Abstract
Innate immunity signaling pathways in both animals and plants are regulated by mitogen-activated protein kinase (MAPK) cascades. In a recent publication we show that MPK4 and its substrate MKS1 interact with WRKY33 in vivo, and that WRKY33 is released from complexes with MPK4 upon infection. Transcriptome analysis of a wrky33 loss-of-function mutant identified a subset of defense-related genes as putative targets of WRKY33. These genes include PAD3 and CYP71A13, which encode cytochrome P450 monoxygenases required for synthesis of the antimicrobial phytoalexin camalexin. Chromatin immunoprecipitation confirmed that WRKY33 bound the promoter of PAD3 when plants were inoculated with pathogens. Here we further discuss the involvement of two other targets of WRKY33, NUDT6 and ROF2 in defense responses against invading pathogens.
Key words: Arabidopsis, MAPK cascade, defense response, transcription factor, target gene
Plants are constantly exposed to external stimuli by a broad range of microbes and have evolved complicated immune systems to defend against invading pathogens.1,2 A common mechanism to convert such external stimuli into cellular responses is the activation of mitogen-activated protein kinase (MAPK) cascades. These cascades consist of MAPKKK-MAPKK-MAPK modules that play central roles in signal transduction mechanisms linking upstream receptors and downstream targets and leading to rapid activation of defense responses upon recognition of invading pathogens.3,4
Recently, MPK4 was shown to be part of a MAPK cascade including MEKK1 and MKK1/MKK2.5,6 MEKK1 acts downstream of the flagellin receptor FLS2 and is required with MKK1/MKK2 for activation of MPK4 by phosphorylation of conserved Thr and Tyr residues in the kinase activation loop.7–9 Previously, MKS1 was identified as a MPK4 substrate, and analysis of transgenic plants and transcript profiling indicated that MKS1 was required for full resistance in mpk4 mutants.10 MKS1 interacts with two transcription factors, WRKY25 and WRKY33, and may function as an adaptor linking MPK4 activity to WRKY-regulated gene expression. Interestingly, wrky33 mutants exhibit enhanced susceptibility to two necrotrophs, Botrytis cinerea and Alternaria brassicicola, whereas overexpression of WRKY33 increases resistance, suggesting a specific role of WRKY33 in plant defense against necrotrophic pathogens.11
To further understand how WRKY33 regulates gene expression, we employed expression profiling using ATH1 GeneChips (Affymetrix) to screen for putative target genes of WRKY33. From this, we identified four transcripts that accumulated in response to BTH (SA analogue) treatment in wild type, but not in wrky33.12 Two of these, PAD3 and CYP71A13, are strongly co-regulated13 and are required for synthesis of the antimicrobial compound camalexin important for resistance against necrotrophic pathogens. Furthermore, we show that MPK4 and MKS1 associate in a complex with WRKY33, and that activation of MPK4 and phosphorylation of MKS1 upon infection leads to a release of MKS1 and WRKY33 from MPK4, followed by recruiting WRKY33 to the promoter of PAD3.
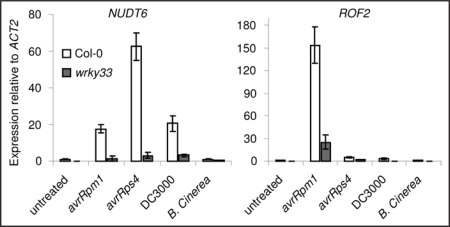
Two other putative targets identified from the microarray analysis are NUDT6, a member of the cytosolic Nudix hydrolase family14 and a peptidyl prolyl cis/trans isomerase (PPIase, named ROF215) that catalyze the cis/trans isomerization of peptide bonds adjacent to a proline.16 To further verify and elucidate the biological relevance of these two targets in defense, we performed real-time RT-PCR of wild-type and wrky33 mutants after infection with different pathogens. In wild-type Col-0 the expression of NUDT6 is barely detectable, but was found to be highly responsive to the biotrophic pathogen, Pseudomonas syringae (Fig. 1, Pst DC3000 or Pst DC3000 expressing the avrRpm1 or avrRps4 effector that triggers resistance through the host resistance genes RPM1 or RPS4, respectively). In contrast, this elevation of NUDT6 accumulation is impaired in wrky33 mutants. Expression of ROF2 is also responsive to Pst DC3000 avrRpm1, and impaired in wrky33, but is barely induced by Pst DC3000 or Pst DC3000 avrRps4 (Fig. 1). In contrast to PAD3 and CYP71A13, neither NUDT6 nor ROF2 expression is induced by B. cinerea infections, suggesting specific roles and potentially different mechanisms of regulating gene targets downstream WRKY33 dependent on different pathogens.
Figure 1.
Real-time PCR quantitation of NUDT6 and ROF2. Expression in Col-0 and wrky33 before or after treatment with Pst DC3000 for 21 h, with Pst DC3000 (avrRpm1) for 4 h, with Pst DC3000 (avrRps4) for 14 h, or with B. cinerea for 48 h. Samples were tested in triplicate and normalized to ACTIN2. Means ± SD are shown.
Previously, NUDT6 was shown to be pathogen responsive, and NUDT6 induction was found to depend on the defense regulators EDS1 and PAD4.17 Furthermore, the nudt7 mutant, a close homologue of NUDT6, shows constitutive resistance against P. syringae and Hyaloperonospora parasitica suggesting these two closely related NUDTs function as repressors of the basal defense response.18 ROF2 is a member of plant co-chaperones FKBPs that function in signal transduction and ROF2 has chaperone activity. Recent research suggests that ROF2 interacts with heat shock protein 90 (HSP90).15 Interestingly, the hsp90 mutant was shown to be impaired in RPM1-specified hypersensitive cell death (hypersensitive response; HR) and disease resistance.19 These data are in agreement with ROF2 being only responsive to Pst DC3000 expressing avrRpm1.
In summary, induction of both NUDT6 and ROF2 appear to be dependent on WRKY33 and both seem to be involved in defense responses upon recognition of invading pathogens. However, further studies are required to determine if these genes are direct targets of WRKY33, as was shown for PAD3.12
Acknowledgements
This study was supported by the Danish Research Councils (23020101, 23030076, 272050367 and 272060049).
Abbreviations
- BTH
benzothiadiazole
- SA
salicylic acid
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/6878
References
- 1.Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell. 2006;124:803–814. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 3.Nakagami H, Pitzschke A, Hirt H. Emerging MAP kinase pathways in plant stress signalling. Trends Plant Sci. 2005;10:339–346. doi: 10.1016/j.tplants.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Jonak C, Okresz L, Bogre L, Hirt H. Complexity, cross talk and integration of plant MAP kinase signalling. Curr Opin Plant Biol. 2002;5:415–424. doi: 10.1016/s1369-5266(02)00285-6. [DOI] [PubMed] [Google Scholar]
- 5.Suarez-Rodriguez MC, Adams-Phillips L, Liu Y, Wang H, Su SH, Jester PJ, Zhang S, Bent AF, Krysan PJ. MEKK1 is required for flg22-induced MPK4 activation in Arabidopsis plants. Plant Physiol. 2007;143:661–669. doi: 10.1104/pp.106.091389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu JL, Zhou L, Yun BW, Nielsen HB, Fiil BK, Petersen K, MacKinlay J, Loake GL, Mundy J, Morris PC. Arabidopsis mitogen-activated protein kinase kinases MKK1 and MKK2 have overlapping functions in defense signaling mediated by MEKK1, MPK4, and MKS1. Plant Physiol. 2008;148:1–12. doi: 10.1104/pp.108.120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen M, Brodersen P, Naested H, Andreasson E, Lindhart U, Johansen B, Nielsen HB, Lacy M, Austin MJ, Parker JE, Sharma SB, Klessig DF, Martienssen R, Mattsson O, Jensen AB, Mundy J. Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell. 2000;103:1111–1120. doi: 10.1016/s0092-8674(00)00213-0. [DOI] [PubMed] [Google Scholar]
- 8.Brodersen P, Petersen M, Bjorn Nielsen H, Zhu S, Newman MA, Shokat KM, Rietz S, Parker J, Mundy J. Arabidopsis MAP kinase 4 regulates salicylic acid- and jasmonic acid/ethylenedependent responses via EDS1 and PAD4. Plant J. 2006;47:532–546. doi: 10.1111/j.1365-313X.2006.02806.x. [DOI] [PubMed] [Google Scholar]
- 9.Caspersen MB, Qiu JL, Zhang X, Andreasson E, Naested H, Mundy J, Svensson B. Phosphorylation sites of Arabidopsis MAP kinase substrate 1 (MKS1) Biochim Biophys Acta. 2007;1774:1156–1163. doi: 10.1016/j.bbapap.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Andreasson E, Jenkins T, Brodersen P, Thorgrimsen S, Petersen NH, Zhu S, Qiu JL, Micheelsen P, Rocher A, Petersen M, Newman MA, Bjorn Nielsen H, Hirt H, Somssich I, Mattsson O, Mundy J. The MAP kinase substrate MKS1 is a regulator of plant defense responses. EMBO J. 2005;24:2579–2589. doi: 10.1038/sj.emboj.7600737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Z, Qamar SA, Chen Z, Mengiste T. Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J. 2006;48:592–605. doi: 10.1111/j.1365-313X.2006.02901.x. [DOI] [PubMed] [Google Scholar]
- 12.Qiu JL, Fiil BK, Petersen K, Nielsen HB, Botanga CJ, Thorgrimsen S, Palma K, Suarez-Rodriguez MC, Sandbech-Clausen S, Lichota J, Brodersen P, Grasser KD, Mattsson O, Glazebrook J, Mundy J, Petersen M. Arabidopsis MAP kinase 4 regulates gene expression through transcription factor release in the nucleus. EMBO J. 2008;27:2214–2221. doi: 10.1038/emboj.2008.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nafisi M, Goregaoker S, Botanga CJ, Glawischnig E, Olsen CE, Halkier BA, Glazebrook J. Arabidopsis cytochrome P450 monooxygenase 71A13 catalyzes the conversion of indole-3-acetaldoxime in camalexin synthesis. Plant Cell. 2007;19:2039–2052. doi: 10.1105/tpc.107.051383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogawa T, Ueda Y, Yoshimura K, Shigeoka S. Comprehensive analysis of cytosolic Nudix hydrolases in Arabidopsis thaliana. J Biol Chem. 2005;280:25277–25283. doi: 10.1074/jbc.M503536200. [DOI] [PubMed] [Google Scholar]
- 15.Aviezer-Hagai K, Skovorodnikova J, Galigniana M, Farchi-Pisanty O, Maayan E, Bocovza S, Efrat Y, von Koskull-Doring P, Ohad N, Breiman A. Arabidopsis immunophilins ROF1 (AtFKBP62) and ROF2 (AtFKBP65) exhibit tissue specificity, are heat-stress induced, and bind HSP90. Plant Mol Biol. 2007;63:237–255. doi: 10.1007/s11103-006-9085-z. [DOI] [PubMed] [Google Scholar]
- 16.Harrar Y, Bellini C, Faure JD. FKBPs: at the crossroads of folding and transduction. Trends Plant Sci. 2001;6:426–431. doi: 10.1016/s1360-1385(01)02044-1. [DOI] [PubMed] [Google Scholar]
- 17.Bartsch M, Gobbato E, Bednarek P, Debey S, Schultze JL, Bautor J, Parker JE. Salicylic acid-independent ENHANCED DISEASE SUSCEPTIBILITY1 signaling in Arabidopsis immunity and cell death is regulated by the monooxygenase FMO1 and the Nudix hydrolase NUDT7. Plant Cell. 2006;18:1038–1051. doi: 10.1105/tpc.105.039982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ge X, Li GJ, Wang SB, Zhu H, Zhu T, Wang X, Xia Y. AtNUDT7, a negative regulator of basal immunity in Arabidopsis, modulates two distinct defense response pathways and is involved in maintaining redox homeostasis. Plant Physiol. 2007;145:204–215. doi: 10.1104/pp.107.103374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hubert DA, Tornero P, Belkhadir Y, Krishna P, Takahashi A, Shirasu K, Dangl JL. Cytosolic HSP90 associates with and modulates the Arabidopsis RPM1 disease resistance protein. EMBO J. 2003;22:5679–5689. doi: 10.1093/emboj/cdg547. [DOI] [PMC free article] [PubMed] [Google Scholar]