Abstract
Lipid transfer proteins (LTPs) play important roles in cellular biology, and fluorescence spectroscopy has found wide range use as a facile means for time-resolved monitoring of protein-lipid interactions[1]. Here, we show how the fluorescence emission properties of dansyl-DHPE can be exploited to characterize lipid extraction and lipid transfer kinetics. The GM2 activator protein serves as an example LTP where the ability to independently characterize lipid extraction from donor vesicles, formation of a protein:lipid complex in solution, and release of lipid from the complex to acceptor liposomes is crucial for full kinetic characterization of lipid transfer.
Lipid transfer, lipid trafficking and lipid sorting all play important roles in cellular biology, and examples include but are not limited to the following: lipid metabolism, organelle membrane structure, cholesterol metabolism and trafficking, food and pollen allergens, and lipoprotein metabolism, to name a few[2–6]. As such, facile assays for monitoring kinetics of lipid extraction and of lipid release are desired. Here we show that the fluorescence properties of dansyl-DHPE, specifically the sensitivity of the emission wavelength and quantum yield to the polarity of the local environment[7], can be exploited as a means for monitoring lipid extraction from and lipid release to POPC liposomes.
In general, the study of lipid transfer includes but is not limited to the following steps: binding of the LTP to the donor vesicle, extraction of a ligand and release of the LTP:ligand complex, binding of the LTP:ligand complex to the acceptor vesicle, and release of the ligand to the acceptor vesicle with subsequent dissociation of the protein. Full characterization of the process requires the ability to track and quantify the relocation of the given ligand from donor to acceptor vesicles, including characterization of the protein:ligand complex in solution.
GM2AP is a non-enzymatic accessory protein in the degradation of neuronal gangliosides. Its primary function is to extract GM2 out of intralysosomal vesicle membranes forming the GM2-AP:GM2 complex, which is the substrate for GM2 hydrolysis by a water soluble hydrolyase[8]. GM2AP is also known to transfer GM2 between donor and acceptor vesicles[9]. The molecular level details of the mechanism by which GM2AP functions remain unclear. Hence, we are interested in understanding how this protein binds to lipid vesicles, recognizes specific lipid ligands, extracts lipids (specific or non-specific) from membranes and transfers/releases its ligands. Here, we have utilized GM2AP as an example LTP to demonstrate the utility of the fluorescent properties of dansyl-DHPE for monitoring the kinetics of lipid transfer and extraction.
In addition to its specific ligand, GM2AP has been shown in vitro to bind to other lipids and fatty acids[10]. This crystal structure analysis of GM2AP with various ligands bound reveals a unique β-cup hydrophobic cavity which can accommodate lipid molecules in different binding modes. For example, when GM2 binds to GM2AP, the ceramide tails of GM2 occupy a hydrophobic pocket within the β-cup protein and the tetrasaccharide moiety projects out of the protein surface into the aqueous environment. When phosphatidylglycerol binds to GM2AP, a different orientation of the lipid ligand is observed, where the distal end of the fatty acid chains occupies a similar position as the ceramide tail, however the phosphoglycerol head group is completely buried within the hydrophobic pocket[10].
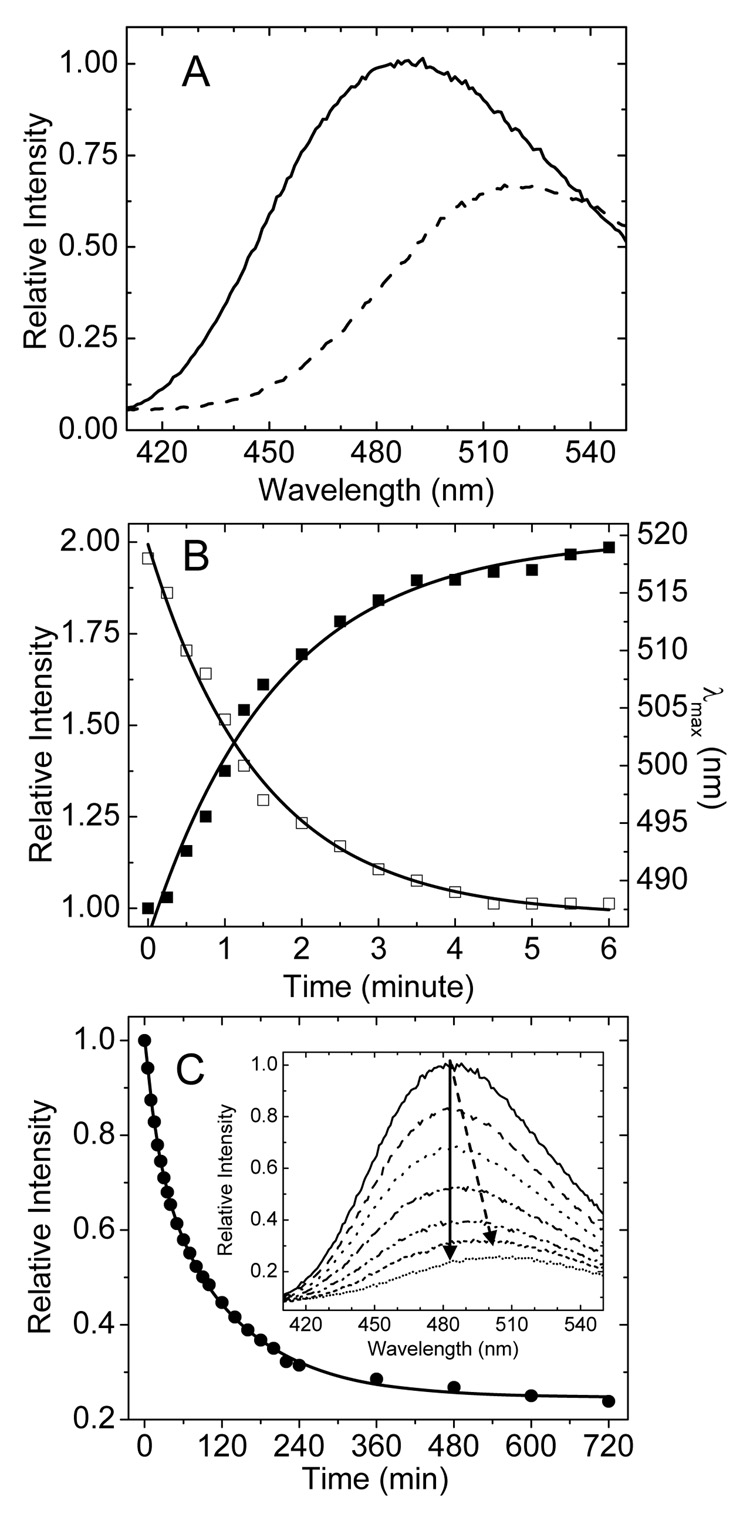
We found that GM2AP also binds dansyl-DHPE. The complex is formed by injecting dansyl-DHPE in ethanol into a solution of GM2AP (pH 4.8), and unbound ligand is removed by gel-filtration chromatography. UV-VIS absorption spectra show a GM2AP:dansyl-DHPE ratio of 1:1, which is consistent with analysis of the GM2AP:POPG crystal structure. For the protein:lipid complex, the maximal emission fluorescence wavelength is near 484 nm, which is similar to dansyl-DHPE dissolved in benzene[7]. A red-shift to 518 nm is obtained for extruded 100 nm large unilamellar vesicles of POPC:dansyl-DHPE(4:1), and is similar the emission wavelength when dansyl-DHPE is dissolved in methanol. Figure 1A shows fluorescence emission spectra for dansyl-DHPE in these environments. The differences in fluorescence intensity and emission wavelengths are consistent with previous literature reports characterizing the fluorescent properties of this fluorophore[7], and these findings provide a model picture for dansyl-DHPE sequestration into the hydrophobic binding pocket of GM2AP. When dansyl-DHPE is in POPC LUVs, the head-group labeled fluorophore is in contact with the relatively high polarity aqueous solution. When the ligand is bound to GM2AP, it likely adopts a conformation similar to the model of phosphatidylglycerol, which has the head group buried within the hydrophobic pocket of GM2AP, consistent with a polarity similar to a solution of benzene. These changes in spectral properties can be exploited to monitor the kinetics of both the extraction of dansyl-DHPE from POPC liposomes as well as the release/transfer of the fluorescence lipid from the GM2AP:dansyl-DHPE complex to liposomes.
Fig.1.
Changes in fluorescence emission spectra during extraction and release of dansyl-DHPE. (A) The fluorescence emission spectra of 0.2 µM GM2AP:dansyl-DHPE complex (solid line) and 1 µM POPC:dansyl-DHPE (4:1) vesicles (dashed line). The spectra were recorded on a FLUOROMAX-3 fluorometer with excitation at 340 nm with a 90° and a 0° orientation of the excitation and emission polarizer, respectively. All experiments were performed at 20°C by using a water bath circulator. GM2AP functions in the acidic compartment of the lysosome; therefore all studies herein are performed in 50 mM sodium acetate buffer at pH 4.8 (B) Plots of the relative emission intensity at 484 nm (filled squares) and the value of maximal emission wavelength (λmax) (open square) as a function of time during the extraction of dansyl-DHPE from 1 µM(final concentration) POPC:dansyl-DHPE (4:1) vesicles by GM2AP. GM2AP was added to 5 µM final concentration. Solid lines show fits to a first order single exponential rate process. (C) Plot of the relative emission intensity as a function of time for the release of dansyl-DHPE from 0.25 µM GM2AP:dansyl-DHPE complex with 20 µM POPC vesicles present (filled circle). The solid line is the best fit to a two-component first order processes. The inset plots representative spectra over a 600 minute time period showing how the spectral intensity decreases and the wavelength red shifts overtime (indicted by the arrows).
Results for lipid extraction are shown in Figure 1B. Here, purified GM2AP is mixed with extruded POPC:dansyl-DHPE (4:1) LUVs. Upon extraction from the membrane and formation of the protein:lipid complex, both a blue shift in the maximum emission wavelength and an increase in fluorescence intensity are seen. In Figure 1B, the molar ratio of GM2AP to total dansyl-DHPE is 25:1, where GM2AP is added in excess to ensure nearly complete extraction from the outer leaflet. (However, note that dansyl-DHPE is likely distributed on both leaflets of the vesicle and we assume that only the outer leaflet is accessible for ligand extraction.) After GM2AP was added and rapidly mixed, spectral scans were recorded every 15 seconds. Figure 1B plots as a function of time, both the intensity at 484 nm and the maximum emission wavelength for the extraction process. The maximal emission wavelength shifts from a value of 518 nm, corresponding to dansyl-DHPE in the liposome membrane, to 486 nm, which is very close that for the purified GM2AP:dansyl-DHPE complex (484 nm). Both plots can be fit fairly well with a single exponential first order process with halt life of 1.5±0.1 min.
The fluorescence change of dansyl-DHPE can be exploited when studying ligand release from the protein complex with transfer to the POPC vesicle. Here, the GM2AP:dansyl-DHPE complex was prepared, and after recording the spectrum of the complex, POPC vesicles were added and spectra were recorded over a time period of 600 minutes. Figure 1C plots the fluorescence emission intensity at 484 nm as a function of time, and a two component first order process fits the data fairly well, with half lives of t1=21±2 min and t2=124±4 min. The inset in Figure 1C shows representative spectra over this time period. By comparing the kinetic results in Figure 1, we clearly see that the extraction of dansyl-DHPE from POPC vesicles is much faster than the release from the protein complex to POPC vesicles. We are currently investigating the relevance of the two component release process for the mechanism of GM2AP lipid transfer, and those experiments are beyond the scope of the work presented here. Nevertheless, the significance here is that these data demonstrate the utility and sensitivity of dansyl-DHPE as a general tool for probing the details of lipid extraction and transfer.
The ability of GM2AP to extract dansyl-DHPE from the liposomes forming a solution stable GM2AP:dansyl-DHPE complex was independently confirmed by sedimentation assays using ultracentrifugation of sucrose loaded vesicles coupled to fluorescamine labeling for quantification of the protein remaining in solution and measurement of the fluorescence signal of dansyl-DHPE as a function of protein concentration[11]. For GM2AP, it is important to consider the formation of the protein:lipid complex when characterizing lipid extraction/transfer assays, as the 6 sedimentation assays reveal that nearly 80% of the protein at equilibrium exists in solution (Data are shown in Supp Info). This finding requires that the results of the commonly used octadecylrhodamine (R18) method to assay GM2AP function be interpreted carefully. Typically, in the R18 assays, donor vesicles are prepared that contain 5–15% R18, which is self quenching when incorporated into vesicles at these concentrations. Upon addition of a LTP and acceptor vesicles, the fluorescence intensity increases as ligand is extracted from the acceptor vesicle and transferred to the donor vesicle[9,12].
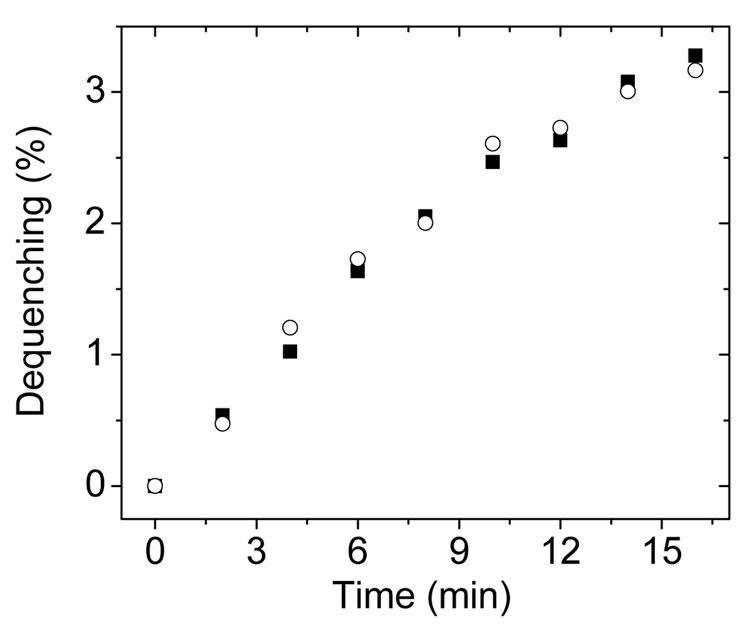
However, as the data in Figure 2 show, it is very important to consider the formation of a protein:lipid complex when analyzing the results from the R18 transfer assays. Figure 2 plots the increase in fluorescence intensity as a function of time observed for two different experiments: (1) donor vesicles of POPC:R18 (19:1) mixed with GM2AP (filled squares) and (2) donor vesicles of POPC:R18 (19:1), acceptor vesicles (POPC only) and GM2AP (open circles). The percentage dequenching observed for both experiments are nearly identical. Hence, care must be taken when interpreting the results from such an assay regarding actual ligand transfer. The observed dequenching is likely arising from the formation of the protein:R18 complex in solution, and NOT transfer to the liposome. This potential drawback of the R18 methodology has also been noted by others[13,14]. Nevertheless, the R18 dequenching assay has found utility as a means of characterizing relative lipid binding affinities for numerous proteins, including GM2AP[9].
Fig. 2.
Results from two different R18 dequenching assays. In the first experiments (filled squares) only donor vesicles and GM2AP were mixed. Spectra were recorded for GM2AP final concentration of 0.75 µM with 20 µM POPC:R18 (95:5) vesicles. In the second experiments, (open circles) donor vesicles, GM2AP and acceptor vesicles were mixed. Here, 20 µM POPC:R18 (95:5) donor vesicles and 20 µM POPC vesicles were incubated for 10 min; GM2AP was added to a final concentration of 0.75 µM. Before addition of GM2AP, the emission intensity at 590 nm was set as 0% dequenching. The intensities after complete solubilization of the POPC:R18 (95:5) vesicles by 0.1% Triton-100 was set as 100% dequenching. Spectra collected with 570 nm excitation wavelength.
It should be noted that the use of the dansyl-DHPE to characterize lipid extraction and transfer does not allow for an independent monitoring of membrane binding/partitioning of the protein or protein:lipid complex. Other biophysical characterizations are necessary to fully characterize each of the steps within the process. In addition, although dansyl-DHPE is not the specific substrate of GM2AP, this assay can be used in future studies in competition based assays where GM2 is present, as well as for investigating the effect that various lipid compositions of both the acceptor and donor vesicle have upon the extraction and transfer processes, respectively.
As shown here, dansyl-DHPE can be utilized to study lipid transfer pathways by separating the procedure into two steps of lipid extraction from liposomes and lipid release from a protein:lipid complex. It is likely that this assay will be of use in the study of a variety of LTPs and provides an alternative to radioactive labeled lipids or other fluorescent based assays.
Supplementary Material
Acknowledgements
The research herein was funded by NIH R01GM077232 to GEF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maier O, Oberle V, Hoekstra D. Fluorescent lipid probes: some properties and applications (a review) Chem. Phys. Lipids. 2002;116:3–18. doi: 10.1016/s0009-3084(02)00017-8. [DOI] [PubMed] [Google Scholar]
- 2.De Matteis MA, Di Campli A, D'Angelo G. Lipid-transfer proteins in membrane trafficking at the Golgi complex. Biochim. Biophys. Acta. 2007;1771:761–768. doi: 10.1016/j.bbalip.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Kolter T, Winau F, Schaible UE, Leippe M, Sandhoff K. Lipid-binding proteins in membrane digestion, antigen presentation, and antimicrobial defense. J. Biol. Chem. 2005;280:41125–41128. doi: 10.1074/jbc.R500015200. [DOI] [PubMed] [Google Scholar]
- 4.Yeats TH, Rose JK. The biochemistry and biology of extracellular plant lipid-transfer proteins (LTPs) Protein Sci. 2008;17:191–198. doi: 10.1110/ps.073300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang XC, Zhou HW. Plasma lipid transfer proteins. Curr. Opin. Lipidol. 2006;17:302–308. doi: 10.1097/01.mol.0000226124.94757.ee. [DOI] [PubMed] [Google Scholar]
- 6.Ile KE, Schaaf G, Bankaitis VA. Phosphatidylinositol transfer proteins and cellular nanoreactors for lipid signaling. Nat. Chem. Biol. 2006;2:576–583. doi: 10.1038/nchembio835. [DOI] [PubMed] [Google Scholar]
- 7.Waggoner AS, Stryer L. Fluorescent probes of biological membranes. Proc. Natl. Acad. Sci. U S A. 1970;67:579–589. doi: 10.1073/pnas.67.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolter T, Sandhoff K. Sphingolipid metabolism diseases. Biochim. Biophys. Acta. 2006;1758:2057–2079. doi: 10.1016/j.bbamem.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Smiljanic-Georgijev N, Rigat B, Xie B, Wang W, Mahuran DJ. Characterization of the affinity of the G(M2) activator protein for glycolipids by a fluorescence dequenching assay. Biochim. Biophys. Acta. 1997;1339:192–202. doi: 10.1016/s0167-4838(97)00002-2. [DOI] [PubMed] [Google Scholar]
- 10.Wright CS, Mi LZ, Lee S, Rastinejad F. Crystal structure analysis of phosphatidylcholine-GM2-activator product complexes: evidence for hydrolase activity. Biochemistry. 2005;44:13510–13521. doi: 10.1021/bi050668w. [DOI] [PubMed] [Google Scholar]
- 11.Buser CA, McLaughlin S. Ultracentrifugation technique for measuring the binding of peptides and proteins to sucrose-loaded phospholipid vesicles. Methods Mol. Biol. 1998;84:267–281. doi: 10.1385/0-89603-488-7:267. [DOI] [PubMed] [Google Scholar]
- 12.Schwarzmann G, Wendeler M, Sandhoff K. Synthesis of novel NBD-GM1 and NBD-GM2 for the transfer activity of GM2-activator protein by a FRET-based assay system. Glycobiology. 2005;15:1302–1311. doi: 10.1093/glycob/cwj018. [DOI] [PubMed] [Google Scholar]
- 13.Epand RF, Schlattner U, Wallimann T, Lacombe ML, Epand RM. Novel lipid transfer property of two mitochondrial proteins that bridge the inner and outer membranes. Biophys. J. 2007;92:126–137. doi: 10.1529/biophysj.106.092353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohki S, Flanagan TD, Hoekstra D. Probe transfer with and without membrane fusion in a fluorescence fusion assay. Biochemistry. 1998;37:7496–7503. doi: 10.1021/bi972016g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.