Abstract
Recent large scale phosphoproteomics studies have helped identify many phosphorylation sites of both membrane and soluble proteins. In most cases the relevance of specific sites has yet to be established whereas in a small number of cases their potency in modulating protein activity is evident. With the increasing amount of data it is becoming clear that phosphosites are often conserved within protein families, pointing to generic regulatory mechanisms. In addition, such mechanisms may be conserved across species. In this addendum evidence is presented for these phenomena occurring in rice and Arabidopsis.
Key words: Arabidopsis, kinase, phosphoproteomics, rice
Introduction
Full scale sequencing of plant genomes not only allows investigations at the nucleic acid level but also freed the way to comprehensive proteomic analyses. Indeed, a large number of proteome studies has appeared in the past 5 to 6 years.
However, a ‘static’ picture of protein expression is limited in its value to understand physiological processes since ultimately, a phenotype is the result of protein activity which depends on a ‘higher order’ of regulation such as posttranslational modification and protein-protein interaction. Reversible protein phosphorylation by the action of kinases and phosphatases, is one of the most prominent mechanisms for the post-translational modulation of protein activity. The activity of many plant proteins has been shown to be subject to phosphorylation including metabolic, regulatory and transport proteins, and it is estimated that at least 30% of all proteins are subject to this kind of modification. With the advent of metal affinity column enrichment of phosphopeptides coupled to MS analysis the tools are available for comprehensive probing of plant phosphoproteomes.1,2 Although such ‘omics’ approaches can have many drawbacks, such as large numbers of false positives, the large scale identification of phosphorylation or phosphosites in plant proteins is likely to generate testable hypotheses for investigating regulation of their activity.
Recently high throughput phosphoproteomic techniques were applied to membrane fractions of Arabidopsis and rice by several groups.3–8 Our lab reported on many new phosphosites in Arabidopsis tonoplast enriched fractions and in the tonoplast and plasma membrane of rice.7,8
Phosphosites are Often Consered within Gene Families
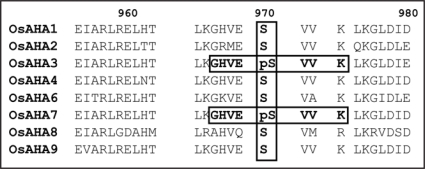
The cumulative data suggest that conservation of phosphosites within gene families occurs frequently. Within the category of membrane transporters, evidence of conservation was particularly strong within the AHA, PIP and NHX families in rice and the CLC, KUP and NHX families in Arabidopsis. For example, two identical C-terminal phosphopeptides (GHEVpSVVK) were detected in the plasma membrane H+-ATPases, OsAHA3 and OsAHA10 (Fig. 1). This motif was found in a highly conserved region that contains a Ser residue at position 970 in all rice AHA proteins (apart from AHA10 which is much shorter). The data therefore suggest that this motif may provide a phosphorylation site in all AHA isoforms.
Figure 1.
In rice plasma membrane H+-ATPases, two overlapping phosphosites are detected in OsAHA3 and OsAHA7. The phosphorylation residue is a Ser at position 970 which is highly conserved across all rice AHA isoforms apart from OsAHA10 which has a shorter C-terminus (not shown).
Reversible phosphorylation of P-type ATPases at a C-terminal threonine in the 14-3-3 protein binding domain is well known to change enzyme activity but the relevance of these newly detected phosphosites has yet to be tested. Nevertheless, recently identified new phosphosites in the C-terminus of AtAHA1/AHA2 were shown to greatly affect pumping capacity of this protein.5
Within the rice PIP (plasma membrane intrinsic protein) family a motif was found around a conserved Ser residue at position 286. This 8–12 residue peptide was found in four isoforms of the PIP2 subfamily of aquaporins which contains eight isoforms in total. Similarly, several overlapping motifs were found in the Arabidopsis KUP (K+ transporter) family. In most instances, the functional significance of these specific phosphosites has yet to be confirmed though general mechanisms of phosphorylation dependent activity may be known. For example, phosphorylation of plant aquaporins such as PIPs, greatly affects membrane water permeability and in yeast KUP-type (TRK1) transporters are modulated by phosphorylation in response to K+ starvation.9 In a few cases the effect of phosphorylation events has been detailed: The Arabidopsis ammonium transporter AMT1;1 contains a threonine residue at position 460 as the phosphorylation target and this was shown to be critical in the allosteric regulation of the AMT1;1 oligomer activity.10
Many Phosphosites May be Conserved Across Species
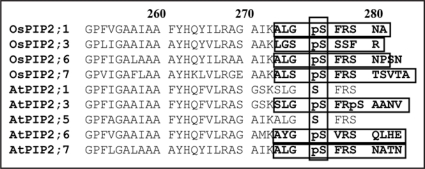
With the emergence of data from multiple species, comparative observations can be made. In both Arabidopsis and rice NHX families, phosphosites were detected, but in different positions. However, the motif in rice has a Ser at position 471 which contains a Thre in the Arabidopsis ortholog and therefore may point to conservation across species. The phosphosite with a Thre residue at position 460 in AtAMT1;1, which is critical for its activity, was found to have an identical counterpart in rice.8 It is thus tempting to assume that phosphorylation at this site in rice could have similar regulatory significance. In Arabidopsis, PIP2;6 was found to have a C-terminal phosphosite.5,7 This site not only overlaps perfectly with a phosphosite in the rice orthologue OsPIP2;6 but is also detected in many other isoforms of the PIP2 subfamily8 (Fig. 2). Interestingly, OsPIP2;6 was detected as a phosphorylated protein in shoots but in non-phosphorylated form in roots.8
Figure 2.
Common phosphorylation sites in Arabidopsis and rice PIP aquaporins. Four overlapping C-terminal phosphopeptides were found in OsPIP2;1, OsPIP2;3, OsPIP2;6 and OsPIP2;7 that cover a highly conserved serine at position 286. Very similar phosphosites, at an identical position, were reported by several groups6,7 in Arabidopsis PIP2 isoforms.
Membrane (phospho) proteomics preparations also include many non-integral membrane proteins. These can be associated with membranes for example through myristoyl or palmitoyl moieties and are therefore often not removed by carbonate washes. Amongst these a large number of kinases can be found which in our work totalled around 40 different proteins from Arabidopsis and rice membrane fractions. Analysis of phosphosites in these showed that a specific class of kinases, Ca-dependent kinases (CDPKs or CPKs), may also show conservation of phosphosites. CDPKs contain several EF hands that can bind Ca2+ and this interaction promotes a conformational change that leads to kinase activity. In addition to calcium, CDPKs are also regulated by phospholipids and 14-3-3 proteins.11 A peptide in the N terminus of CPK8 (SNPFYpSEAYTTNGpSGTGFK) was also identified in CPK7 by Benschop et al.4 studying the elicitor signalling phosphoproteome. Figure 3 shows a highly conserved region of CDPKs that contains the catalytic and activation loop of the protein. Within this domain another phosphopeptide (LGpSK-LTESEIK) was found in AtCPK29. Most other CDPKs (9 out of 11) from both Arabidopsis and rice show a Ser/Thre at the same position. A further, but less well conserved Ser residue is part of the EEFCASALpSVY phosphopeptide found in AtCRK1. These data suggest there may be common residues in Arabidopsis and rice CDPKs for phosphorylation. However, in contrast to for examples MAP kinases, there is as yet no evidence that phosphorylation of CDPKs impacts on their activity
Figure 3.
Amongst protein kinases detected in Arabidopsis and rice membrane preparations, the Ca2+-dependent kinase (CDPK) CPK29 shows a phosphosite with a phosphorylated serine. The motif is in the well conserved catalytic domain of the CDPK family which carries a Ser or Thre amino acid in most isoforms.
Conclusions and Outlook
Although still relatively new, large scale phosphoproteomics studies have already yielded a wealth of information and provided important insights into the regulation of important proteins. Currently, most studies have only reported ‘snap shot’ recordings of the phosphoproteome but increasingly papers are appearing that detail the dynamic changes in protein phosphorylation in response to various stimuli. The latter are obviously far more powerful in revealing the details of cellular regulatory processes. Unfortunately, in either case a demonstration of a direct impact of specific phosphorylation events on protein activity is usually lacking since this is often laborious and can be technically demanding. Similarly, identification of specific kinases that interact with the identified phosphoproteins is a large task ahead. Nevertheless, phosphoproteomic approaches will greatly aid in understanding signalling and regulatory networks1,2 and with the growing mass of data, bioinformatic analyses will become more lucrative and could generate in silico predictions about the involvement of specific types of kinases and their putative substrates.
and
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/6721
References
- 1.Peck SC. Phosphoproteomics in Arabidopsis: moving from empirical to predictive science. J Exp Bot. 2006;57:1523–1527. doi: 10.1093/jxb/erj126. [DOI] [PubMed] [Google Scholar]
- 2.van Bentem SD, Hirt H. Using phosphoproteomics to reveal signalling dynamics in plants. Trends Plant Sci. 2007;12:404–411. doi: 10.1016/j.tplants.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Nuhse TS, Bottrill AR, Jones AME, Peck SC. Quantitative phosphoproteomic analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. Plant J. 2007;51:931–940. doi: 10.1111/j.1365-313X.2007.03192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benschop JJ, Mohammed S, O'Flaherty M, Heck AJR, Slijper M, Menke FLH. Quantitative phosphoproteomics of early elicitor signaling in Arabidopsis. Mol Cell Proteomics. 2007;6:1198–1214. doi: 10.1074/mcp.M600429-MCP200. [DOI] [PubMed] [Google Scholar]
- 5.Niittylae T, Fuglsang AT, Palmgren MG, Frommer WB, Schulze WX. Temporal analysis of sucrose-induced phosphorylation changes in plasma membrane proteins of Arabidopsis. Mol Cell Proteomics. 2007;6:1711–1726. doi: 10.1074/mcp.M700164-MCP200. [DOI] [PubMed] [Google Scholar]
- 6.Nuhse TS, Stensballe A, Jensen ON, Peck SC. Phosphoproteomics of the Arabidopsis plasma membrane and a new phosphorylation site database. Plant Cell. 2004;16:2394–2405. doi: 10.1105/tpc.104.023150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whiteman S, Serazetdinova L, Jones A, Sanders D, Peck S, Maathuis FJM. Identification of novel proteins and phosphorylation sites in the vacuolar membrane of Arabidopsis thaliana. Proteomics. 2008;8:3536–3547. doi: 10.1002/pmic.200701104. [DOI] [PubMed] [Google Scholar]
- 8.Whiteman S, Nühse TS, Ashford DA, Sanders D, Maathuis FJM. A proteomic and phosphoproteomic analysis of Oryza sativa plasma membrane and vacuolar membrane. Plant J. 2008;56:146–156. doi: 10.1111/j.1365-313X.2008.03578.x. [DOI] [PubMed] [Google Scholar]
- 9.Thornton G, Wilkinson CRM, Toone WM, Jones N. A novel pathway determining multidrug sensitivity in Schizosaccharomyces pombe. Genes Cells. 2005;10:941–951. doi: 10.1111/j.1365-2443.2005.00891.x. [DOI] [PubMed] [Google Scholar]
- 10.Loque D, Lalonde S, Looger LL, von Wiren N, Frommer WB. A cytosolic trans-activation domain essential for ammonium uptake. Nature. 2007;446:195–198. doi: 10.1038/nature05579. [DOI] [PubMed] [Google Scholar]
- 11.Li AL, Zhu YF, Tan XM, Wang X, Wei B, Guo HZ, Zhang ZL, Chen XB, Zhao GY, Kong XY, Jia JZ, Mao L. Evolutionary and functional study of the CDPK gene family in wheat (Triticum aestivum L.) Plant Mol Biol. 2008;66:429–443. doi: 10.1007/s11103-007-9281-5. [DOI] [PubMed] [Google Scholar]