Abstract
Studies on direct and indirect defenses of lima bean (Phaseolus lunatus L.) revealed a quantitative trade-off between cyanogenesis and the total quantitative release of herbivore-induced volatile organic compounds (VOCs). In this addendum we focus on the qualitative variability in the VOC bouquets. We found intraspecific and ontogenetic variation. Five out of eleven lima bean accessions lacked particular VOCs in the bouquets released from secondary and/or primary leaves. These compounds (cis-3-hexenyl acetate, methyl salicylate and methyl jasmonate) can induce and prime indirect defenses in neighboring plants. Thus, the variability in VOC quality as described here might have substantial effects on plant-plant communication.
Key words: indirect defense, herbivore-induced plant volatiles, Phaseolus lunatus, trade-off, tritrophic interactions, plant-plant communication
Plants can be attacked by multiple enemies and accordingly have evolved multiple defense strategies comprising direct and indirect mechanisms. Lima bean (Fabaceae: Phaseolus lunatus L.) represents a well established experimental plant for the analysis of three defenses: herbivore-induced volatile organic compounds (VOCs), extrafloral nectar (EFN) and plant cyanogenesis. Herbivore-induced VOCs have manifold functions associated to indirect plant defenses. For example, VOCs attract arthropod predators or parasitoids and thus can act as an indirect defense.1–5 They also may be perceived by neighboring, yet-undamaged plant individuals (plant-plant signaling) or plant parts (within-plant signaling) and they prime or induce defensive responses.6 VOC-exposed plants may upregulate the secretion of EFN7–9 or the release of VOCs.10 In addition to these indirect defenses, lima bean shows cyanogenesis as a direct defense. Cyanogenesis means the release of toxic hydrogen cyanide (HCN) from preformed precursors in response to cell damage11 and is considered a constitutive direct defense against herbivores12 (but see ref. 13).
Recently we demonstrated that cyanogenesis and total release of VOC in lima bean are negatively correlated to each other.14 Accessions characterized by strong cyanogenesis in secondary leaves released little amounts of VOCs in response to jasmonic acid (JA) treatment, whereas total VOC production in accessions with low cyanide concentrations was high. Interestingly, these findings also held true on the ontogenetic level, since primary leaves generally showed low concentrations of cyanide and released high amounts of VOCs. However, the question remained unanswered whether these differences are merely of quantitative or also of qualitative nature. We therefore selected eleven accessions from the larger set of lima beans that had been used in our previous study and searched for qualitative differences in their VOC bouquets.
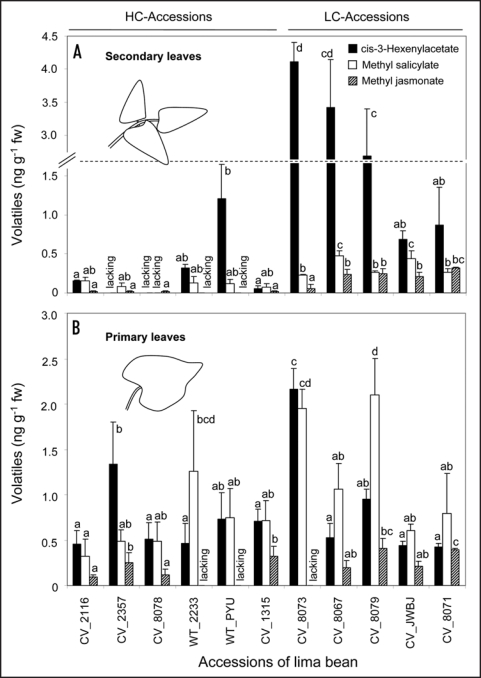
Eight out of the eleven volatiles that were quantified in our study were consistently released from both primary and secondary leaves of all accessions. However, five accessions indeed lacked individual compounds in secondary or primary leaves. For example, we did not detect cis-3-hexenyl acetate in the headspace of secondary leaves of accessions CV_2357 and CV_8078. The latter accession also lacked methyl salicylate in all individual plants analyzed (N = 10 plants). In contrast to secondary leaves, primary leaves of these accessions showed the complete blend of eleven VOCs. While CV_2357 and CV_8078 showed qualitative variability in VOC blends depending on leaf developmental stage, the accessions WT_2233 and WT_PYU consistently lacked methyl jasmonate in both, secondary and primary leaves. Strikingly, accession CV_8073, which was characterized by high quantitative release of cis-3-hexenyl acetate and methyl salicylate from secondary and primary leaves, showed a very low release of methyl jasmonate from secondary leaves—and lacked this compound completely in the headspace of primary leaves (Fig. 1).
Figure 1.
Qualitative variation in VOC mixtures of lima bean. Secondary (A) and primary leaves (B) of cultivated (CV) and wildtype (WT) lima beans characterized by high (HC) or low (LC) concentration of cyanogenic precursors in secondary leaves were analysed for release of three selected VOC compounds. Volatiles were collected in a closed-loop stripping over an experimental period of exactly 24 hrs. Values shown represent the mean (±SE) of five plants per accession. Different letters on top of the columns indicate means that differ significantly (according LSD post-hoc analysis after one-way ANOVA. Statistical analyses were conducted separately for each compound and each leaf stage).
In addition to qualitative differences, the quantitative release of volatiles was significantly different among the accessions. This holds true for the three compounds we have focused on in this addendum (Fig. 1) as well as for all eleven volatiles that we have quantified in our previous study (data not shown). However, while quantitative release of total VOCs from secondary leaves was negatively correlated to their cyanide concentration,14 the qualitative differences in VOC composition were not that strictly correlated to cyanogenesis. Accessions CV_2357, CV_8078, WT_2233 and WT_PYU were characterized by high cyanogenic secondary leaves—and lacked at least one compound. However, also the generally low cyanogenic primary leaves did not always show the complete VOC blend (Fig. 1B). These findings demonstrate a high qualitative variability of VOC composition that did depend neither on cyanogenic leaf features nor on leaf ontogeny.
So far it remains elusive whether the observed intraspecific and ontogenetic variability is of relevance in natural systems, but ecological effects are highly likely. For some tritrophic systems an intriguing degree of sophistication in the communication between plants and the third trophic level has been demonstrated: bouquet compositions can provide specific information on the identity of the attacking herbivore and, hence, on its suitability for the prey-seeking carnivores.15 Variability in the composition of VOC compositions as observed for lima bean (and as also known for corn, cotton or cabbage16–20) may compromise the reliability of herbivore-specific signals across a range of plant genotypes,21 although the ability of parasitoids to learn and associate successful foraging and egg-laying experience with the encountered odor pattern may help them in part overcome this problem.22,23
In contrast to tritrophic interactions, the efficiency of volatiles in plant-plant signaling appears more restricted to specific compounds. Recent studies on lima bean demonstrated that cis-3-hexenyl acetate causes priming or induction of extrafloral nectar.7,24 In other plant species, the release of gaseous methyl jasmonate in response to herbivore attack has been demonstrated to induce the synthesis of proteinase-inhibitors, which represent an efficient defense against herbivores.6,25 Methyl salicylate has been reported to be an important elicitor of resistance responses directed towards pathogens and herbivores26,27 and as a carnivore-attractant.28,29 Thus, the quantitative variation or a complete lacking of single biological active compounds of volatile blends may have dramatic effects on ecological interactions. We suggest that different defense strategies may be realized in these lima bean accessions: some genotypes have evolved strong cyanogenesis in secondary leaves and now ‘pay’ for this efficient direct defense with reduced or lost abilities for indirect defense and/or plant-plant communication, while others invest less in cyanogenesis and are more “communicative” concerning the third trophic level and conspecifics.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/6693
References
- 1.McCall P, Turlings TCJ, Lewis WJ, Tumlinson JH. Role of plant volatiles in host location by the specialist parasitoid Microplitis croceipes Cresson (Braconidae: Hymenoptera) J Insect Behav. 1993;6:625–639. [Google Scholar]
- 2.Steinberg S, Dicke M, Vet LEM. Relative importance of infochemicals from first and second tritrophic level in long-range host location by the larval parasitoid Cotesia glomerata. J Chem Ecol. 1993;19:23–28. doi: 10.1007/BF00987470. [DOI] [PubMed] [Google Scholar]
- 3.Stowe MK, Turlings TCL, Loughrin JH. The chemistry of eavesdropping, alarm and deceit. Proc Natl Acad Sci USA. 1995;92:23–28. doi: 10.1073/pnas.92.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turlings TCJ, Loughrin JH, McCall PJ. How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc Natl Acad Sci USA. 1995;92:4169–4174. doi: 10.1073/pnas.92.10.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heil M. Indirect defence via tritrophic interactions. New Phytol. 2008;178:41–61. doi: 10.1111/j.1469-8137.2007.02330.x. [DOI] [PubMed] [Google Scholar]
- 6.Heil M, Ton J. Long-distance signalling in plant defence. Trends Plant Sci. 2008;13:264–272. doi: 10.1016/j.tplants.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Kost C, Heil M. Herbivore-induced plant volatiles induce an indirect defence in neighbouring plants. J Ecol. 2006;94:619–628. [Google Scholar]
- 8.Heil M, Silva Bueno JC. Within-plant signalling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci USA. 2007;104:5467–5472. doi: 10.1073/pnas.0610266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heil M, Silva Bueno JC. Herbivore-induced volatiles as rapid signals in systemic plant responses. How to quickly move the information? PS & B. 2007;2:191–193. doi: 10.4161/psb.2.3.4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ton J, D'Allesandro M, Jourdie V, Jakab G, Karlen D, Held M, Mauch-Mani B, Turlings TCJ. Priming by airborne signals boosts direct and indirect resistance in maize. Plant J. 2007;49:16–26. doi: 10.1111/j.1365-313X.2006.02935.x. [DOI] [PubMed] [Google Scholar]
- 11.Møller BL, Seigler DS. Biosynthesis of cyanogenic glucosides, cyanolipids and related compounds. In: Singh BK, editor. Plant amino acids, biochemistry and biotechnology. New York: Marcel Dekker; 1999. pp. 563–609. [Google Scholar]
- 12.Patton CA, Ranney TG, Burton JD, Wallenbach JF. Natural pest resistance of Prunus taxa to feeding by adult Japanese beetles—role of endogenous allelochemicals in host plant resistance. J Am Soc Hortic Sci. 1997;122:668–672. [Google Scholar]
- 13.Ballhorn DJ, Heil M, Lieberei R. Phenotypic plasticity of cyanogenesis in lima bean Phaseolus lunatus—Activity and activation of β-glucosidase. J Chem Ecol. 2006;32:261–275. doi: 10.1007/s10886-005-9001-z. [DOI] [PubMed] [Google Scholar]
- 14.Ballhorn DJ, Kautz S, Lion U, Heil M. Trade-offs between direct and indirect defences of lima bean (Phaseolus lunatus) J Ecol. 2008;96:971–980. doi: 10.1111/j1365-2745.2008.01404x. [DOI] [Google Scholar]
- 15.De Moraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH. Herbivore-infested plants selectively attract parasitoids. Nature. 1998;393:570–573. [Google Scholar]
- 16.Turlings TCJ, McCall PJ, Alborn HT, Tumlinson JH. An elicitor in caterpillar secretions that induces corn seedlings to emit chemical signals attractive to parasitic wasps. J Chem Ecol. 1993;19:411–425. doi: 10.1007/BF00994314. [DOI] [PubMed] [Google Scholar]
- 17.Turlings TCJ, Bernasconi ML, Bertossa R, Bigler F, Caloz G, Dorn S. The induction of volatile emissions in maize by three herbivore species with different feeding habits: possible consequences for their natural enemies. Biol Control. 1998;11:122–129. [Google Scholar]
- 18.Mattiacci L, Dicke M, Posthumus MA. Induction of parasitoid attracting synomone in Brussel sprouts plants by feeding of Pieris brassicae larvae: role of mechanical damage and herbivore elicitor. J Chem Ecol. 1994;20:2229–2247. doi: 10.1007/BF02033199. [DOI] [PubMed] [Google Scholar]
- 19.Loughrin JH, Manukian A, Heath RR, Tumlinson JH. Volatiles emitted by different cotton varieties damaged by feeding beet armyworm larvae. J Chem Ecol. 1995;21:1217–1227. doi: 10.1007/BF02228321. [DOI] [PubMed] [Google Scholar]
- 20.Gouinguené S, Degen T, Turlings TCJ. Variability in herbivore-induced odour emissions among maize cultivars and their wild ancestors (teosinte) Chemoecol. 2001;11:9–16. [Google Scholar]
- 21.Degen T, Dillmann C, Marion-Poll F, Turlings TCJ. High genetic variability of herbivore-induced volatile emission within a broad range of maize inbred lines. Plant Physiol. 2004;135:1928–1938. doi: 10.1104/pp.104.039891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vet LEM, Lewis WJ, Cardé RT. Parasitoid foraging and learning. In: Cardé RT, Bell WJ, editors. Chemical Ecology of Insects. New York: Chapman & Hall; 1995. pp. 65–101. [Google Scholar]
- 23.De Boer JG, Snoeren TAL, Dicke M. Predatory mites learn to discriminate between plant volatiles induced be prey and nonprey herbivores. Anim Behav. 2005;69:869–879. [Google Scholar]
- 24.Heil M, Lion U, Boland W. Defense-inducing volatiles: In search of the active motif. J Chem Ecol. 2008;34:601–604. doi: 10.1007/s10886-008-9464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farmer EE, Ryan CA. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci USA. 1990;87:7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shulaev V, Silverman P, Raskin I. Airborne signalling by methyl salicylate in plant pathogen resistance. Nature. 1997;385:718–721. [Google Scholar]
- 27.Schlenk PM, Kazan K, Wilson I, Anderson J, Richmond T, Sommerville SC, Manners JM. Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci USA. 2000;97:11655–11660. doi: 10.1073/pnas.97.21.11655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.James DG. Synthetic herbivore-induced plant volatiles as field attractants for beneficial insects. Environ Entomol. 2003;32:977–982. doi: 10.1603/0046-225X-37.6.1410. [DOI] [PubMed] [Google Scholar]
- 29.Zhu JW, Park KC. Methyl salicylate, a soybean aphid-induced plant volatile attractive to the predator Coccinella septempunctata. J Chem Ecol. 2005;31:1733–1746. doi: 10.1007/s10886-005-5923-8. [DOI] [PubMed] [Google Scholar]