence for apoptotic cell response and differential signals in chromatin condensation and DNA cleavage in victorin-treated oats. Plant J. 2001;28:13–26. doi: 10.1046/j.1365-313x.2001.01109.x. [DOI] [PubMed] [Google Scholar]
- 25.Desikan R, Neill SJ, Hancock JT. Hydrogen peroxide-induced gene expression in Arabidopsis thaliana. Free Radic Biol Med. 2000;28:773–778. doi: 10.1016/s0891-5849(00)00157-x. [DOI] [PubMed] [Google Scholar]
- 26.Desikan R, Mackerness SA-H, Hancock JT, Neill SJ. Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol. 2001;127:159–172. doi: 10.1104/pp.127.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davletova S, Schlauch K, Coutu J, Mittler R. The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signalling in Arabidopsis. Plant Physiol. 2005;139:847–856. doi: 10.1104/pp.105.068254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sweetlove LJ, Heazlewood JL, Holtzapffel R, Day DA, Leaver CJ, Millar AH. The impact of oxidative stress on Arabidopsis mitochondria. Plant J. 2002;32:891–904. doi: 10.1046/j.1365-313x.2002.01474.x. [DOI] [PubMed] [Google Scholar]
- 29.Baxter CJ, Redestig H, Schauer N, Repsilber D, Patil KR, Nielsen J, Selbig J, Liu J, Fernie AR, Sweetlove LJ. The metabolic response of heterotrophic Arabidopsis cells to oxidative stress. Plant Physiol. 2007;143:312–325. doi: 10.1104/pp.106.090431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pnueli L, Liang H, Rozenberg M, Mittler R. Growth suppression, altered stomatal responses, and augmented induction of heat shock proteins in cytosolic ascorbate peroxidase (Apx1)-deficient Arabidopsis plants. Plant J. 2003;34:187–203. doi: 10.1046/j.1365-313x.2003.01715.x. [DOI] [PubMed] [Google Scholar]
- 31.Rizhsky L, Liang H, Mittler R. The water-water cycle is essential for chloroplast protection in the absence of stress. J Biol Chem. 2003;278:38921–38925. doi: 10.1074/jbc.M304987200. [DOI] [PubMed] [Google Scholar]
- 32.Vandenabeele S, Vanderauwera S, Vuylsteke M, Rombauts S, Langebartels C, Seidlitz HK, Zabeau M, Van Montagu M, Inzé D, Van Breusegem F. Catalase deficiency drastically affects gene expression induced by high light in Arabidopsis thaliana. Plant J. 2004;39:45–58. doi: 10.1111/j.1365-313X.2004.02105.x. [DOI] [PubMed] [Google Scholar]
- 33.Murgia I, Tarantino D, Vannini C, Bracale Marcelly, Carravieri S, Soave C. Arabidopsis thaliana plants overexpressing thylakoidal ascorbate peroxidase show increased resistance to paraquat-induced photooxidative stress and to nitric oxide-induced cell death. Plant J. 2004;38:940–953. doi: 10.1111/j.1365-313X.2004.02092.x. [DOI] [PubMed] [Google Scholar]
- 34.Davletova S, Rizhsky L, Liang H, Zhong S, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell. 2005;17:268–281. doi: 10.1105/tpc.104.026971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Umbach AL, Fiorani F, Siedow JN. Characterization of transformed Arabidopsis with altered alternative oxidase levels and analysis of effects on reactive oxygen species in tissue. Plant Physiol. 2005;139:1806–1820. doi: 10.1104/pp.105.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yabuta Y, Motoki T, Yoshimura K, Takeda T, Ishikawa T, Shigeoka S. Thylakoid membrane-bound ascorbate peroxidase is a limiting factor of antioxidative systems under photo-oxidative stress. Plant J. 2002;32:915–925. doi: 10.1046/j.1365-313x.2002.01476.x. [DOI] [PubMed] [Google Scholar]
- 37.Tarantino D, Vannini C, Bracale M, Campa M, Soave C, Murgia I. Antisense reduction of thylakoidal ascorbate peroxidase in Arabidopsis enhances Paraquat-induced photooxidative stress and nitric oxide-induced cell death. Planta. 2005;221:757–765. doi: 10.1007/s00425-005-1485-9. [DOI] [PubMed] [Google Scholar]
- 38.Chamnongpol S, Willekens H, Langebartels C, Montagu MV, Inzé D, Camp WV. Transgenic tobacco with a reduced catalase activity develops necrotic lesions and induces pathogenesis-related expression under high light. Plant J. 1996;10:491–503. [Google Scholar]
- 39.Willekens H, Inzé D, Van Montagu M, Van Camp W. Catalases in plants. Mol Breed. 1995;1:207–228. [Google Scholar]
- 40.Vandenabeele S, Van der Kelen K, Dat J, Gadjev I, Boonefaes T, Morsa S, Rottiers P, Slooten L, Van Montagu M, Zabeau M, Inzé D, Van Breusegem F. A comprehensive analysis of hydrogen peroxide-induced gene expression in tobacco. Proc Natl Acad Sci USA. 2003;100:16113–16118. doi: 10.1073/pnas.2136610100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 42.Møller IM. Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:561–591. doi: 10.1146/annurev.arplant.52.1.561. [DOI] [PubMed] [Google Scholar]
- 43.Maxwell DP, Wang Y, McIntosh L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA. 1999;96:8271–8276. doi: 10.1073/pnas.96.14.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fiorani F, Umbach AL, Siedow JN. The alternative oxidase of plant mitochondria is involved in the acclimation of shoot growth at low temperature: a study of Arabidopsis AOX1a transgenic plants. Plant Physiol. 2005;139:1795–1805. doi: 10.1104/pp.105.070789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meskauskiene R, Nater M, Gislings D, Kessler F, op den Camp, Apel K. FLU: A negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2001;98:12826–12831. doi: 10.1073/pnas.221252798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Przybyla D, Göbel C, Imboden A, Hamberg M, Feussner I, Apel K. Enzymatic, but not non-enzymatic, 1O2 -mediated peroxidation of polyunsaturated fatty acids forms part of the EXECUTER1-dependent stress response program in the flu mutant of Arabidopsis thaliana. Plant J. 2008;5:236–248. doi: 10.1111/j.1365-313X.2008.03409.x. [DOI] [PubMed] [Google Scholar]
- 47.Triantaphylidès C, Krischke M, Hoeberichts FA, Ksas B, Gresser G, Havaux M, Van Breusegem F, Mueller MJ. Singlet oxygen is the major reactive oxygen species involved in photo oxidative damage to plants. Plant Physiol. 2008 doi: 10.1104/pp.108.125690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Conklin PL, Williams EH, Last R. Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc Natl Acad Sci USA. 1996;93:9970–9974. doi: 10.1073/pnas.93.18.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Conklin PL, Norris SR, Wheeler GS, Williams EH, Smirnoff N, Last RT. Genetic evidence for the role of GDP-manose in plant ascorbic acid (vitamin C) biosynthesis. Proc Natl Acad Sci USA. 1999;96:4198–4203. doi: 10.1073/pnas.96.7.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pastori MG, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier P, Noctor G, Foyer CH. Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell. 2003;15:939–951. doi: 10.1105/tpc.010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barth C, Moeder W, Klessig D, Conklin P. The timing of senescence and response to pathogens is altered in the ascorbate-deficient Arabidopsis mutant vitamin c-1. Plant Phys. 2004;134:1784–1792. doi: 10.1104/pp.103.032185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veljovic-Jovanovic SD, Pignocchi C, Noctor G, Foyer CH. Low ascorbic acid in the vtc1 mutant of Arabidopsis is associated with decreased growth and intracellular redistribution of the antioxidant system. Plant Physiol. 2001;127:426–435. [PMC free article] [PubMed] [Google Scholar]
- 53.Porfirova S, Bergmüller E, Tropf S, Lemke R, Dörman P. Isolation of an Arabidopsis mutant lacking vitamin E and identification of a cyclase essential for all tocopherol biosynthesis. Proc Natl Acad Sci USA. 2002;99:12495–12500. doi: 10.1073/pnas.182330899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Havaux M, Eymery F, Porfirova S, Rey P, Dörmann P. Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell. 2005;17:3451–3469. doi: 10.1105/tpc.105.037036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Niyogi KK, Grossman AR, Björkman O. Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell. 1998;10:1121–1134. doi: 10.1105/tpc.10.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li XP, Björkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature. 2000;403:391–395. doi: 10.1038/35000131. [DOI] [PubMed] [Google Scholar]
- 57.Netting AG. pH, abscisic acid and the integration of metabolism in plants under stressed and non-stressed conditions: Cellular responses to stress and their implication for water relations. J Exp Bot. 2000;51:147–158. doi: 10.1093/jexbot/51.343.147. [DOI] [PubMed] [Google Scholar]