Abstract
A new Arabidopsis mutant is characterized (rha1) that shows, in the roots, reduced right-handed slanting, reduced gravitropism and resistance to 2,4-D, TIBA, NPA and ethylene. It also shows reduced length in the shoot and root, reduced number of lateral roots and shorter siliques. The gene was cloned through TAIL-PCR and resulted in a HSF. Because none of the known gravitropic and auxinic mutants result from damage in a HSF, rha1 seems to belong to a new class of this group of mutants. Quantitative PCR analysis showed that the expression of the gene is increased by heat and cold shock, and by presence of 2,4-D in the media. Study of the expression through the GUS reporter gene revealed increased expression in clinostated and gravistimulated plants, but only in adult tissues, and not in the apical meristems of shoots and roots.
Key words: auxin, ethylene, slanting, gravitropism, HSFs
Arabidopsis primary roots, and especially those from some ecotypes (Ws, Landsberg), when grown on an agar dish, tilted on the vertical, show a wavy pattern, and a clear slanting towards a direction that has been considered the right-hand.1–4 In the case of the mutant rha1, the right-handed slanting is notably reduced, its primary roots growing partly to the right-hand, partly straight down and partly to the left-hand, even though a slight preference for the right-hand is apparent.
In addition, its roots show resistance to the inhibitory action of the auxin 2,4-D, ethylene (ACC), and the auxin transport inhibitors TIBA and NPA. These characteristics qualify the mutant as an auxinic one, and therefore a connection between the reduced slanting and the auxinic disturbances could be imagined. It is not known, however, what controls the slanting process itself, even though it appears as the consequence of a chiral circumnutational process. As reported,4 it seems the result of a chiral circumnutation with preference for the right-hand, transformed in a lateral slanting movement, because of the impact of the helix with the hard agar surface. This process results in the formation of waves, when the circumnutation helix impactig the agar reverses direction at every half turn, or the formation of large loops and strict loops (coils) when there is no reversion. The latter case seems to be a consequence of the fact that gravity is no longer “felt”. This has been previously noted in some mutants, or sometimes in old roots.
rha1 is not the only mutant known to show reduction or increase of slanting, because other mutants were reported by Rutherford and Masson,3 and subsequent publications from the same group. Almost all show an increase of slant, with the exception of rhd3 and its alleles that show a complete suppression of the process.5 The mutated gene in rha1 was cloned through TAIL-PCR and shown to be a HSF. No other auxinic mutant, among those for which the gene was cloned, is known to be mutated in a HSF.
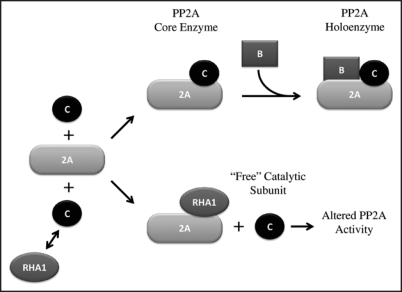
HSFs, that are characteristically involved in the activation of the HSPs (heat shock proteins), which protect the cells from damage arising from high temperature and other stresses, have been shown to be involved also in different processes.6,7 Hence, also in the case of rha1, we can well imagine other different functions, beside that of counteracting the heat shock. In particular, since the connection with auxin regulated processes is evident, we can suppose that the action could be on the PP2A phosphatase, as in the case of the rcn1 mutant, or of the human HSF2. The RCN1 protein corresponds to one unit of the PP2A,8 and the HSF29 has been shown to substitute itself for the C subunit and alter the function of the phosphatase (Fig. 1). Experiments directed to see if a heat shock can modify the slanting of the roots in the wild-type and rha1, gave negative results, even though these experiments will need to be repeated under more widely ranging conditions. These results seem to indicate that the mechanism which induces asymmetric growth in roots is complex, and it is not controlled by a single gene.
Figure 1.
Model proposed for the regulation of the PP2A activity by the RHA1 protein. (modified after Hong and Sarge, 1999).
On the other hand, another puzzling characteristic of rha1 is the fact that its roots are resistant only to the auxin 2,4-D, and not to NAA and IAA. Differences in the response of the primary roots to different auxins have already been reported, and it was suggested that the response to NAA should be different, because this substance can penetrate passively the cell membranes.10 In the case of rha1, however, it seems that IAA can also penetrate the cells passively. This is in line with the chemiosmotic hypothesis,11 but seems in contrast with the previous supposition. The resistance to ethylene, however, could indicate that the reduced inhibitory effect of 2,4-D is a consequence of the ethylene production induced by the synthetic auxin. On the other hand, the resistance to the auxin transport inhibitors TIBA and NPA, cannot be explained so easily. Possibly, in the mutant rha1, there is a reduced level of receptors for the considered substances.
Using semiquantitative PCR analysis it was shown that rha1 retains the function of a HSF, the gene being clearly upregulated by heat and cold stress, and also by 2,4-D, but not by rotation on a clinostat or gravistimulation. The upregulation of the expression by 2,4-D was confirmed by a study of GUS expression in a transformed rha1, and with this technique the effects of gravity and simulated microgravity appeared clearly stimulatory too. No GUS expression however was apparent in the shoot and root meristems, and consequently we propose that the gene does not influence the first part of the graviresponse, but possibly the general transport of auxin through the plant.
Thus, the mutant seems to be disturbed in root gravitropism, as well as in responses to the auxines and circumnutation. However not in the general circumnutation process, but in its chiral aspect, which is the cause of the slanting to the right-hand. Gravitropism, circumnutation and auxin physiology, thus, seem to be in some way connected in a complex integrated process, that, hopefully, will be gradually revealed in all its different aspects, through the future efforts of plant scientists.
Abbreviations
- IAA
indole-3-acetic acid
- 2,4-D
2,4-dichlorophenoxyacetic acid
- TIBA
2,3,5-triiodobenzoic acid
- NPA
1-naphthylphtalamic acid
- NAA
1-naphthaleneacetic acid
- ACC
1-aminocyclopropane-1-carboxylic acid
- HSF
heat shock factor
- PP2A
protein phosphatase 2A
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/6290
References
- 1.Okada K, Shimura Y. Reversible root tip rotation In Arabidopsis seedlings induced by obstacle-touching stimulus. Science. 1990;250:274–276. doi: 10.1126/science.250.4978.274. [DOI] [PubMed] [Google Scholar]
- 2.Simmons C, Soell D, Migliaccio F. Circumnutation and gravitropism cause root waving in Arabidopsis thaliana. J Exp Bot. 1995;46:143–150. [Google Scholar]
- 3.Rutherford R, Masson PH. Arabidopsis thaliana sku mutants seedlings show exaggerated surface dependent alteration in root growth vector. Plant Physiol. 1996;111:987–998. doi: 10.1104/pp.111.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Migliaccio F, Piconese S. Spiralizations and tropisms in Arabidopsis roots. Trends Plant Sci. 2001;6:595–604. doi: 10.1016/s1360-1385(01)02152-5. [DOI] [PubMed] [Google Scholar]
- 5.Yuen CYL, Pearlmen RS, Silo-Suh L, Hilson P, Carrol L, Masson PH. WVD2 and WDL1 modulate helical organ growth and anisotropic cell expression in Arabidopsis. Plant Physiol. 2003;131:493–506. doi: 10.1104/pp.015966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pigliucci M. Buffer zone. Nature. 2002;417:598–599. doi: 10.1038/417598a. [DOI] [PubMed] [Google Scholar]
- 7.Queitsch C, Sangster TA, Lindquist S. Hsp90 as a capacitor of phetotypic variation. Nature. 2002;417:618–624. doi: 10.1038/nature749. [DOI] [PubMed] [Google Scholar]
- 8.Garbers C, De Long A, Deruére J, Bernasconi P, Soell D. A mutation in protein phosphatase 2A regulatory subunit A affects auxin transport in Arabidopsis. EMBO J. 1996;15:2115–2124. [PMC free article] [PubMed] [Google Scholar]
- 9.Hong Y, Sarg KD. Regulation of protein phosophatase 2A activity by heat shock transcription factor 2. J Biol Chem. 1999;274:12967–12970. doi: 10.1074/jbc.274.19.12967. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto M, Yamamoto K. Differential effects of 1-naphthalenacetic acid, Indole-3-acetic acid and 2,4-Dichlorophenoxyacetic acid on the gravitropic response of roots in an auxin-resistant mutant of Arabidopsis, aux1. Plant Cell Physiol. 1998;39:660–664. doi: 10.1093/oxfordjournals.pcp.a029419. [DOI] [PubMed] [Google Scholar]
- 11.Goldshmit MH. The polar transport of auxin. Ann Rev Plant Physiol. 1977;28:439–478. [Google Scholar]