Abstract
In our recent paper in The Plant Journal,1 we described the remobilization of purine metabolites during natural and dark induced senescence in wild type and Atxdh1 mutant lines impaired in xanthine dehydrogenase (XDH), a pivotal enzyme in the purine catabolism pathway. In the light of these observations and additional evidence shown here, we discuss the probable pathways leading to xanthine synthesis in Arabidopsis plants during senescence and the role that purine metabolites play as an ongoing source of nitrogen in plant growth.
Key words: hypoxanthine, purine catabolism, senescence, xanthine, xanthine dehydrogenase
In mammalian purine catabolism, hypoxanthine is oxidized to xanthine by xanthine oxidase.2 In planta, xanthine can be synthesized in the purine degradation pathway, via three alternative precursors, guanine, xanthosine or hypoxanthine3 (Fig. 1A). Thus, the exact pathway leading to xanthine may depend on the species examined, the particular plant organ, developmental stage or specific environmental stimuli. For example, guanine and guanosine were shown to be the main precursor of ureides and CO2 in cacao leaves4 while in tea leaves, elevated amounts of labeled xanthosine were recovered as ureides.5,6 However, when hypoxanthine was used as a substrate for inosine monophosphate (IMP) formation in tobacco protoplasts7 more than 90% of labeled hypoxanthine was recovered as salvage products, nucleotides and RNA and only less then 10% was found as ureides in cacao leaves.4 Furthermore, when [8-14C]-hypoxanthine is supplied to soybean embryo axes or Jerusalem artichoke shoots it selectively labelled the guanine nucleotide pool.3,8,9 These data do not support the possibility of hypoxanthine being a direct precursor for xanthine formation and illustrate the concept of species dependent differences in xanthine biosynthesis.10
Figure 1.
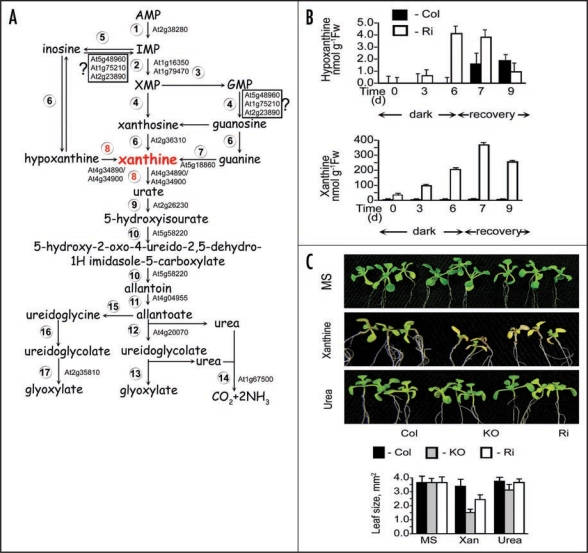
Purine catabolism, xanthine and hypoxanthine accumulation and Arabidopsis plants growth. (A) Purine nucleotide catabolism in plants. Enzymes shown are: (1) AMP deaminase (EC 3.5.4.6), (2) IMP dehydrogenase (EC 1.1.1.205), (3) GMP synthase (EC 6.3.5.2), (4) 5'-nucleotidase, (5) Nucloeside phosphotransferase (EC 2.7.1.77), (6) Inosine-guanosine nucleosidase (EC 3.2.2.2), (7) Guanine deaminase (EC 3.5.4.15), (8) Xanthine dehydrogenase (EC 1.1.1.204), (9) Uricase EC 1.7.3.3, (10) Hydroxyisourate hydrolase (EC 3.5.2.17),1,18 (11) Allantoinase, allantoin amidohydrolase (EC 3.5.2.5), (12) Allantoicase, allantoate amidohydrolase (EC 3.5.3.4), (13) Ureidoglycolate lyase (EC 4.3.2.3), (14) Urease EC 3.5.1.5, (15) Allantoin deaminase (EC 3.5.3.9), (16) Ureidoglycine amidohydrolase (EC 3.5.3.-), (17) Ureidoglycolate hydrolase (EC 3.5.3.19). (B) Analysis of the purine metabolites, hypoxanthine and xanthine, in response to dark stress. Hypoxanthine and xanthine were determined by HPLC1 in rosette leaves of wild-type (Col) and Ri14, XDH1 RNA interference plants after being kept in dark for 6 days and transferred to a 16-h light/8-h dark regime for recovery over an additional 3 days. Values are means ± SEM (n = 3). (C) Wild-type (Col) and XDH-compromised plants (KO, SALK_148364; Ri, XDH1 RNA interference) were germinated on ¼ MS medium and transplanted on the 5 day to a full MS medium (upper panel) or MS medium with 5.0 mM xanthine and urea as the sole nitrogen source. After transplanting the seedlings were left to grow for 14 days under a 16-h light/8-h dark regime (100 µmol m−2 sec−1) and then photographed. Leaf size was estimated using ImageJ software (http://rsb.info.nih.gov/ij/). Values are means ± SEM (n = 3).
To study the possible role of hypoxanthine in xanthine formation in Arabidopsis we utilized XDH1 mutants. The mutants do not show any detectable XDH activity in-vitro when using hypoxanthine and/or xanthine as substrates.1,11 Furthermore, no other enzyme is known to catalyze the conversion of hypoxanthine to xanthine, other than the molybdenum cofactor containing-XDH1. Yet, xanthine accumulation was readily detected in mutant leaves and was up to 100-fold higher than hypoxanthine either in normal growth conditions (Fig. 1B, time 0) or when exposed to dark induced senescence and to a light recovery period thereafter (Fig. 1B). These results indicate that most likely, hypoxanthine is not a major direct source for xanthine formation in Arabidopsis. The results imply that xanthosine or guanine are a source, although, one cannot exclude the possibility that hypoxanthine could be converted to xanthine in a pathway leading to inosine, IMP and then either via guanine or xanthosine, back to xanthine as illustrated in Figure 1A.
In legumes inoculated with rhizobia, nitrogen is fixed initially as NH3/NH4+ that is subsequently incorporated through the purine pathway to form IMP, and finally ureides. The central role of purine catabolism in plant nitrogen metabolism was demonstrated mainly in legumes in which the purine nucleotides are degraded via uric acid and allantoin to urea and then to CO2 and NH3, which is then re-assimilated via the glutamine oxoglutarate aminotransferase (GOGAT) pathway (reviewed in ref. 3). What then is the role of purine catabolism pathways in non leguminous plants? Are the nitrogenous products of the degraded purines re-assimilated in non-legumes as in legume plants? We recently showed in Arabidopsis that a marked transition from assimilation, during the plants normal growth, to a state of rapid metabolite turnover occurs when plants were exposed to extended dark stress, senescence or even during normal diurnal cycles.10 This was depicted by the acceleration of purine catabolic recycling activities in which XDH1 plays a central role.1 To test for a possible role of the accumulating purines as a source of nitrogen metabolites, we grew wild-type Arabidopsis plants and their XDH1 mutants under heterotrophic conditions. The agar plates contained either full MS nutrient solution with nitrate and ammonia or the purine metabolites, hypoxanthine (data not shown), xanthine or urea (Fig. 1C) as sole nitrogen source. The results show that the mutant plants exhibited slower growth in the medium contained xanthine or hypoxanthine compared to wild-type (Fig. 1C, lowest insert). The suboptimal growth of wild type lines is likely due to the low solubility of hypoxanthine and xanthine. In contrast, the growth on urea was the same for wild-type and XDH1 mutant transgenic plants (Fig. 1C). These results suggest that the conversion of xanthine to metabolically active intermediates, such as ureides and urea synthesized through XDH1, can play a role in ensuring nutrient supply for normal plant growth in purine containing media. Indeed, urea has been shown to be essential for the germination of Arabidopsis under nitrogenlimited conditions,12 and recent studies have also shown that uric acid,13 allantoin and allantoate,14–16 can serve as the sole nitrogen source during the growth of Arabidopsis plants. Taken together, the data suggest that ureide formation is an active component of normal plant metabolism facilitating the recovery of nitrogen in stress and non-stressed metabolism in a manner analogous to legumes. Indeed, legumes arose about 50–55 milion years ago17 and likely recruited and amplified existing plant functional purine pathways for their efficient nitrogen distribution system.
Acknowledgements
M.S. and R.F. thankfully acknowledge a grant from the Israel Science Foundation (Grant 417/03) in partial coverage of the costs.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/6304
References
- 1.Brychkova G, Alikulov Z, Fluhr R, Sagi M. A critical role for ureides in dark and senescence-induced purine remobilization is unmasked in the Atxdh1 Arabidopsis mutant. Plant J. 2008;54:496–509. doi: 10.1111/j.1365-313X.2008.03440.x. [DOI] [PubMed] [Google Scholar]
- 2.Harrison R. Structure and function of xanthine oxidoreductase: where are we now? Free Radic Biol Med. 2002;33:774–797. doi: 10.1016/s0891-5849(02)00956-5. [DOI] [PubMed] [Google Scholar]
- 3.Zrenner R, Stitt M, Sonnewald U, Boldt R. Pyrimidine and purine biosynthesis and degradation in plants. Annu Rev Plant Biol. 2006;57:805–836. doi: 10.1146/annurev.arplant.57.032905.105421. [DOI] [PubMed] [Google Scholar]
- 4.Koyama Y, Tomoda Y, Kato M, Ashihara H. Metabolism of purine bases, nucleosides and alkaloids in theobromine-forming Theobroma cacao leaves. Plant Physiol Biochem. 2003;41:977–984. [Google Scholar]
- 5.Ashihara H, Takasawa Y, Suzuki T. Metabolic fate of guanosine in higher plants. Physiol Plant. 1997;100:909–916. [Google Scholar]
- 6.Osamu N, Tetsuo O, Hiroshi I. Guanosine Deaminase and Guanine Deaminase from Tea Leaves. Biosci Biotech Bioch. 1994;58:1277–1281. [Google Scholar]
- 7.Barankiewicz J, Paszkowski J. Purine metabolism in mesophyll protoplasts of tobacco (Nicotiana tabacum) leaves. Biochem J. 1980;186:343–350. doi: 10.1042/bj1860343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Floch F, Lafleuriel J, Guillot A. Interconversion of purine nucleotides in Jerusalem artichocke shoots. Plant Sci Lett. 1982;27:309–316. [Google Scholar]
- 9.Anderson JD. Purine nucleotide metabolism of germinating soybean embryonic axes. Plant Physiol. 1979;63:100–104. doi: 10.1104/pp.63.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanks J, Tolbert NE. Localization of purine degradation animal and plant organelles. Ann NY Acad Sci. 1982;386:420–421. [Google Scholar]
- 11.Yesbergenova Z, Yang G, Oron E, Soffer D, Fluhr R, Sagi M. The plant Mo-hydroxylases aldehyde oxidase and xanthine dehydrogenase have distinct reactive oxygen species signatures and are induced by drought and abscisic acid. Plant J. 2005;42:862–876. doi: 10.1111/j.1365-313X.2005.02422.x. [DOI] [PubMed] [Google Scholar]
- 12.Zonia LE, Stebbins NE, Polacco JC. Essential role of urease in germination of nitrogen-limited Arabidopsis thaliana seeds. Plant Physiol. 1995;107:1097–1103. doi: 10.1104/pp.107.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakagawa A, Sakamoto S, Takahashi M, Morikawa H, Sakamoto A. The RNAi-mediated silencing of xanthine dehydrogenase impairs growth and fertility and accelerates leaf senescence in transgenic Arabidopsis plants. Plant Cell Physiol. 2007;48:1484–1495. doi: 10.1093/pcp/pcm119. [DOI] [PubMed] [Google Scholar]
- 14.Desimone M, Catoni E, Ludewig U, Hilpert M, Schneider A, Kunze R, Tegeder M, Frommer WB, Schumacher K. A novel superfamily of transporters for allantoin and other oxo derivatives of nitrogen heterocyclic compounds in Arabidopsis. Plant Cell. 2002;14:847–856. doi: 10.1105/tpc.010458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Todd CD, Polacco JC. AtAAH encodes a protein with allantoate amidohydrolase activity from Arabidopsis thaliana. Planta. 2006;223:1108–1113. doi: 10.1007/s00425-006-0236-x. [DOI] [PubMed] [Google Scholar]
- 16.Yang J, Han KH. Functional characterization of allantoinase genes from Arabidopsis and a nonureide-type legume black locust. Plant Physiol. 2004;134:1039–1049. doi: 10.1104/pp.103.034637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sprent JI, James EK. Legume evolution: where do nodules and mycorrhizas fit in? Plant Physiol. 2007;144:575–581. doi: 10.1104/pp.107.096156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reumann S, Babujee L, Ma C, Wienkoop S, Siemsen T, Antonicelli GE, Rasche N, Luder F, Weckwerth W, Jahn O. Proteome analysis of Arabidopsis leaf peroxisomes reveals novel targeting peptides, metabolic pathways and defense mechanisms. Plant Cell. 2007;19:3170–3193. doi: 10.1105/tpc.107.050989. [DOI] [PMC free article] [PubMed] [Google Scholar]