Abstract
As a second messenger, H2O2 generation and signal transduction is subtly controlled and involves various signal elements, among which are the members of MAP kinase family. The increasing evidences indicate that both MEK1/2 and p38-like MAP protein kinase mediate ABA-induced H2O2 signaling in plant cells. Here we analyze the mechanisms of similarity and difference between MEK1/2 and p38-like MAP protein kinase in mediating ABA-induced H2O2 generation, inhibition of inward K+ currents, and stomatal closure. These data suggest that activation of MEK1/2 is prior to p38-like protein kinase in Vicia guard cells.
Key words: H2O2 signaling, ABA, p38-like MAP kinase, MEK1/2, guard cell
An increasing number of literatures elucidate that reactive oxygen species (ROS), especially H2O2, is essential to plant growth and development in response to stresses,1–4 and involves activation of various signaling events, among which are the MAP kinase cascades.1–3,5 Typically, activation of MEK1/2 mediates NADPH oxidase-dependent ROS generation in response to stresses,4,6–8 and the facts that MEK1/2 inhibits the expression and activation of antioxidant enzymes reveal how PD98059, the specific inhibitor of MEK1/2, abolishes abscisic acid (ABA)-induced H2O2 generation.6,8,9 It has been indicated that PD98059 does not to intervene on salicylic acid (SA)-stimulated H2O2 signaling regardless of SA mimicking ABA in regulating stomatal closure.2,6,8,10 Generally, activation of MEK1/2 promotes ABA-induced stomatal closure by elevating H2O2 generation in conjunction with inactivating anti-oxidases.
Moreover, activation of plant p38-like protein kinase, the putative counterpart of yeast or mammalian p38 MAP kinase, has been reported to participate in various stress responses and ROS signaling. It has been well documented that p38 MAP kinase is involved in stress-triggered ROS signaling in yeast or mammalian cells.11–13 Similar to those of yeast and mammals, many studies showed the activation of p38-like protein kinase in response to stresses in various plants, including Arabidopsis thaliana,14–16 Pisum sativum,17 Medicago sativa18 and tobacco.19 The specific p38 kinase inhibitor SB203580 was found to modulate physiological processes in plant tissues or cells, such as wheat root cells,20 tobacco tissue21 and suspension-cultured Oryza sativa cells.22 Recently, we investigate how activation of p38-like MAP kinase is involved in ABA-induced H2O2 signaling in guard cells. Our results show that SB203580 blocks ABA-induced stomatal closure by inhibiting ABA-induced H2O2 generation and decreasing K+ influx across the plasma membrane of Vicia guard cells, contrasting greatly with its analog SB202474, which has no effect on these events.23,24 This suggests that ABA integrate activation of p38-like MAP kinase and H2O2 signaling to regulate stomatal behavior. In conjunction with SB203580 mimicking PD98059 not to mediate SA-induced H2O2 signaling,23,24 these results generally reveal that the activation of p38-like MAP kinase and MEK1/2 is similar in guard cells.
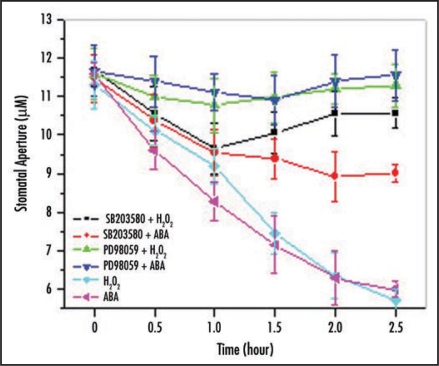
On the other hand, activation of p38-like MAP kinase23,24 is not always identical to that of MEK1/28,25 in ABA-induced H2O2 signaling of Vicia guard cells. For example, H2O2- and ABA-induced stomatal closure was partially reversed by SB203580. The maximum inhibition of both regent-induced stomatal closure were observed at 2 h after treatment with SB203580, under which conditions the stomatal apertures were 89% and 70% of the control values, respectively. By contrast, when PD98059 was applied together with ABA or H2O2, the effects of both ABA- and H2O2-induced stomatal closure were completely abolished (Fig. 1). These data imply that the two members of MAP kinase family are efficient in H2O2-stimulated stomatal closure, but p38-like MAP kinase is less susceptive than MEK1/2 to ABA stimuli.
Figure 1.
Effects of SB203580 and PD98059 on ABA- and H2O2-induced stomatal closure. The experimental procedure and data analysis are according to the previous publication.8,23,24
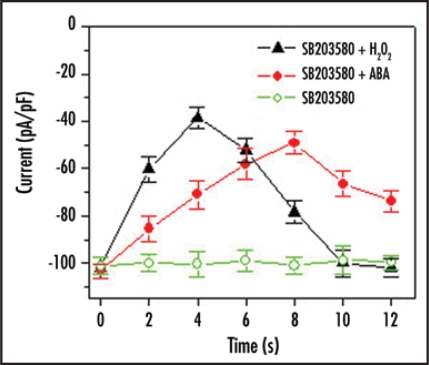
It has been reported that ABA or NaCl activate p38 MAP kinase in the chloronema cells of the moss Funaria hygrometrica in 2∼10 min.26 Similar to this, SB203580 improves H2O2-inhibited inward K+ currents after 4 min and leads it to the control level (100%) during the following 8 min (Fig. 2). However, the activation of p38-like MAP kinase in response to ABA need more time, and only recovered to 75% of the control at 8 min of treatment (Fig. 2). These results suggest that control of H2O2 signaling is required for the various protein kinases including p38-like MAP kinase and MEK1/2 in guard cells,1,2,8,23,24 and the ABA and H2O2 pathways diverge further downstream in their actions on the K+ channels and, thus, on stomatal control. Other differences in action between ABA and H2O2 are known. For example, Köhler et al. (2001) reported that H2O2 inhibited the K+ outward rectifier in guard cells shows that H2O2 does not mimic ABA action on guard cell ion channels as it acts on the K+ outward rectifier in a manner entirely contrary to that of ABA.27
Figure 2.
Effect of SB203580 on ABA- and H2O2-inhibited inward K+ currents. The experimental procedure and data analysis are according to the previous publication.24 SB203580 directs ABA- and H2O2-inactivated inward K+ currents across plasma membrane of Vicia guard cells. Here the inward K+ currents value is stimulated by −190 mV voltage.
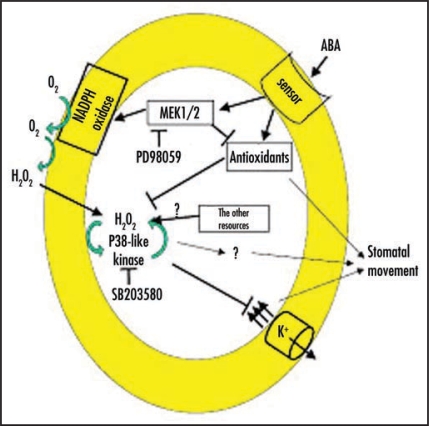
Based on the similarity and difference between PD98059 and SB203580 in interceding ABA and H2O2 signaling, we speculate the possible mechanism is that the member of MAP kinase family specially regulate signal event in ABA-triggered ROS signaling network,1–4 and the signaling model as follows (Fig. 3).
Figure 3.
Schematic illustration of MAP kinase-mediated H2O2 signaling of guard cells. The arrows indicate activation. The line indicates enhancement and the bar denotes inhibition.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant no. 30470895 to Jing Jiang), the Major State Basic Research Program of China (Grant no. 2003CB114305 to Chun-Peng Song), and the Program for New Century Excellent Talents in University of Henan Province (Grant no. 2005HANCET-06 to Jing Jiang).
Abbreviations
- ROS
reactive oxygen species
- ABA
abscisic acid
- MAP kinase
mitogen-activated protein kinase
- MEK
MAP kinase kinase
- SA
salicylic acid
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/6293
References
- 1.Desikan R, Cheung MK, Bright J, Henson D, Hancock JT, Neill SJ. ABA, hydrogen peroxide and nitric oxide signaling in stomatal guard cells. J Exp Bot. 2004;55:205–212. doi: 10.1093/jxb/erh033. [DOI] [PubMed] [Google Scholar]
- 2.Jiang J, Song C-P. Regulation role of reactive oxygen species and mitogen-activated protein kinases in plant stress signaling. J Plant Physiol Mol Biol. 2005;31:1–10. [PubMed] [Google Scholar]
- 3.Nakagami H, Pitzschke A, Hirt H. Emerging MAP kinase pathways in plant stress signaling. Trends Plant Sci. 2005;10:339–346. doi: 10.1016/j.tplants.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Zhang T, Liu Y, Yang T, Zhang L, Xu S, Xue L, An L. Diverse signals converge at MAPK cascades in plant. Plant Physiol Biochem. 2006;44:274–283. doi: 10.1016/j.plaphy.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Mishra NS, Tuteja R, Tuteja N. Signaling through MAP kinase networks in plants. Arch Biochem Biophys. 2006;452:55–68. doi: 10.1016/j.abb.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Grant JJ, Yun B-W, Loake GJ. Oxidative burst and cognate redox signaling reported by luciferase imaging: identification of a signal network that functions independently of ethylene, SA and Me-JA but is dependent on MAPKK activity. Plant J. 2000;24:569–582. doi: 10.1046/j.1365-313x.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- 7.Desikan R, Hancock JT, Ichimura K, Shinozaki K, Neill SJ. Harpin induces activation of the Arabidopsis mitogen-activated protein kinases AtMPK4 and AtMPK6. Plant Physiol. 2001;126:1579–1587. doi: 10.1104/pp.126.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang J, An GY, Wang PC, Wang PT, Han JF, Jia YB, Song C-P. MAP kinase specifically mediates the ABA-induced H2O2 generation in guard cells of Vicia faba L. Chin Sci Bull. 2003;48:1919–1926. [Google Scholar]
- 9.Zhang A, Jiang M, Zhang J, Tan M, Hu X. Mitogen-activated protein kinase is involved in abscisic acid-induced antioxidant defense and acts downstream of reactive oxygen species production in leaves of maize plants. Plant Physiol. 2006;141:475–487. doi: 10.1104/pp.105.075416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mori IC, Pinontoan R, Kawano T, Muto S. Involvement of superoxide generation in salicylic acid-induced stomatal closure in Vicia faba. Plant Cell Physiol. 2001;42:1383–1388. doi: 10.1093/pcp/pce176. [DOI] [PubMed] [Google Scholar]
- 11.Chang L, Karin M. Mammalian MAP kinase signaling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 12.Torres M, Forman HJ. Redox signaling and the MAP kinase pathways. BioFactors. 2003;17:287–296. doi: 10.1002/biof.5520170128. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Gabriel MA, Russell P. Distinct Signaling Pathways Respond to Arsenite and Reactive Oxygen Species in Schizosaccharomyces pombe. Eukaryot Cell. 2005;4:1396–1402. doi: 10.1128/EC.4.8.1396-1402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinozaki K, Yamaguchi-Shinozaki K. Gene expression and signal transduction in water-stress response. Plant Physiol. 1997;115:327–334. doi: 10.1104/pp.115.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ichimura K, Mizoguchi T, Irie K, Morris P, Giraudat J. Isolation of AtMEKK1 (a MAP kinase kinase kinase)-interacting protein and analysis of a MAP kinase cascade in Arabidopsis. Biochem Biophys Res Commun. 1998;253:532–543. doi: 10.1006/bbrc.1998.9796. [DOI] [PubMed] [Google Scholar]
- 16.Urao T, Yakubov B, Satoh R, Yamaguchi-Shinozaki K, Seki M, Hiravam T, Shinozaki K. A transmembrane hybrid-type histidine kinase in Arabidopsis founctions as an osmosensor. Plant Cell. 1999;11:1743–1754. doi: 10.1105/tpc.11.9.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pöpping B, Gibbons T, Watson MD. The Pisum sativum MAP kinase homologue (PsMAPK) rescues the Saccharomyces cerevisiae hog1 deletion mutant under conditions of high osmotic stress. Plant Mol Biol. 1996;31:355–363. doi: 10.1007/BF00021795. [DOI] [PubMed] [Google Scholar]
- 18.Munnik T, Ligterink W, Meskiene I, Calderini O, Beeyerly J, Musgrave A, Hirt H. Distinct osmo-sensing protein kinase pathways are involved in signaling moderate and severe hyperosmotic stress. Plant J. 1999;20:381–388. doi: 10.1046/j.1365-313x.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- 19.Hoyos ME, Zhang S. Calcium-independent activation of salicylic acid-induced protein kinase and a 40-kilodalton protein kinase by hyperosmotic stress. Plant Physiol. 2000;122:1355–1364. doi: 10.1104/pp.122.4.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komis G, Apostolakos P, Gaitanaki C, Galatis B. Hyperosmotically induced accumulation of a phosphorylated p38-like MAPK involved in protoplast volume regulation of plasmolyzed wheat root cells. FEBS Lett. 2004;573:168–174. doi: 10.1016/j.febslet.2004.07.065. [DOI] [PubMed] [Google Scholar]
- 21.Xing T, Malik K, Martin T, Miki BL. Activation of tomato PR and wound-related genes by a mutagenized tomato MAP kinase kinase through divergent pathways. Plant Mol Biol. 2001;46:109–120. doi: 10.1023/a:1010633215445. [DOI] [PubMed] [Google Scholar]
- 22.Matsumura H, Nirasawa S, Kiba A, Urasaki N, Saitoh H, Ito M, Kawai-Yamada M, Uchimiya H, Terauchi R. Overexpression of Bax inhibitor suppresses the fungal elicitorinduced cell death in rice (Oryza sativa L.) cells. Plant J. 2003;33:425–434. doi: 10.1046/j.1365-313x.2003.01639.x. [DOI] [PubMed] [Google Scholar]
- 23.Jiang J, Han S, Song C-P. SB202190 modulate salicylic acid-induced H2O2 generation in Vicia guard cells. Chin Bull Bot. 2007;2:444–451. [Google Scholar]
- 24.Jiang J, Wang PT, An GY, Wang PC, Song C-P. The involvement of a P38-like MAP kinase in ABA-induced and H2O2-mediated stomatal closure in Vicia faba L. Plant Cell Reports. 2008;27:377–385. doi: 10.1007/s00299-007-0449-x. [DOI] [PubMed] [Google Scholar]
- 25.Burnett EC, Desikan R, Moser RC, Neill SJ. ABA activation of an MBP kinase in Pisum sativum epidermal peels correlates with stomatal responses to ABA. J Exp Bot. 2000;51:197–205. doi: 10.1093/jexbot/51.343.197. [DOI] [PubMed] [Google Scholar]
- 26.D'Souza JS, Johri MM. ABA and NaCl activate myelin basic kinase in the chloronema cells of the moss Funaria hygrometrica. Plant Physiol Biochem. 2002;40:17–24. [Google Scholar]
- 27.Köhler B, Hills A, Blatt MR. Control of guard cell ion channels by hydrogen peroxide and abscisic acid indicates their action through alternate signaling pathways. Plant Physiol. 2003;131:385–388. doi: 10.1104/pp.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]