Abstract
In health, emotions are integrated with autonomic bodily responses. Emotional stimuli elicit changes in somatic (including autonomic) bodily states, which feedback to influence the expression of emotional feelings. In patients with spinal cord injury (SCI), this integration of emotion and bodily arousal is partially disrupted, impairing both efferent generation of sympathetic responses and afferent sensory feedback of visceral state via the spinal cord. A number of theoretical accounts of emotion predict emotional deficits in SCI patients, particularly at the level of emotional feelings, yet evidence for such a deficit is equivocal. We used functional MRI (fMRI) and a basic emotional learning paradigm to investigate the expression of emotion-related brain activity consequent upon SC I. We scanned seven SCI patients and seven healthy controls during an aversive fear conditioning task. Subjects viewed randomized presentations of four angry faces. One of the faces (CS + arm) was associated with delivery of electrical shock to the upper arm on 50% of trials. This shock was painful to all subjects. A face of the same gender acted as a ‘safe’ control stimulus (CS − arm). In both control subjects and SCI patients, painful cutaneous stimulation of the arm evoked enhanced activity within components of a central pain matrix, including dorsal anterior cingulate, right insula and medial temporal lobe. However, SCI patients differed from controls in conditioning-related brain activity. SCI patients showed a relative enhancement of activity within dorsal anterior cingulate, periaqueductal grey matter (PAG) and superior temporal gyrus. Conversely, SCI patients showed relative attenuation of activity in subgenual cingulate, ventromedial prefrontal and posterior cingulate cortices to threat of painful arm stimulation (CS + arm > CS − arm). Our findings provide evidence for differences in emotion-related brain activity in SCI patients. We suggest that the observed functional abnormalities including enhanced anterior cingulate and PAG reflect central sensitization of the pain matrix, while decreased subgenual cingulate activity may represent a substrate underlying affective vulnerability in SCI patients consequent upon perturbation of autonomic control and afferent visceral representation. Together these observations may account for motivational and affective sequelae of SCI in some individuals.
Keywords: autonomic arousal, emotion, functional magnetic resonance, spinal cord injury
Introduction
Emotions are associated with the automatic generation of bodily responses, notably those mediated by the autonomic nervous system: changes in heart rate, blood pressure and bodily states contributes to emotional feelings: coupling of emotion with peripheral arousal is highlighted by influential theories of emotion, such as that of James and Lange, who argued that the central representation of bodily arousal states provides emotional colour to otherwise emotionless sensory experiences (James, 1884, 1894). More recently, Damasio and colleagues have provided evidence that peripheral arousal may influence emotion, behaviour and reasoning (Damasio, 1994, 1999; Bechara et al., 1996).
There is increasing interest in neuroanatomy supporting interactions between affect and bodily arousal states. Lesion studies suggest that damage to prefrontal brain regions, particularly orbital and medial prefrontal cortex compromises both generation of peripheral arousal responses and emotional guidance of behaviour (Bechara et al., 1996). Neuroimaging studies implicate anterior cingulate and amygdala in higher order and reflexive generation of autonomic arousal responses to emotional provoking stimuli (Fredrikson et al., 1998; Patterson et al., 2002; Critchley et al., 2000a, b, 2003; Phelps et al., 2001) and insula and OFC in mapping the consequential bodily responses. It is also noteworthy that all these regions have been shown to be sensitive to visceral changes (Craig, 2002; Critchley et al., 2001; 2002, 2004). Brainstem regions appear to provide a tonic homoeostatic autonomic control function (Critchley et al., 2001) and much is known from animal studies about these autoregulatory centres (Spyer, 1999). However, we do not yet have complete understanding of how peripheral arousal is integrated with, and influences, human emotional behaviour. In fact, it is likely that an integration of visceral afferent information and motivational processes underlying emotion occurs within a hierarchy of central structures (Damasio, 1999). Dorsal brainstem structures such as the parabrachial nucleus and periaqueductal grey matter (PAG) support an interface between afferent visceral sensations (including pain), descending autonomic control and ascending neuromodulatory pathways to subcortical and cortical ‘motivational’ centres (Tracey et al., 2002; Allen et al., 1991; Saper, 2002). Thalamus, cingulate, insula and OFC support interoceptive representations that underlie emotional feeling states (Lane et al., 1999; Critchley et al., 2001; 2004; Craig, 2002) and contribute to a general ‘pain matrix’ (Tracey et al., 2002; Rainville, 2002).
Spinal cord injury (SCI) is a devastating neurological disorder, affecting motor, sensory and autonomic systems. In the chronic stage, SCI is characterized by loss of motor and sensory function below the level of injury, which also contributes to a degree of autonomic dysfunction (Mathias and Frankel, 2002). SCI often results in permanent physical and emotional disabilities (Motha et al., 2003). The initial subjective reaction to SCI is depression, despair, bitterness and grief (Mueller, 1962). A high prevalence of anxiety and depressive symptoms persists over the following months (Kennedy and Rogers, 2000). If there is inadequate provision of psychological care during SCI rehabilitation, long-term psychiatric disturbances may ensue (Blanchard et al., 1990; Hickling and Blanchard, 1992; Brom et al., 1993). These sudomotor (sweat gland) activity are themselves associated with changes in regional cerebral activity (Critchley et al., 2000a, b). Subjective experience of these changes in altered emotional disturbances can reasonably be attributable only to the trauma, disability and adjustments of SCI. However, there is a longstanding debate as to whether the very transection of the spinal cord produces or modifies emotional experience as a consequence of decoupling brain from body. It should be noted that descriptions of clinical dissociated deficits are often confounded by mood, which renders this literature unsatisfactory in resolving this debate.
The ‘peripheral’ theory of emotion proposed by James and Lange (James, 1884, 1894) implies that patients with complete high SCI would show decreased emotional responsivity reflecting the absence of ‘emotional colour’ from central representation of bodily responses. While this view persists in clinical anecdotes, many case reports and studies have reported an absence of impairment in SCI patients on emotional tests (Dana, 1921; Lowe and Carroll, 1985; Chwalisz et al., 1988; Cobos et al., 2002; O'Carroll et al., 2003). This contrasts with growing recognition of the importance of ascending bottom-up influences on higher neural processes including emotion (Cameron, 2001, 2002; Bernston et al., 2003; Craig, 2003; Damasio, 1994, 1999), where there is a consensus that the degree to which bodily responses are perceived may modify the expression of emotional feelings in both healthy individuals (Weins et al., 2000) and patients with SCI (Chwalisz et al., 1988).
Our aim in this study was to examine the impact of SCI on central mechanisms of emotional processing. Specifically, SCI is associated with impaired generation of sympathetic autonomic responses and disruption of spinocerebral feedback of states of bodily arousal. We scanned patients with chronic SCI and healthy controls, using functional MRI (fMRI), during performance of an emotional learning task (fear conditioning) to test a hypothesis that absence of afferent spinal information would modulate the expression of brain activity associated with emotional processing.
Methods
Subjects
The study was approved by the Joint Ethics Committee of the National Hospital for Neurology and Neurosurgery and the Institute of Neurology and conformed to the guidelines of the Declaration of Helsinki. All subjects gave fully informed consent and were screened to ensure they were safe to undergo functional magnetic resonance scanning at 1.5 T. Seven patients of the National Hospital for Neurology and Neurosurgery with documented SCI were recruited along with seven control subjects to take part in a neuroimaging study at the Wellcome Department of Imaging Neuroscience. Before being scanned, SCI patients were given a complete neurological examination to determine the characteristics of their sensory and motor impairment following the American Spinal Injury Association (ASIA) Impairment Scale (Maynard et al., 1997). The clinical characteristics of the patients are detailed in Table 1. At the time of the experiment, autonomic dysfunction in all patients was expressed in loss of sweat function below the level of injury. No cardiovascular symptoms were reported at the time of the experiment, although autonomic dysreflexia was previously documented in two patients. Patients did not report active neuropathic pain nor any psychological nor psychiatric disturbances when questioned on direct interview [scores on Hamilton anxiety and depression scales (Hamilton, 1995) did not meet criteria for mood disorder]. One of the subjects (Patient 3) differed from the other SCI patients having suffered from idiopathic acute transverse myelitis which is an inflammatory disorder of the spinal cord resulting in motor, sensory and autonomic dysfunction (Transverse Myelitis Working Group, 2002).
Table 1.
Characteristics of patients with spinal cord injury
| SCI patient |
ASIA neurological level* |
ASIA grade |
Motor score (max 100) |
Sensory score |
Time from injury (weeks) |
Cause of injury |
|
|---|---|---|---|---|---|---|---|
| Pinprick (max 112) | Light touch (max 112) | ||||||
| P | T3 | A | 50 | 46 | 43 | 154 | RTA |
| P2 | C6 | B | 32 | 54 | 42 | 153 | Fall |
| P3 | T4 | D | 100 | 66 | 66 | 159 | TM |
| P4 | C4 | A | 28 | 32 | 32 | 14 | Fall |
| P5 | T5 | C | 50 | 6 | 6 | 332 | RTA |
| P6 | C7 | C | 5 | 32 | 32 | 242 | Sport |
| P7 | C8 | A | 50 | 3 | 3 | 259 | RTA |
TM = acute transverse myelitis. RTA = road traffic accident.
Neurological level: the most caudal segment with normal sensory and motor function.
The ASIA Impairment Scale (Maynard et al., 1997) classifies patients with SCI as: A = complete, when no sensory or motor function is preserved in the sacral segments S4-S5; B = incomplete, when sensory but no motor function is preserved below the neurological level and includes the sacral segments S4-S5; C = incomplete, when motor function is preserved below the neurological level and more than half of key muscles below the neurological level have a muscle grade (MRC scale) <3; D = incomplete; when motor function is preserved below the neurological level and at least half of key muscles below the neurological level have a muscle grade ~3; E = normal, when sensory and motor functions are normal.
Experimental design and task
A structural brain fMRI scan was performed in all subjects to exclude possible brain abnormalities. Each participant underwent one fMRI scanning session in which they made gender discrimination button-press responses to randomized visual presentations of four face stimuli, depicting two male and two female identities bearing angry expressions. The experiment was a classical Pavlovian fear-conditioning paradigm of faces to painful aversive stimulation. Electrical stimulation was achieved following established methods (Seymour et al., 2004) using a custom-built electrical stimulator delivering 20 or 100 Hz trains of electrical pulses (4 ms square waveform pulses, 1 s duration) through a silver chloride electrode. Current levels (ranging between 0.1 and 6 mA) and stimulation frequencies were tailored empirically to each subject to produce subjective pain, but no strong withdrawal reflex.
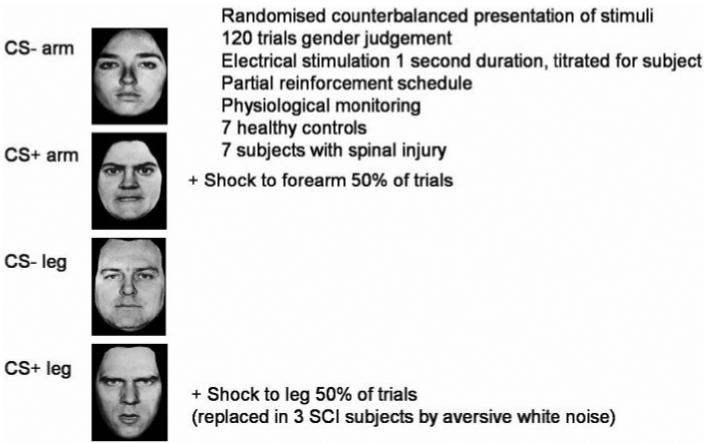
Subjects viewed face stimuli on a projection screen during scanning. Stimuli were presented for 500 ms with an inter-trial interval of 3.2 s. A total of 240 stimuli were presented with an additional 60 null events. The design of the experiment is illustrated in Fig. 1. Presentation of two of the faces (one female, one male) was coupled with delivery of aversive stimulation (US) on half the trials. One face was paired with electrical stimulation of the arm, which was painful to all subjects (CS + arm). The gender/identity of this face was counterbalanced across subjects. A face of the same gender acted as a control (safe) stimulus and was never paired with stimulation (CS − arm). In all the control subjects and in four of the patients a second face was paired with electrical stimulation ofthe leg (CS + leg) and there was a corresponding control (safe CS − leg) stimulus. In fact SCI subjects, in contrast to controls, were completely unable to feel the electrical stimulation applied to the leg. To exclude a possibility that the ‘globally greater’ aversive experience of controls might confound interpretation of group differences, the ‘CS + leg’ stimulus was associated to auditory (headphone) delivery of a burst of aversive white noise [500 ms, 100 dB (Grade A)] for the remaining three SCI patients studied (Patients 5–7; Table 1).
Fig. 1.
Task design. Subjects were scanned during Pavlovian aversive conditioning. Face stimuli (two female, two male, all with angry expressions) were presented in randomized order on a video monitor and the subject judged the sex of each face via two-choice button press response. In a 50% reinforcement schedule, two of the faces (CS + arm, CS + leg) were associated with delivery of aversive electrical stimulation (shock) to the arm and leg, respectively. Same gender faces (CS − arm, CS − leg) represented safe trials where there was no delivery or threat of shock. Stimuli were counterbalanced across subjects. Physiological cardiovascular responses (heart rate, pulse amplitude) were monitored using pulse oximetry. SCI patients were unaware of leg stimulation as this was below the sensory level of their spinal lesion. In the first four SCI patients, no behavioural conditioning to the CS + leg (or related brain activity) was demonstrated. To exclude a possibility that the ‘globally greater’ aversive experience of controls might confound interpretation of group differences, for the remaining three SCI patients studied the ‘CS + leg’ stimulus was associated to a headphone delivered burst of aversive white noise [500 ms, 100 dB (A)].
Physiological monitoring and post-processing
Heart rate was recorded throughout scanning from left ring finger pulse using pulse oximetry (Nonin 8600 Pulse Oximeter; Nonin Medical, Inc., N. Plymouth, MN, USA). The times of each pulse and slice synchronization pulses from the scanner were logged using the CED1401 data acquisition unit and Spike 3 software (CED, Cambridge Electronic Design Limited, Cambridge, UK). The output of the pulse oximeter also enabled us to index changes in peripheral vasoconstriction of the fingers from the amplitude of the pulse waveform. Correspondence with concurrently recorded sympathetic electrodermal activity indicates predominantly sympathetic influences on the amplitude of the pulse waveform. Physiological responses were analysed post hoc using Matlab (Mathworks Inc, Natick MA). Pulse timings and waveform were interpolated to give continuous measures of heart rate and pulse amplitude then resampled to give event-related waveforms. Mean values 0.5–1.5 s following stimulus presentation were used in analyses of physiological response for each stimulus type.
Functional imaging data acquisition, pre-processing and analysis
Participants were scanned at 1.5 T (Siemens Sonata, Erlangen Germany). T2*-weighted echoplanar images optimized for blood-oxygenation level dependent (BOLD) contrast were acquired using a sequence minimizing dropout effects from orbitofrontal regions (Deichmann et al., 2003) (TE 50 ms, TRvol 2.8 s, 30° tilt, 28 · 3.5 mm thick slices). Image pre-processing and subsequent analyses were undertaken using statistical parametric mapping (SPM2) (http://www.fil.ion.ucl.ac.uk/~spm/SPM2.html) on a Matlab platform. Images were initially realigned and unwarped, correcting for motion artefact. Differences in the timing of image slices across each individual volume were corrected and each volume was transformed into standard stereotaxic space and smoothed with a Gaussian filter (full-width-half maximum 12 mm).
Voxel-wide changes in BOLD contrast within the smoothed normalized images were examined using SPM. Individual design matrices were constructed for each subject that modelled these six trial types: (1) Face 1 + stimulation to arm (CS + arm paired), (2) Face 1 + no stimulation (CS + arm unpaired), (3) Face 2 + stimulation to leg (CS + leg paired) (three of the spinal cord patients received white noise auditory stimulation in place of the stimulation to leg), (4) Face 2 + no stimulation (CS + leg unpaired), (5) Face 3 (CS − arm, (6) Face 4 (CS − leg). Each trial type was modelled as an event convolved with a canonical haemodynamic response function. In addition we modelled response interactions with time, modelling exponential decay of the response to each event over the course of the experiment. Statistical images were determined for contrasts of interest for each subject. These were then entered into second-level random effects ANOVA (analysis of variance) (with sphericity correction) to determine significant effects and group differences. For clusters of 10 contiguous voxels or more, threshold significance in these group analyses was set at P < 0.05, corrected for whole brain (false discovery rate), though where relevant we comment descriptively on activations above P < 0.001, uncorrected.
Results
Behavioural and physiological findings
All subjects tolerated the scanning environment and experimental procedure. Controls and SCI patients accurately described the association between CS + arm and painful electrical stimulation, indicating that they not only felt stimulation of the arm, but that they acquired declarative knowledge regarding the contingent association of the CS + arm within this partial reinforcement schedule. However, the first four SCI patients, in contrast to controls, had no conscious awareness of electrical stimulation of the leg (below sensory level) and consequently, could not differentiate between CS + leg and CS − leg stimuli. Furthermore in these SCI patients there was no change in regional brain activation related to leg stimulation, nor autonomic or behavioural discrimination between CS + leg and CS − leg stimuli. To attain a degree for overall aversive stimulation across the experiment, aversive white noise was administered in place of the electrical stimulation of the leg (with CS + leg stimulus) in the remaining three SCI patients. All trials were modelled in individual analyses. However, in second level analyses, as reported in the rest of this manuscript, only activity related to CS + arm (paired and unpaired) and CS − arm is considered.
In the seven control subjects, painful electrical stimulation of arm resulted in transient bradycardia and a peripheral vasoconstrictive vasomotor response in the fingers (Fig. 2). Differences were also present in cardiac and vasomotor responses to the threat of arm stimulation (i.e. conditioning-related) indexed by enhanced responses to unpaired CS + arm compared to CS − stimuli. The mean magnitude of threat-related bradycardia and vasoconstriction responses was intermediate to that of that generated by the CS + paired (shock) and the safe CS − stimuli.
Fig. 2.
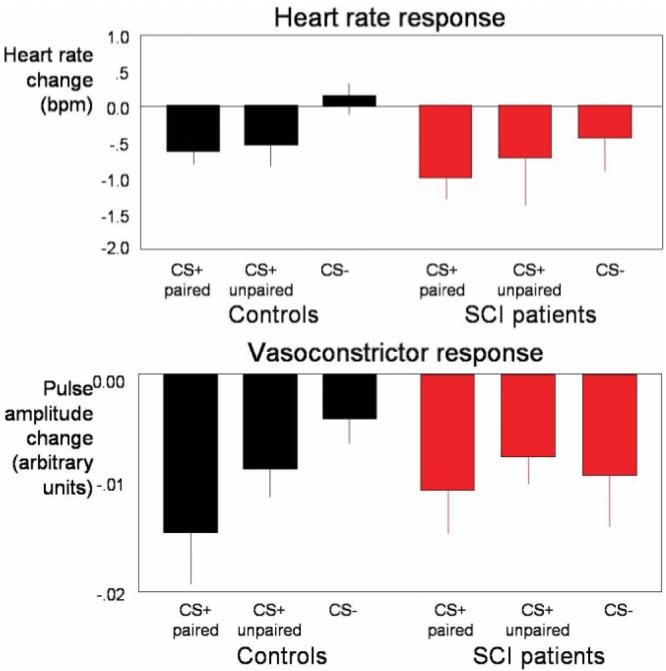
Physiological responses. Bar charts in the upper panel illustrate the heart rate change (parasympathetic bradycardia) (mean 6 SE; beats per minute) within 1.5–3.5 s from stimulus onset, the black bars representing heart rate responses to paired CS + arm (with shock to arm), unpaired CS + arm (threat of shock to arm) and CS − arm (safe) in controls; and the red bars heart rate responses to paired, CS + arm, unpaired CS + arm and CS − arm in patients with SCI. In the lower panel are bar charts of sympathetic vasoconstrictor responses, derived from trial-induced variability in pulse waveform amplitude, measured from finger pulse, for the same trial types (black bars representing controls and red bars patients with SCI). The controls showed a transient bradycardia and a peripheral vasoconstrictive vasomotor response to painful electrical stimulation of arm. Cardiac and vasomotor responses to unpaired CS + arm compared to CS − stimuli were enhanced. The mean magnitude of threat-related bradycardia and vasoconstriction responses was intermediate to that of that generated by the CS + paired (shock) and the safe CS − stimuli. The seven SCI patients showed a bradycardia to the experience of painful arm stimulation that exceeded that observed in controls. Only one patient (Patient 4) showed a discriminatory conditioning-related bradycardia for the CS + arm (unpaired) versus CS − arm and across the group there was no discrimination between CS + arm (unpaired) and CS − stimuli. Vasoconstrictor responses again showed no consistent discriminatory responses across SCI patients. These observations in the SCI patients suggest impairment in autonomic bodily reactions reflecting predictive emotional learning.
Across the seven SCI patients, there was a bradycardia to the experience of painful arm stimulation, exceeding that observed in controls. However, only one patient (surprisingly, Patient 4) showed a discriminatory conditioning-related bradycardia for the CS + arm (unpaired) versus CS − arm and across the group there was no discrimination between CS + arm (unpaired) and CS − stimuli (Fig. 2). Vasoconstrictor responses again showed no consistent discriminatory responses across SCI patients where Patients 1 and 3 demonstrated vasomotor responses to painful arm stimuli, but no patients showed significant conditioning-related vasoconstriction reflected in finger pulse amplitude (Patient 1 demonstrated a trend). These observations suggest impairment across all patients in autonomic bodily reactions, including sympathetic cardiac and electrodermal responses and parasympathetic cardiac responses reflecting predictive emotional learning.
Neuroimaging findings
US-related responses
The precise location of electrical stimulation to the arm was consistent for the controls but differed on a subject by subject basis for SCI patients, being delivered on a dermatome with normal sensory perception (four patients had the arm electrode placed on the anterior shoulder). Each SCI patient showed lateral sensorimotor strip activation in response to electrical stimulation in individual analyses, with more diffuse sensorimotor activation apparent as a group effect.
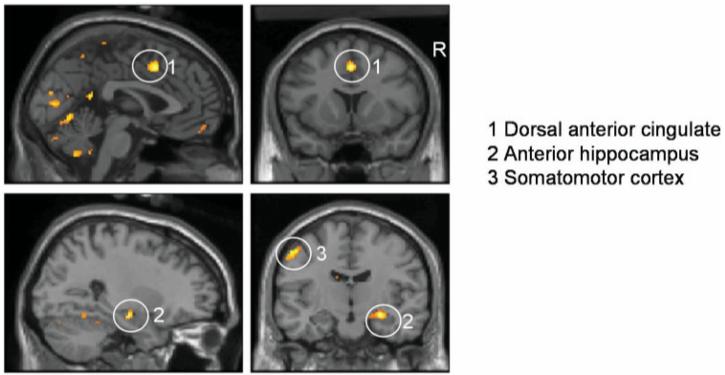
Common responses to CS + arm (paired) trials across SCI patient and control groups revealed a time-dependent activation of an extrastriate visual region sensitive to emotionally salient face stimuli (Noesselt et al., 2005), together with component regions of a pain matrix including medial temporal lobe (right anterior hippocampus), dorsal anterior cingulate cortex, with a common region of somatomotor cortex. Activity within right insula was observed at P < 0.001, uncorrected. (Table 2, Fig. 3).
Table 2.
Activity related to painful stimulation of upper limb in patients and controls [CS + arm (paired)]
| Location | Side | Coordinates of peak activity |
Z-score | ||
|---|---|---|---|---|---|
| Activity common to SCI patients and controls | |||||
| Extrastriate visual cortex | L | −42, −62, −14 | 5.16 | ||
| Amygdalohippocampal junction |
R | 28, −12, −14 | 4.75 | ||
| Dorsal anterior cingulate cortex |
Bilateral | 2, 12, 46 | 4.66 | ||
| Somatomotor cortex |
L | −50, −24, 54 | 4.14 | ||
| Anterior insula | R | 56, 18, −6 | 3.60 | ||
| Controls > SCI patients - nil Z> 3.6 | |||||
| SCI patients > controls | |||||
| Extrastriate visual cortex | L | −34, −78, 0 | 6.10 | ||
| Amygdala | R | 24, −4, − 10 | 4.78 | ||
| Somatomotor cortex | R | 24, −28, 50 | 4.76 | ||
| Midbrain | Bilateral | 4, −10, 8 | 3.90 | ||
Fig. 3.
Regional brain activity to paired CS + arm in SCI patients and controls. The delivery of shock to the arm (coupled with the CS + arm stimulus) enhanced regional brain activity within dorsal cingulate and medial temporal components of the pain matrix in both patients with SCI and controls. Common responses to CS + arm (paired) trials also were observed within an extrastriate visual region, medial temporal lobe (right anterior hippocampus), dorsal anterior cingulate cortex, with a common region of somatomotor cortex. The location of common group activations within dorsal anterior cingulate, medial temporal lobe and somatomotor cortex are illustrated on parasagittal and coronal sections of a normalized template brain scan.
There were no regions surviving corrected threshold that were more active in controls in response to presentation of CS + arm faces paired with electrical arm stimulation. However, cortical regions within left lateral occipital cortex and right somatomotor cortex and subcortical regions of amygdala and midbrain were significantly more active in the SCI patients than controls, consistent with enhanced sensitivity of visual sensory and nociceptive responses to these aversive trials.
Conditioning-related activity
In SCI patients, robust autonomic expression of fear conditioning (differential vagal bradycardia to unpaired CS + arm and CS − arm trials) was compromised. In both SCI patients and controls, corresponding conditioning-related brain responses to threat (unpaired CS + arm versus CS − arm) were observed in bilateral regions of anterior insula extending into lateral OFC and regions including the putamen. This activity pattern is in keeping with evidence implicating insula, lateral OFC and putamen in processing aversive stimuli (Seymour et al., 2004).
Differences between controls and patients with spinal cord injury
We tested for group differences in brain activity between SCI patients and controls during conditioning of faces to the CS + arm: compared to controls, SCI patients demonstrated attenuation of activity during threat (CS + arm unpaired versus CS − arm), predominantly in midline regions including posterior cingulate, motor cingulate, subgenual cingulate and adjacent ventromedial prefrontal cortex. The difference in the subgenual region is notable. Evidence suggests a preferential association of this region with (vagal) autonomic responses (Kaada, 1951; Devinsky et al., 1995). Moreover structural and functional abnormalities of this region in patients with mood disorder indicate a central neurobiological role in the pathoaetiology of depression (Drevets, 1999; Zobel et al., 2005). Our observation of dysfunction of this subgenual region in SCI patients during emotional learning also supports an account that, in health, a representation of visceral arousal state within this region may guide emotional behaviour and feeling states (Damasio, 1994; Bechara et al., 1996; Craig, 2002) (Fig. 4, Table 3).
Fig. 4.
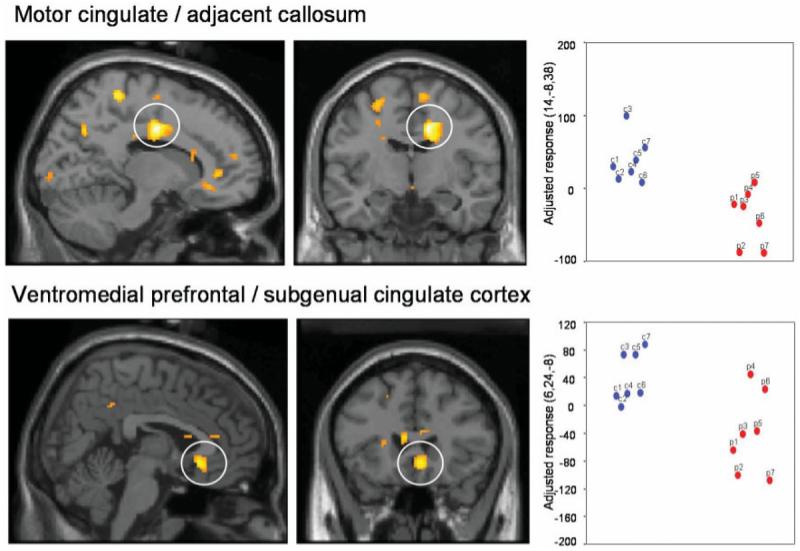
Conditioning-related activity: differences between controls and SCI patients. Conditioning-related activity represents the difference between responses to CS + and CS − stimuli (i.e. learned threat–safe). We tested for group differences in brain activity between SCI patients and controls during conditioning of faces to the CS + arm: controls showed activation of midline regions (posterior cingulate, motor cingulate, subgenual cingulate and ventromedial prefrontal cortex). SCI patients (in red on the scatter plots), compared to controls (in blue on the scatter plots), showed decreased activity during threat (CS + arm unpaired versus CS − arm), mainly in midline regions including posterior cingulate, motor cingulate, subgenual cingulate and adjacent ventromedial prefrontal cortex. Group differences in regional activity are plotted on parasagittal and coronal sections of a template brain next to scatter plots of the parameter estimates (arbitary units, proportional to % signal change) for controls and SCI patients.
Table 3.
Common activity related to processing of threat during fear conditioning [CS + (arm) unpaired versus CS − (arm)]
| Location | Side | Coordinates of peak activity |
Z-score | |
|---|---|---|---|---|
| Activity common to SCI patients and controls | ||||
| Anterior insula lateral OFC |
R | 46, 32, −6 | 6.25 | |
| L | −44, 28, − 12 | 6.08 | ||
| Premotor cortex | L | −46, 0, 34 | 6.00 | |
| Fusiform cortex | R | 42, −46, −12 | 5.85 | |
| Inferior parietal lobule | R | 50, −42, 38 | 5.61 | |
| Putamen | R | 30, 10, −6 | 4.30 | |
| Controls > SCI patients | ||||
| Motor cingulate cortex | R | 14, −8, 38 | 5.90 | |
| Extrastriate visual cortex | R | 20, −88, 4 | 5.26 | |
| Posterior cingulate cortex | R | 10, −38, 28 | 5.18 | |
| Precuneus | R | 10, −66, 34 | 4.95 | |
| Subgenual cingulate / ventromedial prefrontal cortex |
Bilateral | 6, 24, −8 | 4.50 | |
| SCI patients > controls | ||||
| Superior temporal gyrus | L | −46, −16, 0 | 7.34 | |
| Periaqueductal grey | Bilateral | −2, −22, 0 | 5.29 | |
| Dorsal anterior cingulate cortex |
Bilateral | 6, 6, 44 | 5.22 | |
| Lateral occipital cortex | L | −48, −56, − 16 | 4.99 | |
SCI patients, in contrast to controls, showed an enhancement of brain activity to the conditioned threat within dorsal anterior cingulate cortex and PAG regions commonly activated by pain. In addition, greater activity was also observed within visual cortices of superior temporal and lateral occipital gyri that encode salient face stimuli and expression. Together these findings suggest a hypersensitivity of brain responses within the central pain matrix and affective sensory representation to predictors of aversive stimulation (Fig. 5, Table 3).
Fig. 5.
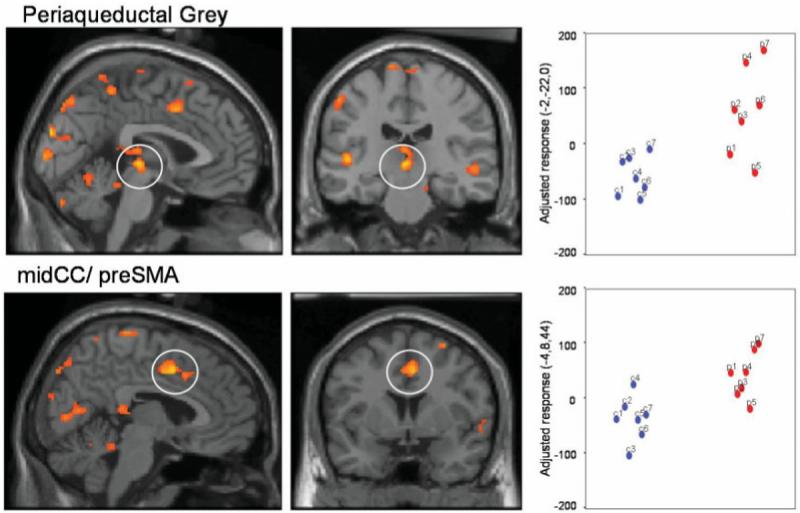
Conditioning-related activity: differences between SCI patients and controls. SCI patients (in red on the scatter plots), in contrast to controls (in blue on the scatter plots), demonstrated enhanced brain activity to the conditioned threat [CS + arm (unpaired) > CS − arm] within dorsal anterior cingulate cortex and periaqueductal grey (PAG), regions commonly activated by pain. Greater activity was also observed within visual cortices of superior temporal and lateral occipital gyri that encode salient face stimuli and expression. These findings imply a hypersensitivity of brain responses within the central pain matrix and affective sensory representation to predictors of aversive stimulation. Group differences in regional activity are plotted on parasagittal and coronal sections of a template brain next to scatter plots of the parameter estimates (arbitary units, proportional to % signal change) for controls and SCI patients.
Discussion
To the best of our knowledge, this study represents the first fMRI study of emotional processing in patients with SCI. We provide evidence for abnormalities in functional activity within brain regions associated with emotional processing. We demonstrate differences in patterns of evoked activity between SCI patients and controls including underactivity of subgenual and posterior cingulate cortex and enhanced responses within dorsal anterior cingulate cortex and PAG during the processing of learned threat. Significantly, all these regions are implicated in supporting emotional processes, notably representation of subjective emotional feelings and the control of motivational behaviour (Damasio, 1994; Craig, 2002).
Recent neuroimaging and lesion studies have highlighted the role of insula, anterior cingulate and ventromedial prefrontal cortical regions in the generation, representation and integration of autonomic arousal responses with emotional processes (Critchley, 2003, 2004). The present study provides clues to potential neural mechanisms through which a perturbation of autonomic response and visceral feedback may account for anecdotal reports of emotional changes in SCI patients. Additionally, our findings speak to theoretical issues by examining neuroanatomical substrates for central representation of spinal afferent information thereby testing the robustness of peripheral theories of emotion (James, 1884, 1894).
Behaviourally, we demonstrated impairment of sympathetic and parasympathetic autonomic bodily responses in SCI patients during emotional learning. This was anticipated for responses to the threat of stimulation below the level of lesion; if the patients could not feel the pain, they could not learn which cues predicted the pain. Leg stimulation in four of the seven SCI patients allowed us to test whether afferent fibres of the vagus conveyed sensory information about local spinal reflexes (induced below the spinal lesion) giving rise to compensatory autonomic responses. We found no evidence for such a process with brief cutaneous shocks, but acknowledge evidence for such effects associated with deep visceral stimulation or dysreflexic responses (Janig, 1996). More surprisingly, however, was the absence of significant conditioned autonomic responses to the threat of painful arm stimulation in SCI patients. Both the controls and SCI patients perceived the arm stimulation as painful and learned the association with CS + arm face stimulus. Each group also showed a bradycardia response to the painful stimulation of the arm indicating intact psychophysiological responses to pain mediated (parasympathetically) by the vagus nerve. However, six of the seven SCI patients, in contrast to controls, did not show a similar cardiac response to the threat of pain. This observation suggests an underlying abnormality in SCI patients in central emotional mechanisms supporting implicit emotional learning and its psychophysiological expression. It is worth noting that no patient generated a significant conditioning-related vasomotor (vasoconstriction) response, perhaps indicating disruption of paravertebral sympathetic outflow (yet puzzlingly two patients appeared to respond to the painful US, indicative of intact sympathetic responses to pain).
The above psychophysiological findings extend observations from studies indexing sympathetic function by electrodermal activity (sympathetic skin response, SSR). The SSR is a non-invasive electrophysiological technique to study efferent sympathetic cholinergic pathways to sweat glands of hand and foot (Shahani et al., 1984). In SCI patients, stimuli applied above the level of lesion can evoke an SSR only where there is preserved sympathetic innervation, whereas there is no SSR (in hand or foot) to stimulation below the level of lesion where sympathetic innervation is impaired (Cariga et al., 2002). As predicted, our SCI patients had absent SSRs when the stimulus was applied below the level of lesion. The SCI patients also showed abnormal generation of SSRs following stimulation above lesion level, even in the two patients with vasomotor responses. These findings suggest efferent sympathetic impairment across noradrenergic vasomotor and cholinergic electrodermal sympathetic subaxes.
Our functional neuroimaging study first confirmed common activation within components of the pain matrix in patients and controls by the arm stimulation, including dorsal anterior cingulate cortex and medial temporal lobe (amygdalohippocampal region). These brain areas are known to be sensitive to the absence of peripheral arousal responses (Critchley et al., 2002) and are implicated in generating autonomic responses to pain and threat (LeDoux et al., 1998; Phelps et al., 2001; Asahina et al., 2003). It is noteworthy that SCI patients did not share with controls pain-induced activation of insular cortex, a region implicated in the central mapping of bodily arousal responses. This suggests abnormalities in the wider pain matrix but further experiments are warranted to clarify the implications.
Our principal findings relate to differences between SCI patients and controls in conditioning-related brain activity, since they highlight abnormalities in emotion-related processing consequent upon the partial uncoupling between body and brain. These analyses refer exclusively to comparisons of the ‘threat’ (unpaired CS + arm) with ‘safe’ (CS − arm) stimuli. During fear conditioning the controls showed predictable patterns of brain activation including activation of cingulate, insula and amygdala to the threat of shock. In contrast, SCI patients showed significantly attenuated activity compared to controls in subgenual and posterior cingulate cortices when processing threat. The subgenual cingulate and adjacent ventromedial prefrontal cortical region is implicated in self-referential processing and monitoring (Gusnard et al., 2001) and lies adjacent to orbitofrontal and insular cortex involved in central mapping of visceral arousal states (Craig, 2002). This medial frontal region is crucially implicated in motivational behaviour including learning and decision-making following punishing outcomes (Bechara et al., 1996; Damasio, 1994) and representation of reward and positive social signals (O'Doherty et al., 2003). Significantly, dysfunction of subgenual cingulate and ventromedial prefrontal cortex is associated with depressive symptoms and vulnerability (Drevets, 2000). Thus, the abnormal activity of this region observed in SCI patients during threat may underlie a predisposition to depressive symptoms arising from maladaptive responses to aversive predictors.
A further important observation was a more widespread enhancement, in response to threat, of cortical and subcortical activity in SCI patients compared to controls. This hyperactivity was particularly observed within dorsal anterior cingulate, PAG and regions of extrastriate and temporal visual cortices. While this dorsal anterior cingulate region cortex is implicated in high-level cognitive functions, it is also activated by emotional, notably during pain, processing (Rainville, 2002; Tracey et al., 2002). The same region participates in conscious processing of emotional feelings, correlating autonomic indices of emotional intensity with cingulate activity (Lane et al., 1999) and is functionally integrated with peripheral autonomic arousal response (Critchley et al., 2000a, 2003). In patients with peripheral autonomic denervation, leading to an absence of peripheral autonomic response during stress, there is compensatory enhancement of anterior cingulate activity (Critchley et al., 2000b). The latter observation suggests that abnormal feedback of autonomic response may also account for heightened activity in dorsal anterior cingulate, which we observed in the SCI patients. However, it is noteworthy that we also observed enhanced activity of PAG (and, at a lower threshold, thalamus) in SCI patients during threat processing suggesting a sensitization of pain related pathways in this patient group. Whether sensitization is directly attributable, at a neural level, to diminished afferent spinal information flow, or a consequence of psychological and emotional adjustment, remains to be clarified. Of interest in this regard was a ‘hypersensitive’ response within visual areas for processing threat which suggests an increasing gain to threat stimuli in these patients that is arguably more in keeping with a psychologically mediated, than neurophysiological, process. Psychological factors, such as attention, distraction, stress and arousal modulate the perception of pain both in normal subjects and in patients. Valet et al. (2004) showed that the PAG and posterior thalamus receive influences from fronto-cingulate cortices that enable the gating of pain during distraction conditions. Altered descending inhibition and facilitatory modulation of nociceptive responses from PAG and brainstem may represent a plausible mechanism underlying chronic somatic and visceral pain syndromes (Dunckley et al., 2005). Tracey et al. (2002) demonstrated activation changes in PAG can be induced by attention to painful stimulation and, in this context, the magnitude of PAG activity correlated with perceptual decreases in pain intensity. Our observations therefore suggest abnormalities within the pain matrix and attentional/emotional systems in patients with SCI during processing of threat, even in the absence of a clinically reported pain syndrome. These functional changes may ultimately reflect a failure in integration of external emotional information with afferent viscerosensory (spinal) signals for emotional self-reference and are ultimately attributable to high spinal lesion.
A number of technical and logistical factors were addressed during the course of this first fMRI study of emotional processing in SCI patients. Nevertheless, the relatively small number of SCI patients studied represents a limitation and the results raise many more questions. We did not in this instance pursue the analyses of individual subject difference to relate to the different clinical characteristics of the patients. However, the effects observed and plotted do not suggest a simple relationship between emotion-related brain activity and ASIA score or lesion level. Deficits in autonomic and sensory function may provide a better account of observed group differences in activity, but such inferences generated by our findings may require further exploration in a larger, more homogeneous patient group. Combining neuroimaging, visual task, aversive electrical stimulation and autonomic monitoring in SCI patients represented a technical challenge. Naturally enhanced consideration of body position and maintained posture was required to prevent involuntary contractions and dysreflexic responses from confounding data acquisition and interpretation. In spite of these extra demands, our study demonstrated the feasibility and usefulness of functional brain neuroimaging with fMRI as a means to gain valuable clinical insight into emotional functioning in an important patient population.
We did not directly address mood or affective behaviour in patients with SCI. This area of research is fraught with confounds, while emotional deficits are clearly subtle in an experimental setting (Lowe and Carrol, 1985; Chwalisz et al., 1988; Bermond et al., 1991; Cobos et al., 2002). Individual differences in emotional sensitivity to bodily reactions may determine the expression of emotional changes (Wiens et al., 2000; Critchley et al., 2004) and be overlooked in group investigations of SCI patients. Our findings highlight potential neural mechanisms through which abnormalities in autonomic control and feedback of bodily spinal information may modulate emotional function. This evidence extends our understanding of emotional (and autonomic) consequences of SCI and may thus inform therapeutic strategies and provision of psychological support for SCI patients.
In conclusion, our study tested for differences between SCI patients and healthy controls to understand how emotional processing may be modulated by the relative absence of feedback from the body following SCI. Our findings highlight abnormalities in brain regions implicated in emotional control and depressive vulnerability and components of the pain matrix, suggestive of sensitization of central pain regulation. We also observed a more general impairment in emotion-related generation of autonomic bodily responses that suggests dysfunction of implicit emotional learning in patients with SCI. Development of these early findings can inform programming psychological support to prevent the occurrence of emotional dysfunction in SCI.
Acknowledgements
This study was supported by a Wellcome Trust Programme grant to R.J.D. and by an International Spinal Research Trust grant to C.J.M. and P.H. Ellaway.
Abbreviations
- fMRI
functional MRI
- OFC
orbitofrontal cortex
- PAG
periaqueductal grey matter
- SCI
spinal cord injury
References
- Asahina M, Suzuki A, Mori M, Kanesaka T, Hattori T. Emotional sweating response in a patient with bilateral amygdala damage. Int J Psychophysiol. 2003;47:87–93. doi: 10.1016/s0167-8760(02)00123-x. [DOI] [PubMed] [Google Scholar]
- Allen GV, Saper CB, Hurley KM, Cechetto DF. Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol. 1991;311:1–16. doi: 10.1002/cne.903110102. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cereb Cortex. 1996;6:215–25. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Bermond B, Nieuwenhuyse B, Fasotti L, Schuerman J. Spinal cord lesions, peripheral feedback, and intensities of emotional feelings. Cogn Emot. 1991;5:201–20. [Google Scholar]
- Berntson GG, Sarter M, Cacioppo JT. Ascending visceral regulation of cortical affective information processing. Eur J Neurosci. 2003;18:2103–9. doi: 10.1046/j.1460-9568.2003.02967.x. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Hickling EJ, Taylor AE, Loos WR, Gerardi RJ, et al. Psychological morbidity associated with motor vehicle accidents. Behav Res Ther. 1990;32:283–90. doi: 10.1016/0005-7967(94)90123-6. [DOI] [PubMed] [Google Scholar]
- Brom D, Kleber RJ, Hofman MC. Victims of traffic accidents: incidence and prevention of post-traumatic stress disorder. J Clin Psychol. 1993;49:131–40. doi: 10.1002/1097-4679(199303)49:2<131::aid-jclp2270490202>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Buechel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–57. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Cameron OG. Interoception: the inside story—a model for psychosomatic processes. Psychosom Med. 2001;63:697–710. doi: 10.1097/00006842-200109000-00001. [DOI] [PubMed] [Google Scholar]
- Cameron OG. Visceral sensory neuroscience. Oxford: Oxford University Press; 2002. [Google Scholar]
- Cariga P, Catley M, Mathias CJ, Savic G, Frankel HL, Ellaway PH. Organisation of the sympathetic skin response in spinal cord injury. J Neurol Neurosurg Psychiatr. 2002;72:356–60. doi: 10.1136/jnnp.72.3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwalisz K, Diener E, Gallagher D. Autonomic arousal feedback and emotional experience: evidence from the spinal cord injured. J Pers Soc Psychol. 1988;54:820–8. doi: 10.1037//0022-3514.54.5.820. [DOI] [PubMed] [Google Scholar]
- Cobos P, Sanchez M, Garcia C, Vera MN, Vila J. Revisiting the James versus Cannon debate on emotion: startle and autonomic modulation in patients with spinal cord injuries. Biol Psychol. 2002;61:251–69. doi: 10.1016/s0301-0511(02)00061-3. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation. J Physiol Lond. 2000a;523:259–70. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Elliott R, Mathias CJ, Dolan RJ. Neural activity to the generation and representation of galvanic skin conductance response: a functional magnetic resonance imaging study. J Neurosci. 2000b;20:3033–40. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neural correlates of first and second-order representation of bodily states. Nat Neurosci. 2001;2:207–12. doi: 10.1038/84048. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Fear-conditioning in humans: the influence of awareness and arousal on functional neuroanatomy. Neuron. 2002;33:653–63. doi: 10.1016/s0896-6273(02)00588-3. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Josephs O, O'Doherty J, Zanini S, Dewar B-K, Mathias CJ, et al. Human cingulate cortex and autonomic cardiovascular control: converging neuroimaging and clinical evidence. Brain. 2003;216:2139–52. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes' error: emotion, reason and the human brain. New York: Grosset/Putnam; 1994. [Google Scholar]
- Damasio AR. The feeling of what happens: body and emotion in the making of consciousness. New York: Harcourt Brace; 1999. [Google Scholar]
- Dana C. The anatomical seat of the emotions: a discussion of the James-Lange theory. Arch Neurol Psychiatr. 1921;6:634–39. [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. [Review] Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage. 2003;19:430–41. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614–37. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Dunckley P, Wise RG, Fairhurst M, Hobden P, Aziz Q, Chang L, et al. A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. J Neurosci. 2005;25:7333–41. doi: 10.1523/JNEUROSCI.1100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrikson M, Furmark T, Olsson MT, Fischer H, Andersson J, Langstrom B. Functional neuroanatomical correlates of electrodermal activity: a positron emission tomographic study. Psychophysiology. 1998;35:179–85. [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MC. Hamilton anxiety scale [HAMA] In: Schutte NS, Malouff JM, editors. Sourcebook of adult assessment. (Applied clinical psychology. New York: Plenum Press; 1995. pp. 154–7. [Google Scholar]
- Hickling EJ, Blanchard EB. Post-traumatic stress disorder and motor vehicle accidents. J Anxiety Dis. 1992;6:285–91. [Google Scholar]
- James W. What is an emotion? Mind. 1884;9:188–205. [Google Scholar]
- James W. Physical basis of emotion. Psychol Rev. 1994;1:516–529. doi: 10.1037/0033-295x.101.2.205. reprinted in. Psychol Rev 1894; 101: 205–10. [DOI] [PubMed] [Google Scholar]
- Janig W. Neurobiology of visceral afferent neurons: neuroanatomy, functions, organ regulations and sensations. Biol Psychol. 1996;42:29–51. doi: 10.1016/0301-0511(95)05145-7. [DOI] [PubMed] [Google Scholar]
- Kaada BR. Somato-motor, autonomic and electrocorticographic responses to electrical stimulation of rhinencephalic and other structures in primates, cat and dog. Acta Physiol Scand. 1951;24:1–262. [PubMed] [Google Scholar]
- Kennedy P, Rogers BA. Anxiety and depression after spinal cord injury: a longitudinal analysis. Arch Phys Med Rehabil. 2000;81:932–7. doi: 10.1053/apmr.2000.5580. [DOI] [PubMed] [Google Scholar]
- Lane RD, Chua PM, Dolan RJ. Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia. 1999;37:989–97. doi: 10.1016/s0028-3932(99)00017-2. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioural correlates of conditioned fear. J Neurosci. 1998;8:2517–29. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J, Carroll D. The effects of spinal cord injury on the intensity of emotional experience. Br J Clin Psychol. 1985;24:135–6. doi: 10.1111/j.2044-8260.1985.tb01327.x. [DOI] [PubMed] [Google Scholar]
- Mathias CJ, Frankel HL. Autonomic disturbances in spinal cord lesions. In: Mathias CJ, Bannister R, editors. Autonomic failure. A textbook of clinical disorders of the autonomic nervous system. Fourth edition New York: Oxford University Press; 2002. pp. 494–513. [Google Scholar]
- Maynard FM, Jr, Bracken MB, Creasey G, Gitunno JF, Jr, Donovan WH, Ducker TB, et al. International standards for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Spinal Cord. 1997;35:266–74. doi: 10.1038/sj.sc.3100432. [DOI] [PubMed] [Google Scholar]
- Mohta M, Sethi AK, Tyagi A, Mohta A. Psychological care in trauma patients. Injury. 2003;34:17–25. doi: 10.1016/s0020-1383(02)00377-7. [DOI] [PubMed] [Google Scholar]
- Mueller AD. Psychologic factors in rehabilitation of paraplegic patients. Arch Phys Rehabil. 1962;43:151–9. [Google Scholar]
- Noesselt T, Driver J, Heinze HJ, Dolan R. Asymmetrical activation in the human brain during processing of fearful faces. Curr Biol. 2005;15:424–9. doi: 10.1016/j.cub.2004.12.075. [DOI] [PubMed] [Google Scholar]
- O'Carroll RE, Ayling R, O'Reilly SM, North NT. Alexithymia and sense of coherence in patients with total spinal cord transection. Psychosom Med. 2003;65:151–5. doi: 10.1097/01.psy.0000039332.98543.3d. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci. 2003;23(793):1–9. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JC, Ungerleider LG, Bandettini PA. Task-independent functional brain activity correlation with skin conductance changes: an fMRI study. Neuroimage. 2002;17:1797–806. doi: 10.1006/nimg.2002.1306. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O'Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2001;4:437–41. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Rainville P. Brain mechanisms of pain affect and pain modulation. Curr Opin Neurobiol. 2002;12:195–204. doi: 10.1016/s0959-4388(02)00313-6. [DOI] [PubMed] [Google Scholar]
- Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2002;25:433–69. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- Seymour B, O'Doherty JP, Dayan P, Koltzenburg M, Jones AK, Dolan RJ, et al. Temporal difference models describe higher-order learning in humans. Nature. 2004;429:664–7. doi: 10.1038/nature02581. [DOI] [PubMed] [Google Scholar]
- Shahani BT, Halperin JJ, Boulu P, Cohen J. Sympathetic skin response—a method of assessing unmyelinated axon dysfunction in peripheral neuropathies. J Neurol Neurosurg Psychiatry. 1984;47:536–42. doi: 10.1136/jnnp.47.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyer KM. Central nervous control of the cardiovascular system. In: Mathias CJ, Bannister R, editors. Autonomic failure. Third edition New York: Oxford University Press; 1999. pp. 45–55. [Google Scholar]
- Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, et al. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci. 2002;22:2748–52. doi: 10.1523/JNEUROSCI.22-07-02748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Transverse Myelitis Consortium Working Group Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002;59:499–505. doi: 10.1212/wnl.59.4.499. [DOI] [PubMed] [Google Scholar]
- Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, et al. Distraction modulated connectivity of the cingulo-frontal cortex and the midbrain during pain—an fMRI analysis. Pain. 2004;109:399–408. doi: 10.1016/j.pain.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Wiens S, Mezzacappa E, Katkin ES. Heartbeat detection and the experience of emotion. Cogn Emot. 2000;14:417–27. [Google Scholar]
- Zobel A, Joe A, Faymann N, Clusmann H, Sehzamm J, Reinhardt H, et al. Changes in regional cerebral blood flow by therapeutic vagus nerve stimulation in depression: an exploratory approach. Psychiatry Res. 2005;139:165–79. doi: 10.1016/j.pscychresns.2005.02.010. [DOI] [PubMed] [Google Scholar]