Abstract
Phenotypes of Arabidopsis thaliana that carry mutations in CYCLOARTENOL SYNTHASE 1 (CAS1) which is required in sterol biosynthesis have been described. Knockout mutant alleles are responsible of a male-specific transmission defect. Plants carrying a weak mutant allele cas1-1 accumulate 2,3-oxidosqualene, the substrate of CAS1, in all analyzed organs. Mutant cas1-1 plants develop albino inflorescence shoots that contain low amount of carotenoids and chlorophylls. The extent of this albinism, which affects Arabidopsis stems late in development, may be modulated by the light/dark regime. The fact that chloroplast differentiation and pigment accumulation in inflorescence shoots are associated with a low CAS1 expression could suggest the involvement of 2,3-oxidosqualene in a yet unknown regulatory mechanism linking the sterol biosynthetic segment, located in the cytoplasm, and the chlorophyll and carotenoid biosynthetic segments, located in the plastids, in the highly complex terpenoid network. CAS1 loss of function in a mosaic analysis of seedlings further demonstrated that leaf albinism associated with an accumulation of 2,3-oxidosqualene is a novel phenotype for plant sterol deficient mutant.
Key words: albinism, cell viability, sterol, terpenoid, light
A Genetic Approach to Investigate Sterol Functions
Sterol-mediated signaling and molecular regulation of sterol synthesis, transport, metabolism and cell homeostasis is described with many details in mammals1 but is not fully understood in plants. The sequence of biosynthetic steps that leads from acetyl-CoA to the C5 building blocks isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) via mevalonate and then generates the C30 precursor squalene further converted into 24-alkyl-Δ5-sterols (Fig. 1) is similar in the model species Arabidopsis thaliana and in cells of mammals, although CAS1 and sterol-C24-methyltransferases are plant-specific. A genetic approach to address the question of the role of sterols was quite informative in recent years. Mutants affected in various steps of sterol biosynthesis have a modified composition of sterol to which are associated pleiotropic macroscopic phenotypes. Arabidopsis mutants deficient in biosynthetic steps that convert cycloartenol into Δ7-sterol intermediates (episterol, Δ7-avenasterol) are embryo or seedling lethals.2–4 It has been shown recently that a cyclopropylsterol isomerase (CPI1) mutant is affected in post-cytokinetic acquisition of an auxin transporter by endocytosis, which in turn alters root gravitropism.5 Arabidopsis mutants deficient in biosynthetic steps that convert the Δ7-sterol intermediates into campesterol and sitosterol are characterized by a typical dwarf phenotype due to lack of the bioactive brassinosteroids synthesized from campesterol.6–9 Modulation of the expression of genes implicated in the relative composition of the 24-alkyl-Δ5-sterols (cholesterol, campesterol, sitosterol, stigmasterol) has been shown to affect growth, branching and fertility,10 vascular patterning11 or endoreduplication.12 However, the biological role of sterols at the molecular level (i.e., mechanism of action) is scarcely described.13
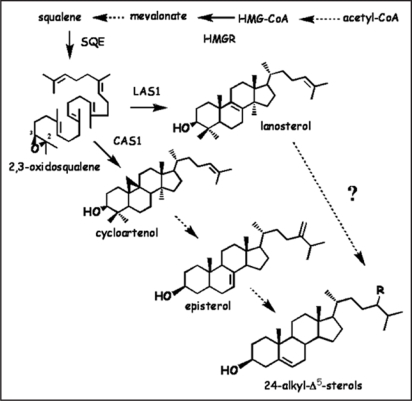
Figure 1.
Sterol biosynthetic pathway emphasizing cyclization of 2,3-oxidosqualene. HMG-CoA, 3-hydroxy-3-methylglutarylcoenzyme A; HMGR, HMG-coA reductase; SQE, squalene epoxydase; LAS, lanosterol synthase; CAS, cycloartenol synthase.
Cell Viability and Plastid Biogenesis in Cycloartenol Synthase 1 Mutants
Two biosynthetic routes have been described that generate tetracyclic steroidal end-products from 2,3-oxidosqualene. Mammals and fungi cyclize it into lanosterol, a tetracyclic triterpenol, whereas plants cyclize it into the pentacyclic steroidal intermediate cycloartenol, a 9β,19-cyclopropyl derivative14(for review) (Fig. 1). Cycloartenol-derived sterols are then produce through the mandatory cyclopropyl sterol isomerase.15 The reason why plants synthesize their sterols according to a major cycloartenol route and do not favor the lanosterol one16,17 is not understood. A series of allelic mutants deficient in the expression of the Arabidopsis cycloartenol synthase (CAS1) has been reported recently.18 In this work, albinism is described as a novel phenotype for a plant sterol mutant. A weak cas1-1 allele is characterized by an organ-dependent albinism late in development. Cortical cells of the part of stems that carry flowers and siliques contain plastids with a morphology reminiscent of photooxidation, in full accordance with a severe reduction in total carotenoids and chlorophylls in these white tissues18 (Fig. 2). This cellular phenotype correlates with the accumulation of 2,3-oxidosqualene, the substrate of CAS1. Loss of function of cycloartenol synthase occurs also in green tissues of cas1-1 but to a lesser extent than in the albino parts. Interestingly, a set of transgenic lines with mutant phenotypes that can be explained by co-suppression of the CAS1 gene were obtained.18 The most common of these phenotypes affecting co-suppressed lines was albinism of upper stems, as it is the case of cas1-1. Less lines had the entire length of the stem affected, even less lines had albino leaf petiole. Although it is shown that CAS1 is ubiquitously expressed in the wild-type,18 the chemical phenotype observed in green versus non-green tissues might indicate different sterol requirements in different organs. Strong, most probably null, cas1-2 and cas1-3 alleles used in genetic crosses show that knockout mutations in CAS1 are causing a male-specific transmission defect. It is therefore assumed that cycloartenol-derived metabolites play major roles for all cell types and developmental stages. A knockout mutant was used in a mosaic analysis approach18 which further demonstrated that progressive depletion of CAS1 in young growing leaves caused an accumulation of 2,3-oxidosqualene associated with albinism. Consequently, the onset of albinism in Arabidopsis is an immediate consequence of a cycloartenol synthase deficiency.
Figure 2.
Stem albinism of a hypomorphic cycloartenol synthase mutant (left) compared to the wild-type.
Light Influences the Albino Phenotype in a Cycloartenol Synthase Mutant
In spite of the CAS1 defect in cas1-1 hypomorphic allele, it is shown that 24-alkyl-Δ5sterols are synthesized in amounts similar to those of the wild-type.18 This observation could suggest the existence of a positive feed-back regulatory interaction between sterol biosynthetic intermediates and upstream enzymes of the pathway. Previous studies have shown that HMGR enzymatic activity is limiting for the accumulation of sterols in plants.19,20 In the case of cas1-1 flowering stems, we detected a 30% increase of HMGR activity compared with the wild-type.18 Regulatory aspects of HMGR and more generally of the mevalonate pathway indicate that light is implicated in its control. Indeed, HMGR in Arabidopsis is downregulated by light21 and this process was shown to occur via photoreceptors: a phytochrome B (PHY B) knockout mutant was isolated in a screening for resistance to inhibition of mevalonate biosynthesis by mevinolin, a potent inhibitor of HMGR.22 Likewise, mutants affected in PHY genes displayed elevated enzyme activity for HMGR.22
In a simple approach to test whether light influences the onset of the albino phenotype in the cas1-1 mutant, we compared the development of plants grown under different day length conditions. Wild-type and cas1-1 plants were grown in 12-hour light conditions until they reached the rosette stage 6.00,23 then transferred (or not) in long day conditions (16-hour light). After four weeks of growth, relevant morphological parameters were recorded (Table 1). Interestingly, we noted a reduction of 25% of the total length of cas1-1 white stems in plants grown in long day conditions, as compared to the length of white stems in plants grown in 12-hour light conditions. Likewise, the ratio of green to white stem lengths showed an increase of 76% for plants grown in long day conditions. We also recorded the rank from bottom to top of the first silique attached to the white part of the stem. It is striking to observe that the bleaching of cas1-1 stems started at silique 5 under 12-hour light conditions whereas it started at silique 15 under long-day conditions (Table 1). This experiment showed clearly that the bleaching of cas1-1 developing stems can be influenced by light and points out that complex regulatory processes are at play in the molecular regulation of terpenoid biosynthetic networks.24
Table 1.
Phenotype of wild-type and cas1-1 plants grown under different light conditionsa
| 12 h light/12 h dark | 16 h light/8 h dark | |||
| wt | cas1-1 | wt | cas1-1 | |
| Height above ground in cm | 38.5 ± 3.5 | 28.6 ± 5.4 | 44.1 ± 2.2 | 32.6 ± 2.7 |
| Number of branches per main stem | 8.4 ± 2.0 | 7.2 ± 1.5 | 9.5 ± 1.4 | 7.9 ± 1.2 |
| Number of white branches | 0 | 6.7 ± 2.3 | 0 | 7.8 ± 1.3 |
| Length of white stems per plant in cm | 0 | 39.5 ± 15.6 | 0 | 29.6 ± 9.9 |
| Ratio of green to white stem lengths | 1.95 | 3.44 | ||
| Number of siliquesb per plant | 25.4 ± 4.6 | 25.3 ± 4.5 | 35.1 ± 2.9 | 25.5 ± 4.4 |
| Rankc of the first silique on white stem | 5.2 ± 2.3 | 15.0 ± 5.5 | ||
experiments included at least 15 plants per genotype and were done three times. One representative experiment is shown.
siliques over 0.5 cm were counted.
position number from bottom to top.
Acknowledgements
This work was supported by the Agence Nationale de la Recherche Grant TERPENE ANR-05-BLAN-0217-02, an European Union Grant EXOTIC QLG2-CT-1999-000351, and a JSPS-CNRS Joint-Project PC212.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/6173
References
- 1.Goldstein JL, DeBose Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 2.Schrick K, Mayer U, Horrichs A, Kuhnt C, Bellini C, Dangl J, Schmidt J, Jürgens G. FACKEL is a sterol C14 reductase required for organized cell division and expansion in Arabidopsis embryogenesis. Genes Dev. 2000;14:1471–1484. [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HB, Schaller H, Goh CH, Kwon M, Choe S, An CS, Durst F, Feldmann KA, Feyereisen R. Arabidopsis cyp51 mutant shows postembryonic seedling lethality associated with lack of membrane integrity. Plant Physiol. 2005;138:2033–2047. doi: 10.1104/pp.105.061598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Souter M, Topping J, Pullen M, Friml J, Palme K, Hackett R, Grierson D, Lindsey K. hydra Mutants of Arabidopsis are defective in sterol profiles and auxin and ethylene signaling. Plant Cell. 2002;14:1017–1031. doi: 10.1105/tpc.001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Men S, Boutté Y, Ikeda Y, Li X, Palme K, Stierhof YD, Hartmann MA, Moritz T, Grebe M. Sterol-dependent endocytosis mediates post-cytokinetic acquisition of PIN2 auxin efflux carrier polarity. Nat Cell Biol. 2008;10:237–244. doi: 10.1038/ncb1686. [DOI] [PubMed] [Google Scholar]
- 6.Choe S, Noguchi T, Fujioka S, Takatsuto S, Tissier CP, Gregory BD, Ross AS, Tanaka A, Yoshida S, Tax FE, Feldmann KA. The Arabidopsis dwf7/ste1 mutant is defective in the delta7 sterol C-5 desaturation step leading to brassinosteroid biosynthesis. Plant Cell. 1999;11:207–221. [PMC free article] [PubMed] [Google Scholar]
- 7.Choe S, Tanaka A, Noguchi T, Fujioka S, Takatsuto S, Ross AS, Tax FE, Yoshida S, Feldmann KA. Lesions in the sterol delta reductase gene of Arabidopsis cause dwarfism due to a block in brassinosteroid biosynthesis. Plant J. 2000;21:431–443. doi: 10.1046/j.1365-313x.2000.00693.x. [DOI] [PubMed] [Google Scholar]
- 8.Klahre U, Noguchi T, Fujioka S, Takatsuto S, Yokota T, Nomura T, Yoshida S, Chua NH. The Arabidopsis DIMINUTO/DWARF1 gene encodes a protein involved in steroid synthesis. Plant Cell. 1998;10:1677–1690. doi: 10.1105/tpc.10.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Nagpal P, Vitart V, McMorris TC, Chory J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science. 1996;272:398–401. doi: 10.1126/science.272.5260.398. [DOI] [PubMed] [Google Scholar]
- 10.Schaeffer A, Bronner R, Benveniste P, Schaller H. The ratio of campesterol to sitosterol that modulates growth in Arabidopsis is controlled by STEROL METHYLTRANSFERASE 2;1. Plant J. 2001;25:605–615. doi: 10.1046/j.1365-313x.2001.00994.x. [DOI] [PubMed] [Google Scholar]
- 11.Carland FM, Fujioka S, Takatsuto S, Yoshida S, Nelson T. The identification of CVP1 reveals a role for sterols in vascular patterning. Plant Cell. 2002;14:2045–2058. doi: 10.1105/tpc.003939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hase Y, Fujioka S, Yoshida S, Sun G, Umeda M, Tanaka A. Ectopic endoreduplication caused by sterol alteration results in serrated petals in Arabidopsis. J Exp Bot. 2005;56:1263–1268. doi: 10.1093/jxb/eri122. [DOI] [PubMed] [Google Scholar]
- 13.Beck JG, Mathieu D, Loudet C, Buchoux S, Dufourc EJ. Plant sterols in rafts: a better way to regulate membrane thermal shocks. FASEB J. 2007;21:1714–1723. doi: 10.1096/fj.06-7809com. [DOI] [PubMed] [Google Scholar]
- 14.Phillips DR, Rasbery JM, Bartel B, Matsuda SP. Biosynthetic diversity in plant triterpene cyclization. Curr Opin Plant Biol. 2006;9:305–314. doi: 10.1016/j.pbi.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Heintz R, Benveniste P. Plant sterol metabolism. Enzymatic cleavage of the 9beta, 19beta-cyclopropane ring of cyclopropyl sterols in bramble tissue cultures. J Biol Chem. 1974;249:4267–4274. [PubMed] [Google Scholar]
- 16.Kolesnikova MD, Xiong Q, Lodeiro S, Hua L, Matsuda SPT. Lanosterol biosynthesis in plants. Arch Biochem Biophys. 2006;447:87–95. doi: 10.1016/j.abb.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki M, Xiang T, Ohyama K, Seki H, Saito K, Muranaka T, Hayashi H, Katsube Y, Kushiro T, Shibuya M, Ebizuka Y. Lanosterol synthase in dicotyledonous plants. Plant Cell Physiol. 2006;47:565–571. doi: 10.1093/pcp/pcj031. [DOI] [PubMed] [Google Scholar]
- 18.Babiychuk E, Bouvier Navé P, Compagnon V, Suzuki M, Muranaka T, Van Montagu M, Kushnir S, Schaller H. Allelic mutant series reveal distinct functions for Arabidopsis cycloartenol synthase 1 in cell viability and plastid biogenesis. Proc Natl Acad Sci USA. 2008;105:3163–3168. doi: 10.1073/pnas.0712190105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gondet L, Weber T, Maillot Vernier P, Benveniste P, Bach TJ. Regulatory role of microsomal 3-hydroxy-3-methylglutaryl-coenzyme A reductase in a tobacco mutant that overproduces sterols. Biochem Biophys Res Commun. 1992;18:888–893. doi: 10.1016/0006-291x(92)90829-a. [DOI] [PubMed] [Google Scholar]
- 20.Schaller H, Grausem B, Benveniste P, Chye ML, Tan CT, Song YH, Chua NH. Expression of the Hevea brasiliensis (H.B.K.) Mull. Arg. 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase 1 in Tobacco Results in Sterol Overproduction. Plant Physiol. 1995;109:761–770. doi: 10.1104/pp.109.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Learned RM, Conolly EL. Light modulates the spatial patterns of 3-hydroxy-3-methylglutaryl-coenzyme A reductase gene expression in Arabidopsis thaliana. Plant J. 1997;11:499–511. doi: 10.1046/j.1365-313x.1997.11030499.x. [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez Conceptíon M, Fores O, Martinez Garcia JF, Gonzales V, Phillips MA, Ferrer A, Boronat A. Distinct light-mediated pathways regulate the biosynthesis and exchange of isoprenoid precursors during Arabidopsis seedling development. Plant Cell. 2006;16:115–144. doi: 10.1105/tpc.016204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Görlach J. Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell. 2001;13:1499–1510. doi: 10.1105/TPC.010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laule O, Fürholz A, Chang HS, Zhu T, Wang X, Heifetz PB, Gruissem W, Lange BM. Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2003;100:6866–6871. doi: 10.1073/pnas.1031755100. [DOI] [PMC free article] [PubMed] [Google Scholar]