Abstract
Intracellular transmission of extracellular signals is mediated by a complex network of second messengers, interacting proteins and signaling cascades that cumulatively govern essentially all stimulated cellular processes. Two important signaling components in the network are the extracellular signal-regulated kinase (ERK) signaling cascade and elevation of calcium concentrations, which transmit signals of hormones, growth factors and other ligands. Each of these components is known to independently regulate a large number of targets in various cellular organelles. The cooperation between them, however, seems to modulate the fate of their individual signals, and accordingly, their regulated processes. We have recently shown that calcium modulates the protein interaction properties of ERKs, which further affect the subcellular localization of the latter and as a consequence also the distribution of their targets. These effects of calcium are important in determining the specificity of the ERK cascade, and thereby, play important roles in the regulation of ERK-dependent cellular processes. Our findings, as well as their possible implications, are further discussed in this addendum.
Key words: MAPK, calcium, subcellular localization, nuclear translocation
The ERK cascade is a central signaling pathway that transmits signals from a variety of extracellular agents to regulate processes, such as proliferation, differentiation, development and more.1–3 Upon stimulation, the cascade is activated by various mechanisms, which usually results in the activation of the small GTPases Ras. Those central proteins, in turn, transmit the signal further via sequential phosphorylation and activation of protein kinases, known as the ERK cascade. The components of this cascade are usually Raf kinases that are directly influenced by Ras, followed by MAPK/ERK kinase 1/2 (MEKs), ERK1/2 (ERKs), and finally also MAPKAPKs, such as RSK, MNK and MSK. Upon activation, ERKs and the downstream kinases phosphorylate hundreds of regulatory targets in the cytoplasm, or nuclear targets that are reached upon translocation of ERKs and the MAPKAPKs into the nucleus. These phosphorylations, in turn, regulate transcription, translation or stability of the downstream targets to allow their action in a variety of ERK-dependent cellular processes.
One of the signaling events that may influence the ERK cascade is a rise in free intracellular calcium concentrations, induced by many extracellular ligands.4–6 Calcium is a versatile second messenger that affects many signaling processes in the cell. Its concentrations are induced by various stimulations that activate phospholipase C (PLC), which produces IP3 that, in turn, releases calcium from its internal stores (Fig. 1). In addition, elevation of calcium concentrations is mediated by membranal calcium channels, which are stimulated by extracellular ligands via other mechanisms. Interestingly, elevated calcium concentrations can either activate or, less frequently, inhibit the ERK cascade.7,8 Molecular mechanisms that mediate the activation process include: (i) Activation of the calcium-affected kinase PYK2.9 (ii) Stimulation of the nucleotide exchange factor Ras-GRF.10 (iii) Inhibition of Ras-GAPs, which is a GTPase activating protein of Ras.11,12 (iv) Activation of both calmodulin-dependent kinases I and II that operate independently either by stabilization of EGF-receptor,13 or by other unknown mechanisms.14
Figure 1.
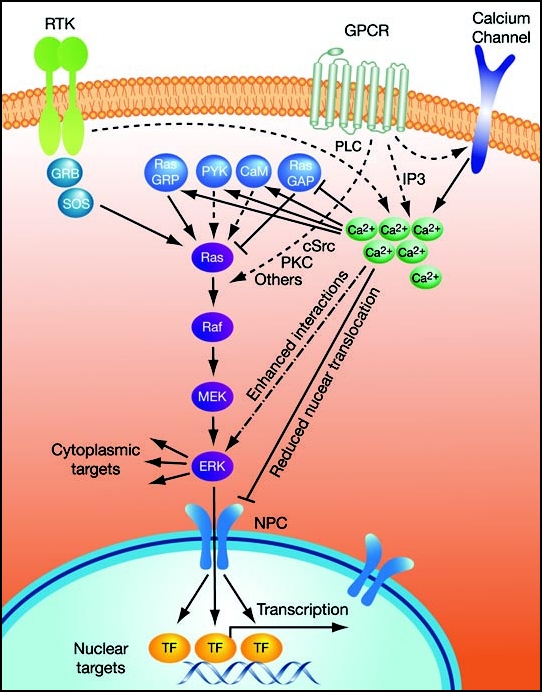
Schematic representation of the mechanisms by which calcium affects the ERK cascade. For more details see text.
Interaction of ERKs with various proteins, such as scaffolds and phosphatases plays an important role in regulating the specificity of the ERK cascade.15,16 In the course of a wide search for ERK interacting proteins, we observed that changes in calcium concentrations significantly affect the binding of various proteins to ERK2.17 Thus, the repertoire of proteins that bind to an immobilized ERK2, and then released with a high salt concentration, was significantly different between extracts from non-treated cells or cells with modified calcium concentrations. In particular, the number of bound proteins was increased when cellular calcium concentrations were elevated by calcium ionophore before extraction. Fewer proteins, however, were bound from extracts of cells in which calcium concentrations were reduced by a calcium chelator. The identity of some of the proteins with calcium-affected binding was determined, and found to include various protein groups such as protein kinases, cytoskeletal elements, scaffold proteins and ERK substrates. In vivo binding assay with GST-ERK2 confirmed that calcium increases the binding of proteins, such as MEK1 and vinculin, but not Elk1, to ERK2.
Due to the fact that protein-protein interactions play a crucial role in the determination of the subcellular distribution of ERKs,18 we undertook to examine the influence of calcium on the dynamic changes in the subcellular localization of ERKs in resting and stimulated cells. We found that elevated calcium concentrations prevent the nuclear translocation of ERKs upon stimulation, whereas reduced calcium accelerates this translocation. We further demonstrated that this effect might be mediated by increased binding affinity of ERK2 to the nuclear pore protein NUP153, which may block the penetration of ERKs through the nuclear pores. These results raised the possibility that calcium also participates in the localization-mediated regulation of ERK-dependent targets. Indeed, this possibility was supported by our findings that the ligand LPA, which significantly elevates calcium concentrations, primarily induces activation of the cytoplasmic ERK substrate RSK, but not the nuclear substrate Elk1. This preferential activation of RSK was inhibited by calcium chelation, while EGF that induces a very small elevation of calcium concentration, mainly activated Elk1 in a calcium-independent manner.
In summary, we established that besides the ability of calcium to activate, and in some cases inhibit, the ERK cascade, it may also participate in the regulation of the protein-protein interactions, and therefore, also the subcellular localization of ERKs. This, in turn, may modify the repertoire and localization of ERKs' substrates under varying conditions, and in this way, the changes of calcium concentrations may play an important role in the determination of signaling specificity of the cascade and its functional outcomes.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/6107
References
- 1.Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- 2.Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 3.Avruch J. MAP kinase pathways: The first twenty years. Biochim Biophys Acta. 2007;1773:1150–1160. doi: 10.1016/j.bbamcr.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rottingen J, Iversen JG. Ruled by waves? Intracellular and intercellular calcium signalling. Acta Physiol Scand. 2000;169:203–219. doi: 10.1046/j.1365-201x.2000.00732.x. [DOI] [PubMed] [Google Scholar]
- 5.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 6.Munaron L. Calcium signalling and control of cell proliferation by tyrosine kinase receptors. Int J Mol Med. 2002;10:671–676. [PubMed] [Google Scholar]
- 7.Agell N, Bachs O, Rocamora N, Villalonga P. Modulation of the Ras/Raf/MEK/ERK pathway by Ca2+, and calmodulin. Cell Signal. 2002;14:649–654. doi: 10.1016/s0898-6568(02)00007-4. [DOI] [PubMed] [Google Scholar]
- 8.Cullen PJ, Lockyer PJ. Integration of calcium and Ras signalling. Nat Rev Mol Cell Biol. 2002;3:339–348. doi: 10.1038/nrm808. [DOI] [PubMed] [Google Scholar]
- 9.Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 10.Farnsworth CL, Freshney NW, Rosen LB, Ghosh A, Greenberg ME, Feig LA. Calcium activation of Ras mediated by neuronal exchange factor Ras-GRF. Nature. 1995;376:524–527. doi: 10.1038/376524a0. [DOI] [PubMed] [Google Scholar]
- 11.Chen HJ, Rojas-Soto M, Oguni A, Kennedy MB. A synaptic Ras-GTPase activating protein (p135 SynGAP) inhibited by CaM kinase II. Neuron. 1998;20:895–904. doi: 10.1016/s0896-6273(00)80471-7. [DOI] [PubMed] [Google Scholar]
- 12.Liu Q, Walker SA, Gao D, Taylor JA, Dai YF, Arkell RS, Bootman MD, Roderick HL, Cullen PJ, Lockyer PJ. CAPRI and RASAL impose different modes of information processing on Ras due to contrasting temporal filtering of Ca2+ J Cell Biol. 2005;170:183–190. doi: 10.1083/jcb.200504167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tebar F, Villalonga P, Sorkina T, Agell N, Sorkin A, Enrich C. Calmodulin regulates intra-cellular trafficking of epidermal growth factor receptor and the MAPK signaling pathway. Mol Biol Cell. 2002;13:2057–2068. doi: 10.1091/mbc.01-12-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abraham ST, Benscoter HA, Schworer CM, Singer HA. A role for Ca2+/calmodulin-dependent protein kinase II in the mitogen-activated protein kinase signaling cascade of cultured rat aortic vascular smooth muscle cells. Circ Res. 1997;81:575–584. doi: 10.1161/01.res.81.4.575. [DOI] [PubMed] [Google Scholar]
- 15.Chuderland D, Seger R. Protein-protein interactions in the regulation of the extracellular signal-regulated kinase. Mol Biotechnol. 2005;29:57–74. doi: 10.1385/MB:29:1:57. [DOI] [PubMed] [Google Scholar]
- 16.Kolch W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol. 2005;6:827–837. doi: 10.1038/nrm1743. [DOI] [PubMed] [Google Scholar]
- 17.Chuderland D, Marmor G, Shainskaya A, Seger R. Calcium-mediated interactions regulate the subcellular localization of extracellular signal-regulated kinases. J Biol Chem. 2008;283:11176–11188. doi: 10.1074/jbc.M709030200. [DOI] [PubMed] [Google Scholar]
- 18.Rubinfeld H, Hanoch T, Seger R. Identification of a cytoplasmic-retention sequence in ERK2. J Biol Chem. 1999;274:30349–30352. doi: 10.1074/jbc.274.43.30349. [DOI] [PubMed] [Google Scholar]


