Abstract
Our goal was to examine the gait patterns of older adults with Down syndrome (DS) for precocious stabilizing adaptations during comfortable over-ground walking and in more challenging conditions. Twelve participants with DS and 12 with typical development (TD) were matched for height, weight and age (range 35 to 62 years). We used a 6-camera motion capture system to assess foot trajectories over obstacles. Participants first walked at their preferred speed over a 5.3 m instrumented gait mat (unperturbed condition). Subsequent walking trials included perturbations mid-walkway: a) minimal obstacle to step over (12 cm high), b) moderate obstacle to step onto with both feet and then off (standard step), c) maximum obstacle to step onto with only one foot and over with the other (standard step).
Adults with DS walked slower with shorter, wider strides while spending more time in both stance and double support. These adaptations increased during the moderate and maximal perturbations. They stepped with the minimal perturbation obstacle further forward in their crossing step and produced a lower, flatter trajectory of the lead foot, with less dorsiflexion at crossing. This strategy decreased trailing toe clearance but did not alter leading heel clearance.
The combined effects of ligamentous laxity, low tone, obesity, inactivity and physiological decrements associated with aging lead to these stability-enhancing adaptations at a younger chronological age in adults with DS. We believe intervention to increase overall stability will be beneficial in helping adults with DS maintain optimal functional mobility and health.
Introduction
Persons with Down syndrome (DS) are now living well into adulthood. The median age at death has increased to nearly 60 years, with some people with DS living into their 70s [1, 2]. Premature aging has been noted in this population; the rate of primary aging is nearly twice that of adults with typical development (TD)[3].
Adults with DS show premature age-related changes in several respects. They demonstrate decreased ability to perform activities of daily living by 40 years of age [4] while adults with only intellectual disability do not demonstrate a decline until 50 years of age [5]. They often also experience problems with osteoarthritis [6], increased body weight [7] and Alzheimer’s type dementia [8–10].
Our goal in this study was to determine if the gait patterns of adults with DS show precocious age-related changes. In older adults with TD, age-related changes in gait include reduced stride length, reduced velocity and increased step width and are observed starting around 64 years of age [11, 12]. Older adults also spend more time in double support [13]. Together, these adjustments reflect strategies to increase walking stability [13–15].
Locomotor stability is an issue for people with DS across their lifespan. Due to low tone and ligamentous laxity, achieving stable upright locomotion is a more difficult problem than for their peers with TD; average onset of independent walking in those with DS is 2 years of age [16, 17]. Their solution to this stability problem has unique attributes; toddlers with DS after one month of walking experience show shorter stride length, slower velocity and a trend towards wider step width than peers with DS [16]. Following years of walking practice, however, some of these group differences disappear, only step width remains larger in preadolescents with DS [18, 19].
In pre-adolescents, merely increasing stride width is an adequate adaptation to provide the necessary stability for self-paced, unperturbed over-ground walking [18, 19]. There are many reasons to believe walking, which is important for maintaining many activities of daily living, may require stability-enhancing adaptations as adults with DS age.
In addition to the stability challenges inherent to DS, factors known to affect gait in adults with TD that may also be relevant include a sedentary lifestyle [20], obesity [7] and neurophysiological changes associated with aging. Overall, the gait characteristics of sedentary older adults suggest that they adopt a more cautious walking style than active ones, exhibiting shorter step lengths and slower step velocities [21]. Obese persons walk slower [22] with wider step width [22, 23]. Neurophysiological changes associated with aging, such as decreased tactile sensation, proprioception, vision and strength, as well as increased reaction time all may contribute to changes in body control [24–28] and the adoption of a walking pattern selected to enhance stability [29, 30].
As people with DS age, the constraints affecting their ability to maintain walking stability increase. Accordingly, they may adopt strategies to increase walking stability at an earlier age than their peers with TD. Our aim is to perturb their gait patterns to probe for adaptations during over-ground walking in conditions of gradually increasing challenge. We propose that older adults with DS will make distinct changes in their foot trajectories at a younger age than their peers with TD. We designed this study because we believe it is important to know when and how declines in gait occur with age in order for clinicians to screen for and, perhaps, develop interventions to help persons with Down syndrome maintain healthy, safe mobility.
Method
Procedures
Twelve adults with DS and 12 with TD between ages 35 and 62 years participated in the study. Individuals were matched a priori for height, weight and age. Inclusion/exclusion criteria were only that participants had to be able to understand the tasks and perform them independently, as well as have no uncorrected visual or hearing deficits and no neurological deficits or operative procedures of the lower extremities. Participants with DS were recruited through local family support groups and word of mouth, while adults with TD were recruited through a campus research volunteer website.
Participants with DS came to our laboratory for 1.5 to 2 hours of testing on two occasions. As they required less time to acclimate to the laboratory setting and learned tasks much faster, adults with TD performed all testing in one visit. The University of Michigan Institutional Review Board approved all procedures. Prior to participation, we explained the purposes of our study and all procedures to participants and any caregivers or legal guardians. Participants signed an assent or consent form as appropriate, with consent for assenting adults provided by a legal guardian.
As detailed below, the data collection consisted of walking comfortably over ground, stepping over obstacles, walking on a treadmill and collection of background information. We collected reflective marker data for 3-D motion analysis of all body segments and bilateral EMG data for the leg muscles. Results for treadmill walking, EMG and resultant center of mass data will not be discussed further here.
When participants arrived in the lab they changed into a bathing suit or close-fitting shorts and a tank top. We attached, bilaterally, reflective markers at the temperomandibular joint, shoulder at acromion process, elbow at lateral humeral epicondyle, wrist at styloid process, greater trochanter, femoral condyle, ankle at 10 cm above lateral malleolus, heel at bony prominence and third metatarsophalangeal joint.
Participants walked barefoot and performed 4–6 repetitions of each condition. First, they walked at their preferred speed over a 5.3-meter GAITRite mat (CIR Systems, Inc., Havertown, PA) (unperturbed condition). We then placed a foam-covered rod (14 cm circumference) 12 cm above and perpendicular to the walkway at its midpoint (minimal perturbation condition). Participants stepped over the obstacle in any manner they chose. Next, we placed a standard step (20-cm high, 91-cm wide, 28-cm deep, made of wood) across the walkway at its midpoint. Participants a) stepped up onto the step with two feet and then down from it (moderate perturbation condition) followed by b) stepping onto the step with only one foot and swinging the other leg over to step on the floor (maximum perturbation condition). We explained and demonstrated each task. We allowed participants to practice, giving them visual (demonstration), auditory (verbal encouragement and feedback) and tactile cues (touching a leg) in response to their needs until each was able to perform the task. Adults with DS tended to require instruction, practice, and encouragement to complete the more challenging conditions.
We used a 6-camera Peak Motus real-time system (Peak Performance Technologies, Centennial, CO) to collect reflective marker position data at a sampling rate of 60 Hz. Three cameras were distributed evenly on each side, and a video camera provided backup visual records of performance.
Participants also completed the Berg Balance Scale, a standardized balance assessment instrument [31, 32] with demonstrated reliability for adults with DS [33]. With necessary assistance from caregivers, they completed a paper survey addressing general health and medication questions and the Stanford Health Questionnaire Disability Index and Pain Scale [34–36]. During an in-person interview we asked about living arrangements, employment status, and exercise or recreational activities. We took anthropometric measurements using a Healthometer (Precision Weighing Balances, Bradford, MA) beam scale to obtain body weight and a GPM anthropometer (Siber Hegner and Co., Zurich, Switzerland) to record height and body segment lengths.
Data Reduction and Analysis
As part of the assessment of foot clearance, raw marker position data were converted to 3D data via the Peak system software and filtered with a second order Butterworth filter at a cutoff frequency of 6 Hz. We used GAITRite software to edit steps and to calculate the following over-ground gait parameters: walking speed, stride length, step width, proportion of stride spent in stance and in double support. The GAITRite system measures step width as the perpendicular distance from the center of one foot to the line of progression of the center of the other foot. We normalized walking speed, stride length and step width by converting them to dimensionless values, using published formulas (see Appendix).
To determine baseline gait parameters, we included all strides during unperturbed walking. As our focus was participants’ anticipatory reactions, we analyzed only strides approaching and crossing the minimal perturbation obstacle and strides approaching the step obstacle (moderate and maximal perturbations).
We used SPSS Version 14.0 (Chicago, IL.) for statistical tests with an alpha level of significance at 0.05.
Results
Participants
We confirmed the a priori participant matching had been effective. To examine the potential for other body structure differences, we compared seven body segment lengths using one-way (group) multivariate analyses of variance (MANOVA). A second MANOVA included three body segment ratios. Significant main effects resulted for the body segment ratios analysis. Follow-up univariate analyses revealed a significant group difference for upper extremity to trunk ratio, with persons with DS having shorter upper limbs relative to their trunks than their peers with TD. Group mean values and statistically significant results are shown in Table 1.
Table 1.
Mean anthropometric data of adults with DS and TD
| DS Mean(SD) |
TD Mean(SD) |
|
|---|---|---|
| Lengths (cm) | ||
| Head and neck | 26.34(2.98) | 26.41(1.04) |
| Trunk | 56.37(3.64) | 57.64(2.38) |
| Thigh | 35.58(2.70) | 38.47(3.10) |
| Shank | 33.24(3.45) | 35.03(2.38) |
| Foot | 21.74(1.87) | 22.44(1.10) |
| Upper Arm | 25.37(2.41) | 28.52(1.87) |
| Forearm | 21.5(1.97) | 22.69(0.91) |
| UE/trunk ratio* | 0.83(0.07) | 0.89(0.04) |
| LE/trunk ratio | 1.23(0.09) | 1.27(0.04) |
| Foot/height ratio | 0.14(0.01) | 0.14(0.01) |
| Weight (kg) | 76.54(21.22) | 68.64(16.94) |
| Height (m) | 1.53(0.09) | 1.60(0.05) |
| BMI (kg/m2) | 32.37(7.67) | 26.96(6.86) |
| Age (yrs) | 43.33(8.35) | 44.83(7.04) |
| Gender | 6 M, 6 F | 1 M, 11 F |
p< 0.05
Significant main effects resulted for the body segment ratios analysis (Wilks’ Lambda = 0.64, F[3,20] = 3.66, p = 0.03, partial eta squared = 0.36). Follow-up univariate analyses revealed a significant group difference for upper extremity to trunk ratio (F[1,22] = 6.33, p = 0.02).
Five adults with DS lived at home with their parents, 7 lived in supervised apartments (2–3 residents) or group home (7–20 residents) settings. Adults with TD lived independently. Of the 12 participants with DS, 9 had corrected vision problems, 5 had flat feet (2 wore orthotics), 7 reported obesity and 2 reported dementia. According to our Stanford Scale results, approximately 25% needed some assistance with shampooing, cutting meat, opening milk cartons, bathing and dressing, shopping and other errands. Six reported no pain, while 6 described pain in the legs and hips, with an intensity ranging from 10 to 50 on a scale of 1 to 100.
Ten participants with DS had jobs consisting of light physical activity (e.g. housekeeping, packing boxes). Amount of work per week ranged from 3 hours for 1 day to 7 hours for 5 days. In terms of recreational physical activity, 7 reported regular physical activity, mostly walking, 2–7 days per week from 5 minutes to 1 hour in duration.
Over-ground Walking Characteristics
We used a one-way MANOVA with Bonferroni adjustments to compare groups’ unperturbed walking characteristics (dependent variables were the mean value of each adult’s speed, stride frequency, stride length, step width, percent stance phase, percent double support phase, dimensionless speed, dimensionless stride frequency, dimensionless stride length and dimensionless step width). We obtained a significant group effect (Wilks’ Lambda = 0.26, F[10,13] = 3.65, p = 0.02, partial eta squared = 0.74). Follow-up ANOVAs revealed significant group differences for all variables except absolute and dimensionless stride frequency. Inspection of the means (See Table 2) revealed adults with DS walked slower with shorter, wider strides while spending more time in both stance and double support (speed F[1,22] = 20.96, p < 0.01, stride length F[1,22] = 39.57, p < 0.01, step width F[1,22] = 20.08, p < 0.01, percent stance phase F[1,22] = 10.28, p < 0.01, percent double support phase F[1,22] = 9.29, p < 0.01, dimensionless speed F[1,22] = 18.79, p < 0.01, dimensionless stride length F[1,22] = 32.61, p < 0.01 and dimensionless step width F[1,22] = 25.43, p < 0.01).
Table 2.
Characteristics of over-ground gait for adults with DS and TD
| DS | TD | M Difference | DS | TD | |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | DS-TD | 95% Confidence Interval | 95% Confidence Interval | |
| Stride Length (m)* | 0.75 (0.17) | 1.13 (0.15) | −0.38 | 0.65–0.85 | 1.04–1.21 |
| Dimensionless Stride Length* | 1.09 (0.23) | 1.54 (0.2) | −0.45 | 0.96–1.22 | 1.43–1.65 |
| Step Width (m)* | 0.16 (0.04) | 0.11 (0.03) | 0.05 | 0.14–0.18 | 0.09–0.13 |
| Dimensionless Step Width* | 0.23 (0.06) | 0.15 (0.04) | 0.08 | 0.20–0.26 | 0.13–0.17 |
| Velocity (m/s)* | 0.63 (0.2) | 0.99 (0.21) | −0.36 | 0.52–0.74 | 0.87–1.11 |
| Dimensionless Velocity* | 0.24 (0.08) | 0.37 (0.08) | −0.13 | 0.19–0.28 | 0.32–0.41 |
| Stride Frequency (Hz) | 0.82 (0.15) | 0.87 (0.1) | −0.05 | 0.73–0.90 | 0.81–0.93 |
| Dimensionless Frequency | 0.22 (0.04) | 0.24 (0.03) | −0.02 | 0.20–0.24 | 0.22–0.26 |
| Percent Stance* | 0.67 (0.05) | 0.63 (0.03) | 0.04 | 0.64–0.70 | 0.61–0.65 |
| Percent Double Support* | 0.33 (0.09) | 0.26 (0.05) | 0.07 | 0.28–0.38 | 0.23–0.29 |
p< 0.05
See Appendix for formulas used to convert absolute to dimensionless values.
Perturbation Condition Gait Characteristics
We used a 2 (Group) × 3 (Condition) MANOVA with Bonferroni adjustments to compare adults’ walking characteristics during the perturbation conditions (dependent variables were the same as those used for unperturbed walking). The main effects group (Wilks’ Lambda = 0.42, F[10,43] = 5.84, p < 0.01, partial eta squared =0.58) and condition (Wilks’ Lambda = 0.14, F[20,86] = 7.28, p < 0.01, partial eta squared =0.63) were significant, the interaction was not. Post hoc ANOVA results are organized by dependent variable in Table 3 for ease of interpretation.
Table 3.
Post hoc ANOVA results for gait characteristics during obstacle conditions.
| Condition | Group | |
|---|---|---|
| Stride Length | NS | F[1,52] = 38.96, p < 0.01 |
| Dimensionless Stride Length | F[2,52] = 3.57 p = 0.04 | F[1,52] = 19.73, p < 0.01 |
| Step Width | NS | F[1,52] = 29.73, p < 0.01 |
| Dimensionless Step Width | NS | F[1,52] = 40.18, p < 0.01 |
| Velocity | NS | F[1,52] = 27.50, p < 0.01 |
| Dimensionless Velocity | NS | F[1,52] = 12.92, p < 0.01 |
| Frequency | F[2,52] = 5.60, p = 0.01 | NS |
| Dimensionless Frequency | F[2,52] = 5.86, p = 0.01 | NS |
| Percent Stance Phase | F[2,52] = 27.52, p < 0.01 | F[1,52] = 19.64, p < 0.01 |
| Percent Double Support Phase | F[2,52] = 6.43, p < 0.01 | F[1,52] = 15.75, p < 0.01 |
p< 0.05, NS = not significant.
As with unperturbed walking, the groups were significantly different for all variables except absolute and dimensionless stride frequency. Adults with DS walked slower with shorter, wider strides while spending more time in both stance and double support. For the condition effect, post-hoc Tukey tests revealed 1) during both the moderate (p = 0.01) and maximal perturbations (p < 0.01) participants spent more of stance in double support 2) in the moderate perturbation condition absolute velocity (p = 0.04) and percent stance values (p < 0.01) were less than in the minimal perturbation condition and 3) during the maximal perturbation condition absolute (p = 0.02) and dimensionless stride length (p = 0.01) values were less and absolute (p < 0.01) and dimensionless frequency (p < 0.01) values were higher than the moderate perturbation condition.
Differential Scaling for Perturbations
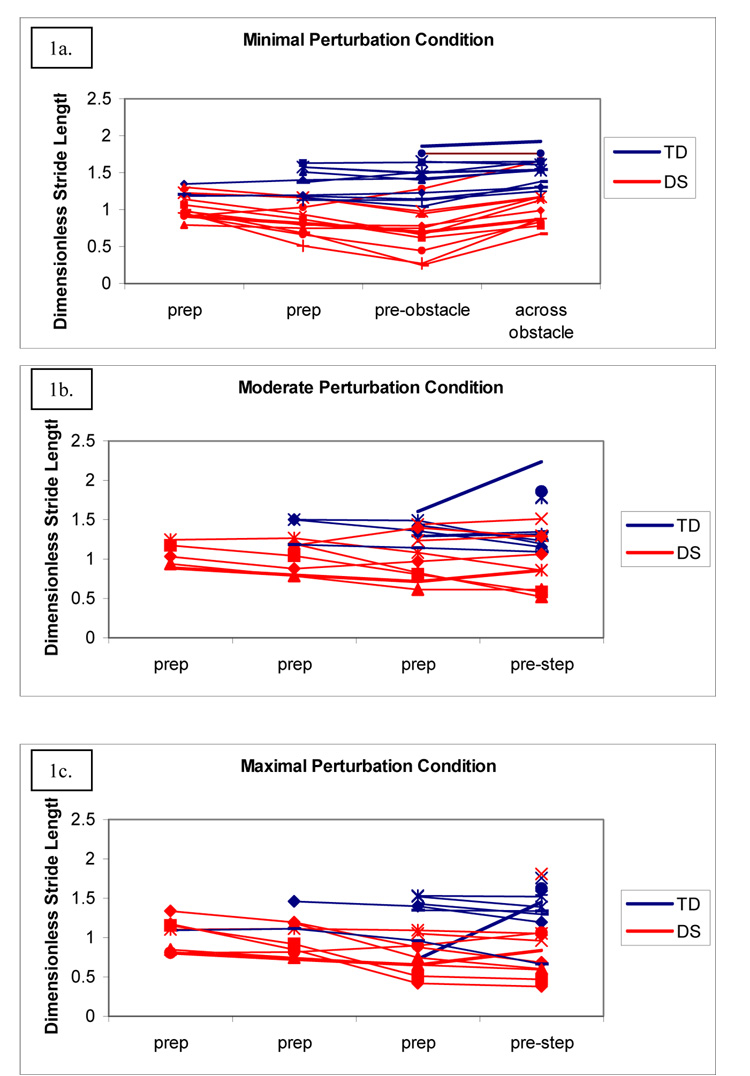
The minimal perturbation condition elicited an observable adaptation in stride length from adults with DS, but not from adults with TD (Figure 1a). In contrast, all participants showed gait adjustments for the moderate (Figure 1b) and maximal (Figure 1c) perturbation conditions. The maximal perturbation condition was even more challenging and two adults with DS were unable to perform it. Only two variables, stride length and stance phase, clearly differentiated group performance in the perturbation conditions.
Figure 1. Mean values of dimensionless stride length for each participant as they approach the obstacle.
Participants with DS show a decrease in dimensionless stride length as they approach the minimal perturbation (1a); those with TD do not. Participants with DS and TD generally show a decrease in dimensionless stride length as they approach the step for both the moderate (1b) and maximal (1c) perturbations. Group and condition effects are significant.
We used a 2 (Group) × 2 (Stride) ANOVA with repeated measures on stride to test for differences in stride length adjustments as participants approached the minimal perturbation obstacle. We included the last two strides as participants approached the obstacle. We obtained a significant group main effect (F[1,18]= 17.70, p < 0.01, partial eta squared =0.50), a significant stride main effect (F[1,18]= 5.47, p = 0.03, partial eta squared =0.23), and a significant group by stride interaction (F[1,18]= 4.62, p = 0.04, partial eta squared =0.20). Figure 1a illustrates the group by stride interaction and shows that while participants with DS decreased their dimensionless stride length from the prep stride to the pre-obstacle stride, participants with TD demonstrated longer dimensionless stride lengths that did not change.
Both groups needed to adjust stance phase in preparation for perturbation conditions (see Figure 2). However, they used different strategies to prepare for the moderate and maximal perturbation conditions. Adults with TD approached the obstacle and then adjusted their stance phase when they got close while most adults with DS showed a slow decrease in percent stance phase throughout their approach.
Figure 2. Mean values of proportion stance phase for each participant approaching the obstacles.
Participants show similar strategies for the minimal perturbation (2a) and different strategies (mostly specific to group) for the moderate (2b) and maximal (2c) perturbation conditions. Condition and group effects are both significant (see Table 2).
Minimal perturbation clearance
We used a one-way MANOVA with Bonferroni adjustments to examine group differences in foot clearance over the minimal perturbation obstacle. Six dependent variables were (in cm); maximum vertical height of the leading toe and heel markers and vertical distance between the top of the obstacle and the leading and trailing toe and heel markers as they crossed. The MANOVA was significant (Wilks’ Lambda = 0.37, F[6,65] = 18.03, p <0.01, partial eta squared = 0.63). Follow-up ANOVAs revealed significant differences for maximum vertical height of the leading heel (F[1,70] = 36.49, p < 0.01), vertical distance between the obstacle and the leading toe (F[1,70] = 5.37, p = 0.02), trailing toe (F[1,70] = 5.15, p = 0.03) and trailing heel (F[1,70] = 26.50, p < 0.01). Inspection of the means revealed adults with DS had smaller values for all.
Balance and Weight
As expected, persons with DS scored lower on the balance assessment (one-way [Group] ANOVA (F[1,23]= 41.66, p < 0.01). Out of a maximum possible score of 56 on the Berg Balance Scale, scores of participants with DS ranged from 38–54, with a mean of 47. Participants with TD scored 55 (2 participants) or 56 (10 participants).
For those with DS, we found a significant negative rank-order correlation between age and balance score (Pearson correlation −0.67, one-tailed significance = 0.01), indicating poorer performance with age. Specifically, Items 6 (stand eyes closed), 7 (stand feet together), 12 (stepping on stool) and 13 (stand one foot in front) were significantly correlated (respective Pearson correlations −0.70, −0.89, −0.68, −0.57, and one-tailed significance = 0.01, < 0.01, 0.01, 0.03).
Surprisingly, neither total Berg balance scores nor scores on Items 6, 7, 12 or 13 were correlated with percent stance phase, percent double support phase, or dimensionless stride width, frequency, stride length or velocity values for over-ground walking in those with DS, nor was weight. In adults with TD, however, weight had an effect on gait patterns. During over-ground walking, percent stance phase, percent double support phase and dimensionless stride width were positively correlated with weight (Respective Pearson correlations 0.61, 0.63, 0.59 and one-tailed significance = 0.02, 0.01, 0.02). Dimensionless stride frequency, stride length and velocity values were not.
Discussion
Overall our results show that older adults with DS demonstrate precocious stability-enhancing adaptations in gait. To achieve this increased stability, they adapt in ways seen across the lifespan in those with DS (e.g. wider step width) and in ways used by elderly adults with TD (e.g. shorter stride lengths, slower speed, more time in stance and double support). These changes take place, however, at a much younger chronological age in adults with DS compared to their peers with TD.
Adults with DS demonstrate the ability to anticipate and scale adaptations when perturbations increase, as in our minimal perturbation obstacle condition. Interestingly, they show obstacle clearance patterns similar to elderly adults with TD. Visual observation of trajectories of the leg show our adults with DS stepped with the obstacle further forward in their crossing step and produced a lower, flatter trajectory of the lead foot, with less dorsiflexion at crossing than adults with TD. This trajectory produced decreased trailing toe clearance and no difference in leading heel clearance. Chen and colleagues [37] showed healthy older adults (M age 71 years) exhibited a significantly more conservative strategy than young adults (M age 22 years) when crossing obstacles, with slower crossing speed, shorter step length, and shorter obstacle-heel strike distance.
In a follow-up study, Chen and colleagues [38] found that older adults modulated their step length and time, on average, one step ahead of the crossing step and one step earlier than younger adults. Our participants with DS adjusted their strides nearly as soon as they started walking toward the obstacle (3 to 4 strides, the equivalent of 6 to 8 steps), while the adults with TD did not adjust their strides at all. This suggests stepping over the minimal perturbation challenged only the adults with DS, and, although we cannot make a direct comparison, they may have adjusted their gait even earlier than elderly adults with TD, indicating they needed even more time to prepare for this challenge.
As none of the participants made contact with the obstacle, we can conclude that their stability-enhancing adaptations were successful. However, there is a limit to the ultimate adaptability of compensatory strategies. As the effects of age accumulate and/or inactivity increases failure begins to appear. In the Chen study mentioned above [38], 4 out of the 24 older adults caught their heel on the obstacle. In a study involving 25 young (20–37 years) and 99 older adults (65–88 years) where participants walked on a treadmill and an obstacle was dropped in front of the left foot at various phases in the step cycle, successful obstacle clearance rates decreased with increasing age. Only the 65–69 year olds were not different from young adults with respect to success rate, despite marked changes in the other parameters measured. The youngest of the older adults showed gait changes and no decrease in success rates while their older peers showed gait changes and decreased success rates [39].
Where do these stability-enhancing adaptations come from, and why does their success seem to diminish over time? For adults with TD, scholars suggest these stability seeking behaviors are related to a global definition of stability that involves fear of falling and confidence in one’s ability to avoid falling in addition to more physical changes [40]. This idea is supported by Rosengren’s work [21] showing that inactive older adults adopt a more cautious walking style than active ones. It appears that inactivity, confidence and physical performance are intertwined; the less active you are, the more your confidence and physical ability to respond decrease. Conversely, decreased confidence can make you less active.
Although stabilizing adaptations are sufficient initially, with further decline in the system they become inadequate. These changes are subtle at first, and difficult to detect with gross clinical measures. Rosengren [21] found, for example, that Berg balance scores clustered in a narrow range (group means 52.2 and 55.2) and did not differentiate performance. Cromwell and Newton [13], however, found a significant correlation between alternate stepping on a stool (Item 12) and both walking velocity and number of steps per meter. Our adults with DS walked slower and scored lower on the Berg than Cromwell’s older adult participants; however we found no correlation between the alternate stepping on a stool item and walking velocity. This indicates that the issues contributing to walking performance in adults with DS are multifactorial and involve more than the ability to perform alternate stepping movements.
For our adults with DS, weight was not significantly correlated with gait variables for over-ground walking as it was in adults with TD. This again indicates that the issues contributing to their walking performance are numerous and difficult to trace to one or two cause and effect relationships. Before we consider how they might adapt their gait for obesity, we have to remember they are already showing greater percent stance phase, percent double support phase and dimensionless stride width values than their peers with TD. These existing strategies may be sufficient when faced with increased body weight, or they may have reached maximum adaptability and are unable to adjust further.
Conclusion
The combined effects of ligamentous laxity, low tone, obesity, inactivity and body structure and function decrements associated with aging leave adults with DS with many barriers to maintaining a healthy, active, pain-free lifestyle. Although they demonstrate stability-enhancing adaptations during the activities of perturbed and unperturbed walking, information allowing us to link this to increased fall risk does not exist for this population. Adults with DS may experience an increased risk of falling at an earlier age than their peers with TD as they face multiple constraints to walking stability earlier in life. Our findings so far are in agreement with Hale and colleagues [41], who concluded that the increased risk of falling in adults with various intellectual disabilities is multi-factorial. In future research, we plan to explore the link between decreased stability and risk of falling in adults with DS, as well as the effectiveness of intervention to increase overall stability. We believe intervention incorporating variations in task and environment (for further discussion see Huxham, Goldie, & Patla [42]) will be beneficial in helping adults with DS maintain optimal functional mobility and health.
Acknowledgements
This research was supported by funds from the National Institutes of Health grant HD42728 awarded to Beverly D. Ulrich. Beth A. Smith was financially supported by grant H424C010067 from the US Office of Special Education and Rehabilitative Services awarded to Dale Ulrich. We would like to thank Allison McIntyre for her help with data analysis.
Appendix
Formulas for Normalization
where v̂ (velocity), fSTRIDE (stride length), f̂STRIDE (stride frequency), and ŵSTEP (step width) are converted gait variables, lo is leg length (sum of thigh length and shank length) and g is acceleration due to gravity. For further information on the derivation of these formulas please see Ulrich et al. [18].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Beth A Smith, University of Michigan, Division of Kinesiology, 401 Washtenaw Ave., Ann Arbor, MI 48109-2214, bethas@umich.edu.
Beverly D Ulrich, University of Michigan, Division of Kinesiology, 1402 Washington Heights, Ann Arbor, MI 48109-2013, bdulrich@umich.edu
References
- 1.Glasson EJ, Sullivan SG, Hussain R, Petterson BA, Montgomery PD, Bittles AH. The changing survival profile of people with Down's syndrome: implications for genetic counselling. Clin Genet. 2002;62:390–393. doi: 10.1034/j.1399-0004.2002.620506.x. [DOI] [PubMed] [Google Scholar]
- 2.Day SM, Strauss DJ, Shavelle RM, Reynolds RJ. Mortality and causes of death in persons with Down syndrome in California. Dev Med Child Neurol. 2005;47:171–176. doi: 10.1017/s0012162205000319. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura E, Tanaka S. Biological ages of adult men and women with Down’s syndrome and its changes with aging. Mech Ageing Dev. 1998;105:89–103. doi: 10.1016/s0047-6374(98)00081-5. [DOI] [PubMed] [Google Scholar]
- 4.Maaskant MA, van der Akker M, Kessels AGH, Haveman MJ, van Schrojenstein Lantman-de Valk HMJ, Urlings HFJ. Care dependence and activities of daily living in relation to ageing: results of a longitudinal study. J Intellect Disabil Res. 1996;40:535–543. doi: 10.1046/j.1365-2788.1996.807807.x. [DOI] [PubMed] [Google Scholar]
- 5.Janicki MP, Jacobson JW. Generational trends in sensory, physical, and behavioral abilities among older mentally retarded persons. Am J Ment Defic. 1986;90:490–500. [PubMed] [Google Scholar]
- 6.Diamond LS, Lynne D, Sigman B. Orthopedic Disorders in Patients with Down’s syndrome. Orthop Clin North Am. 1981;12:57–71. [PubMed] [Google Scholar]
- 7.Melville CA, Cooper SA, McGrother CW, Thorp CF, Collacott R. Obesity in adults with Down syndrome: a case-control study. J Intellect Disabil Res. 2005;49:125–133. doi: 10.1111/j.1365-2788.2004.00616.x. [DOI] [PubMed] [Google Scholar]
- 8.Lott IT, Head E. Down Syndrome and Alzheimer’s Disease: A link between development and aging. Ment Retard Dev Disabil Res Rev. 2001;7:172–178. doi: 10.1002/mrdd.1025. [DOI] [PubMed] [Google Scholar]
- 9.Schupf N, Pang D, Patel BN, Silverman W, Schubert R, Lai F, Kline JK, Stern Y, Ferin M, Tycko B, Mayeux R. Onset of dementia is associated with age at menopause in women with Down’s syndrome. Ann Neurol. 2003;54:433–438. doi: 10.1002/ana.10677. [DOI] [PubMed] [Google Scholar]
- 10.Teipel SJ, Alexander GE, Schapiro MB, Moller HJ, Rapoport SI, Hampel H. Age-related cortical grey matter reductions in non-demented Down’s syndrome adults determined by MRI with voxel-based morphometry. Brain. 2004;127:811–824. doi: 10.1093/brain/awh101. [DOI] [PubMed] [Google Scholar]
- 11.Stolze H, Friedrich HJ, Steinauer K, Vieregge P. Stride parameters in healthy young and old women--measurement variability on a simple walkway. Exp Aging Res. 2000;26:159–168. doi: 10.1080/036107300243623. Apr–Jun. [DOI] [PubMed] [Google Scholar]
- 12.Samson MM, Crowe A, de Vreede PL, Dessens JA, Duursma SA, Verhaar HJ. Differences in gait parameters at a preferred walking speed in healthy subjects due to age, height and body weight. Aging Clin Exp Res. 2001;13:16–21. doi: 10.1007/BF03351489. Feb. [DOI] [PubMed] [Google Scholar]
- 13.Cromwell RL, Newton RA. Relationship between balance and gait stability in healthy older adults. J Aging Phys Act. 2004;12:90–100. doi: 10.1123/japa.12.1.90. [DOI] [PubMed] [Google Scholar]
- 14.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. J Am Geriatr Soc. 1997;45:313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 15.Winter DA, Patla AE, Frank JS, Walt SE. Biomechanical walking pattern changes in the fit and healthy elderly. Phys Ther. 1990;70:340–347. doi: 10.1093/ptj/70.6.340. [DOI] [PubMed] [Google Scholar]
- 16.Kubo M, Ulrich BD. Early stage of walking: development of control in mediolateral and anteroposterior directions. J Mot Behav. 2006;38:229–237. doi: 10.3200/JMBR.38.3.229-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulrich DA, Ulrich BD, Angulo-Kinzler RM, Yun J. Treadmill training of infants with Down syndrome: evidence-based developmental outcomes. Pediatrics. 2001 Nov;108:E84. doi: 10.1542/peds.108.5.e84. [DOI] [PubMed] [Google Scholar]
- 18.Ulrich BD, Haehl V, Buzzi UH, Kubo M, Holt KG. Modeling dynamic resource utilization in populations with unique constraints: preadolescents with and without Down syndrome. Hum Mov Sci. 2004 Sep;23:133–156. doi: 10.1016/j.humov.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Smith BA, Kubo M, Black DP, Holt KG, Ulrich BD. Effect of practice on a novel task--walking on a treadmill: preadolescents with and without Down syndrome. Phys Ther. 2007;87:766–777. doi: 10.2522/ptj.20060289. [DOI] [PubMed] [Google Scholar]
- 20.Fujiura GT, Fitzsimons N, Marks B, Chicoine B. Predictors of BMI among adults with Down syndrome: The social context of health promotion. Res Dev Disabil. 1997;18:261–274. doi: 10.1016/s0891-4222(97)00008-5. [DOI] [PubMed] [Google Scholar]
- 21.Rosengren KS, McAuley E, Mihalko SL. Gait adjustments in older adults: activity and efficacy influences. Psychol Aging. 1998;13:375–386. doi: 10.1037//0882-7974.13.3.375. [DOI] [PubMed] [Google Scholar]
- 22.Spyropoulos P, Pisciotta JC, Pavlou KN, Cairns MA, Simon SR. Biomechanical gait analysis in obese men. Arch Phys Med Rehabil. 1991;72:1065–1070. [PubMed] [Google Scholar]
- 23.Browning RC, Kram R. Energetic Cost and Preferred Speed of Walking in Obese vs. Normal Weight Women. Obes Res. 2005;13:891–899. doi: 10.1038/oby.2005.103. [DOI] [PubMed] [Google Scholar]
- 24.Delbono O. Neural control of aging skeletal muscle. Aging Cell. 2003;2:21–29. doi: 10.1046/j.1474-9728.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- 25.Lord SR, Clark RD, Webster IW. Postural stability and associated physiological factors in a population of aged persons. J Gerontol. 1991;46:M69–M76. doi: 10.1093/geronj/46.3.m69. [DOI] [PubMed] [Google Scholar]
- 26.Matsumura BA, Ambrose AF. Balance in the elderly. Clin Geriatr Med. 2006;22:395–412. doi: 10.1016/j.cger.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Speers RA, Kuo AD, Horak FB. Contributions of altered sensation and feedback responses to changes in coordination of postural control due to aging. Gait Posture. 2002;16:20–30. doi: 10.1016/s0966-6362(02)00003-6. [DOI] [PubMed] [Google Scholar]
- 28.Woollacott MH, Tang PF. Balance control during walking in the older adult: research and its implications. Phys Ther. 1997;77:646–660. doi: 10.1093/ptj/77.6.646. [DOI] [PubMed] [Google Scholar]
- 29.Menz HB, Lord SR, Fitzpatrick RC. Age-related differences in walking stability. Age Ageing. 2003;32:137–142. doi: 10.1093/ageing/32.2.137. [DOI] [PubMed] [Google Scholar]
- 30.Menz HB, Lord SR, Fitzpatrick RC. A structural equation model relating impaired sensorimotor function, fear of falling and gait patterns in older people. Gait Posture. 2007;25:243–249. doi: 10.1016/j.gaitpost.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Berg K, Wood-Dauphinee S, Williams JI, Gayton D. Measuring balance in the elderly: preliminary development of an instrument. Physiother Can. 1989;41:304–311. [Google Scholar]
- 32.Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health. 1992;83 Suppl 2:S7–S11. [PubMed] [Google Scholar]
- 33.Sackley C, Richardson P, McDonnell K, Ratib S, Dewey M, Hill HJ. The reliability of balance, mobility and self-care measures in a population of adults with a learning disability known to a physiotherapy service. Clin Rehabil. 2005;19:216–223. doi: 10.1191/0269215505cr815oa. [DOI] [PubMed] [Google Scholar]
- 34.Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: The Health Assessment Questionnaire, disability and pain scales. J Rheumatol. 1982;9:789–793. [PubMed] [Google Scholar]
- 35.Fries JF, Spitz PW, Kraines G, Holman H. Measurement of Patient Outcome in Arthritis. Arthritis Rheum. 1980;23:137–145. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 36.Ramey DR, Fries JF, Singh G. The Health Assessment Questionnaire 1995 -- Status and Review. In: Spilker B, editor. Quality of Life and Pharmacoleconomics in Clinical Trials. 2nd ed. Philadelphia: Lippincott-Raven Publishers; 1996. pp. 227–237. [Google Scholar]
- 37.Chen HC, Ashton-Miller JA, Alexander NB, Schultz AB. Stepping over obstacles: gait patterns of healthy young and old adults. J Gerontol. 1991;46:M196–M203. doi: 10.1093/geronj/46.6.m196. [DOI] [PubMed] [Google Scholar]
- 38.Chen HC, Ashton-Miller JA, Alexander NB, Schultz AB. Effects of age and available response time on ability to step over an obstacle. J Gerontol. 1994;49 doi: 10.1093/geronj/49.5.m227. M227-33. [DOI] [PubMed] [Google Scholar]
- 39.Weerdesteyn V, Nienhuis B, Duysens J. Advancing age progressively affects obstacle avoidance skills in the elderly. Hum Mov Sci. 2005;24:865–880. doi: 10.1016/j.humov.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 40.Balash Y, Hadar-Frumer M, Herman T, Peretz C, Giladi N, Hausdorff JM. The effects of reducing fear of falling on locomotion in older adults with a higher level gait disorder. J Neural Transm. 2007. e-published ahead of print. [DOI] [PubMed]
- 41.Hale L, Bray A, Littmann A. Assessing the balance capabilities of people with profound intellectual disabilities who have experienced a fall. J Intellect Disabil Res. 2007;51:260–268. doi: 10.1111/j.1365-2788.2006.00873.x. [DOI] [PubMed] [Google Scholar]
- 42.Huxham FE, Goldie PA, Patla AE. Theoretical considerations in balance assessment. Aust J Physiother. 2001;47:89–100. doi: 10.1016/s0004-9514(14)60300-7. [DOI] [PubMed] [Google Scholar]