Abstract
Response of root system architecture to nutrient availability is an essential way for plants to adapt to soil environments. Nitrogen can affect root development either as a result of changes in the external concentration, or through changes in the internal nutrient status of the plant. Low soil N stimulates root elongation in maize. Recent evidence suggests that plant hormones auxin and cytokinin, as well as NO signaling pathway, are involved in the regulation of root elongation by low nitrogen nutrition.
Key words: nitrogen, root growth, auxin, cytokinin, NO
Nitrogen acquisition is determined by N demand for plant growth. At low N stress, N demand for maximum plant growth rate is not matched by plant N uptake. To acquire adequate N, plants may increase root length density to explore a larger soil volume and/or increase N uptake activity. High root density is also an important root trait for competition with soil microorganisms.1 Since nitrate is a highly mobile, non-adsorbing ion, theoretic analysis predicts that its uptake is not limited by transport through soil, and a small root system is sufficient for nitrate acquisition.2–4 In field conditions, however, genotypes that are efficient in N acquisition generally had a larger root system and higher root length density.5,6 Under conditions of insufficient N supply, N mass flow to roots may not be adequate to meet the N demand for plant growth. Even in N-sufficient soils, various soil constraints (low water content, etc) may reduce the N mass flow rate. In these cases, large root size and high density will be very important for the utilization of the spatially distributed N, especially newly mineralized N, and the competition for organic N with soil microorganisms.7,8
The development of lateral roots in Arabidopsis in response to nitrate supply has been widely studied.9 Less attention has been paid to primary root growth in response to N, possibly because root elongtion is insensitive to increased N supply in Arabidopsis.10,11 In maize, however, root elongation was sigificantly promoted by suboptimal N supply, and inhibited by overdose supply of N (Fig. 1).12,13 Until recently less is known about the underlying physiological mechansms. It is well documented that cytokinin is a root-to-shoot signal communicating N availability in addition to nitrate itself.14 Exogenous cytokinin application suppresses the elongation of primary roots.15 Recent work in Arabidopsis overexpressing cytokinin synthase (IPT) demonstrate that long-term CK overproduction inhibited primary root elongation by reducing quantitative parameters of primary root meristem.16 By comparing two maize inbred lines whose root elongation had a differential response to low N stress, it was found that the change of cytokinin content in roots was closely related to low-N induced root elongation.13 In the N-sensitive genotype 478, cytokinin (Zeatin + Zeatin riboside) content was significantly lower at low N condition. While in N-insensitive genotype Wu312, cytokinin content was hardly affected at various N supplies. Higher N supply shortened the distance from root apex to the first visible lateral roots, a phenomenen similar to that caused by exogenous cytokinins. Furthermore, exogenous cytokinin 6-benzylaminopurine (6-BA) completely reversed the stimulatory effect of low nitrate on root elongation. All the data suggests that the inhibitory effect of high concentration of nitrate on root elongation is, at least in part, mediated by increased cytokinin level in roots.
Figure 1.
Root elongation is inhibited at high nitrate supply.
Auxin regulates many cellular responses crucial for plant development. Auxin plays a key role in establishing and elaborating patterns in root meristems.17,18 Root elongation of Arabidopsis is enhanced by exogenous auxin at low concentrations, but is inhibited at high concentrations.19 In an earlier report, a high external nitrate supply (8 mM) did cause a 70% decrease in the auxin concentration of the root in soybean.20 In maize, inhibition of root growth by high nitrate was found closely related to the reduction of IAA levels in roots and exogenous NAA and IAA restored primary root growth in high nitrate concentrations.21 Interesting, it was found that auxin concentrations in phloem exudates were reduced by a greater nitrate supply, suggesting that shoot-to-root auxin transport may be inhibited by high N supply. Considering the antagonism between auxin and cytokinin.22 it was possible that, by increasing the cytokinin level and decreasing the auxin level, high nitrate supply may have negative influences on root apex activity so that root apical dominance is weakened and, therefore, root elongation is suppressed and lateral roots grow closer to the root apex.
Nitric oxide (NO) is emerging as an important messenger molecule associated with many biochemical and physiological processes in plants. The involvement of NO in IAA-induced adventitious root development has also been reported.23 Given that nitrate is a substrate for NR-catalysed NO production, and root development and growth are closely related to NO, it is expected that NO may play a role in nitrate-dependent root growth. Surprisingly, endogenous levels of NO in the root apices of maize seedlings grown in high nitrate solution were much lower than those in apices grown in low nitrate. The nitrate-induced inhibition of root elongation in maize was markedly reversed by treatments of the roots with a NO donor (SNP) and IAA.24 These data suggest that the arrest of root elongation by high levels of external nitrate concentrations may result from an alteration of endogenous NO levels in root apical cells. NR mediated NO production is unlikely to be involved in the nitrate-dependent NO production and root elongation because NR activity is lower at low N supply. A NO synthase (NOS) inhibitor reduced root elongation in maize plants grown in the low-nitrate medium, suggest that NOS activity may be inhibited in plants grown in high-nitrate solution, thus leading to a reduction of the endogenous NO levels.
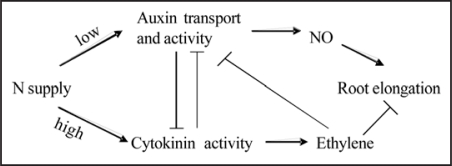
Taken together, high nitrogen supply increases cytokinin level, but decreases auxin and NO levels in roots of maize. Besides, it was well documented ethylene has a negative effect on root elongation of various plants.25–27 Exogenous supply of cytokinin increase ethylene production (Stenlid 1982; Bertell et al., 1990). Recently, it was demonstrated in Arabidopsis that auxin transport from the root apex via the lateral root cap is required for ethylene-mediated inhibition of root growth.28 Therefore, a complex multiple siganlling pathways may be involved in N-mediated root elongation (Fig. 2). Further study is required to understand how these pathways interact with each other to reduce root elongation in response to high nitrate supply.
Figure 2.
A simplified model explaining nitogen-mediated root elongation in maize.
Acknowledgments
We would like to thank Daniel Schachtman for helpful comments on our manuscript. The work was supported by the Ministry of Science and Technology of China (No. 2005CB120900) and the NNSFC (No.30771289). F. Zhang is supported by Program for Changjiang Scholars and Innovative Research Team in University (IRT0511).
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/6800
References
- 1.Hodge A. The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol. 2004;162:9–24. [Google Scholar]
- 2.Burns IG. Influence of the spatial distribution of nitrate on the uptake of N by plants: a review and a model for rooting depth. J Soil Sci. 1980;31:155–173. [Google Scholar]
- 3.Fitter AH, Stickland TR, Harvey ML, Wilson GW. Architectural analysis of plant root systems. 1. Architectural correlates of exploitation efficiency. New Phytol. 1991;118:375–382. [Google Scholar]
- 4.Robinson D, Rorison IH. Relationships between root morphology and nitrogen availability in a recent theoretical model describing nitrogen uptake from soil. Plant Cell Environ. 1983;6:641–647. [Google Scholar]
- 5.Mackay AD, Barber SA. Effect of nitrogen on root growth of two corn genotypes in the field. Agronomy J. 1986;78:699–703. [Google Scholar]
- 6.Wiesler F, Horst WJ. Root growth and nitrate utilization of maize cultivars under field conditions. Plant Soil. 1994;163:267–277. [Google Scholar]
- 7.Lawlor DW. Carbon and nitrogen assimilation in relation to yield: mechanisms are the key to understanding production systems. J Exp Bot. 2002;53:773–787. [PubMed] [Google Scholar]
- 8.Marschner H. Role of root growth, arbusular mycorrhiza, and root exudates for the efficiency in nutrient acquisition. Field Crops Res. 1998;56:203–207. [Google Scholar]
- 9.Zhang H, Rong H, Pilbeam D. Signalling mechanisms underlying the morphological responses of the root system to nitrogen in Arabidopsis thaliana. J Exp Bot. 2007;58:2338–2629. doi: 10.1093/jxb/erm114. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Jennings A, Barlow PW, Forde BG. Dual pathways for regulation of root branching by nitrate. PNAS. 1999;96:6529–6534. doi: 10.1073/pnas.96.11.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bao J, Chen F, Gu R, Wang G, Zhang F, Mi G. Lateral root development of two Arabidopsis auxin transport mutants, aux 1-7 and eir 1-1, in response to nitrate supplies. Plant Sci. 2007;173:417–425. [Google Scholar]
- 12.Chun L, Mi G, Li J, Zhang F, Chen F. Genetic analysis of maize root characteristics in response to low nitrogen stress. Plant Soil. 2005;276:369–382. [Google Scholar]
- 13.Tian Q, Chen F, Zhang F, Mi G. Possible involvement of cytokinin in nitrate-mediated root growth in maize. Plant Soil. 2005;277:185–196. [Google Scholar]
- 14.Takei K, Takahashi T, Sugiyama T, Yamaya T, Sakakibara H. Multiple routes communicating nitrogen availability from roots to shoots: a signal transduction pathway mediated by cytokinin. J Exp Bot. 2002;53:971–977. doi: 10.1093/jexbot/53.370.971. [DOI] [PubMed] [Google Scholar]
- 15.Stenlid G. Cytokinin as inhibitors of root growth. Physiol Plant. 1982;56:500–506. [Google Scholar]
- 16.Kuderová A, Urbánková I, Válková M, Malbeck J, Brzobohatý B, Némethová D, Hejátko J. Effects of Conditional IPT-Dependent Cytokinin Overproduction on root Architecture of Arabidopsis Seedlings. Plant Cell Physiol. 2008;49:570–582. doi: 10.1093/pcp/pcn029. [DOI] [PubMed] [Google Scholar]
- 17.Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- 18.Friml J. Auxin transport—Shaping the plant. Curr Opin Plant Biol. 2003;6:7–12. doi: 10.1016/s1369526602000031. [DOI] [PubMed] [Google Scholar]
- 19.Evans ML, Ishikawa HI, Estelle MA. Response of Arabidopsis roots to auxin studied with high temporal resolution: comparison of wild type and auxinresponse mutants. Planta. 1994;194:215–222. [Google Scholar]
- 20.Caba JM, Centeno ML, Fernandez B, Gresshoff PM, Ligero F. Inoculation and nitrate alter phytohormone levels in soybean roots: differences between a supernodulation mutant and the wild type. Planta. 2000;211:98–104. doi: 10.1007/s004250000265. [DOI] [PubMed] [Google Scholar]
- 21.Tian Q, Chen F, Liu J, Zhang F, Mi G. Inhibition of maize root growth by high nitrate supply is correlated to reduced IAA levels in roots. J Plant Physiol. 2008;165:942–951. doi: 10.1016/j.jplph.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Coenen C, Lomax TL. Auxin-cytokinin interactions in higher plants: old problem and new tools. Trends Plant Sci. 1997;2:351–356. doi: 10.1016/S1360-1385(97)84623-7. [DOI] [PubMed] [Google Scholar]
- 23.Pagnussat GC, Lanteri ML, Lamattina L. Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol. 2003;132:1241–1248. doi: 10.1104/pp.103.022228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao D, Tian QY, LI L, Zhang W. Nitric Oxide is involved in nitrate-induced inhibition of root elongation in Zea mays. Ann Bot. 2007;100:497–503. doi: 10.1093/aob/mcm142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berttell G, Bolander E, Eliassion Factors increasing ethylene production enhance the sensitivity of root growth to auxins. Physiol Plant. 1990;79:255–258. [Google Scholar]
- 26.Hahn A, Zimmermann R, Wanke D, Harter K, Edelmann HG. The root cap determines ethylene-dependent growth and developmen tin maize roots. Molecular plant. 2008;1:359–367. doi: 10.1093/mp/ssm027. [DOI] [PubMed] [Google Scholar]
- 27.Mulkey T, Kuzmanoff KM, EVANS ML. Promotion of growth and hydrogen ion efflux by auxin in roots of maize pretreated with ethylene biosynthesis inhibitors. Plant Physiol. 1982;70:186–188. doi: 10.1104/pp.70.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swarup R, Perry P, Hagenbeek D, Straeten D, Beemster GTS, Sandberg G, Bhalerao R, Ljung K, Bennetta MJ. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell. 2007;19:2186–2196. doi: 10.1105/tpc.107.052100. [DOI] [PMC free article] [PubMed] [Google Scholar]