Abstract
Variation in floral traits including odor, color and morphology, demonstrate the selective pressures imposed by specific pollinator taxa, such as insects and birds. In southern Arizona, Manduca sexta (Sphingidae) hawkmoths are associated with Datura wrightii (Solanaceae) at both the larval (herbivore) and adult (nectar feeding) stages. However during most of the summer Manduca feeds on “bat-adapted” Agave spp. (Agaveacea) flowers, and only use Datura when it is at peak bloom. Manduca's nectar-host use appears to be mediated through innate odor preferences and olfactory learning; they prefer Datura's “hawkmoth-adapted” traits, which facilitate the maintenance of their coevolutionary relationship, yet they are flexible enough to explore and learn to utilize novel resources, such as agave. This behavioral flexibility is likely responsible for the frequent observation of generalized, or mixed, pollination systems. Given that Manduca visit agave species in southern Arizona, we hypothesize that the differences in flower phenotype between two closely related agave species may be associated with the importance of hawkmoths relative to bats. The southernmost agave, Agave palmeri (Agavacea), exhibits floral traits typical of bat pollination, whereas the northernmost species, Agave chrysantha (Agavacea), exhibits mixed floral traits which appear to be adapted to insects, and to a lesser extent, bats. The differences between these agaves are likely correlated with the geographic overlap in migratory bats from Mexico and resident hawkmoth populations. Thus D. wrightii, A. palmeri and A. chrysantha populations represent a unique system in which to examine the evolution of floral traits in both specialized and mixed pollination systems associated with spatially variable pollinator assemblages.
Key words: agave, hawkmoth, pollination, floral characters, floral odors, floral evolution
Floral Advertisements and Pollinator Visitation
Floral traits are signals that animal pollinators use to locate important food resources. Flower phenotypes are believed to be non-random combinations of colors, odors and morphologies hypothesized to reflect selective pressure imposed by certain classes of pollinators.1 For example, some of the well-studied examples of floral signals come from plants with nocturnal antithesis thought to reflect adaptations for hawkmoth or bat pollination. Flowers having hawkmoth pollinated traits are white and highly reflective, emit a strong pleasant scent, and have sucrose-dominant nectar.2–4 In contrast, bat pollinated flowers are dull yellow or cream-colored, emit a strong fetid or pungent scent, and have hexose- (fructose and glucose) dominant nectar.5,6 Phylogenetic and morphological studies have demonstrated that these characteristics have evolved independently in many different plant families.7 The convergence of these common floral features makes night blooming plants excellent model systems in which to examine plant-pollinator interactions and the evolution of floral traits.
In the semi-arid environments of southern Arizona USA, several hawkmoth and bat-adapted plants occur in sympatry. An example of this is Palmer's Agave (Agave palmeri, Agavaceae), and jimsonweed (Datura wrightii, Solanaceae), which exhibit bat and hawkmoth-adapted floral characters, respectively. D. wrightii floral traits include a sweet smelling odor composed of terpenoids (mono- and sesquit-erpenoids) (79%) and benzenoids (18%), a sucrose-rich nectar, and highly reflective corollas (reflectance > 50%) that appear white to the human eye (Fig. 1A). In contrast, A. palmeri traits consist of a foul floral scent dominated by esters (30%), sulfur compounds (<1%), and monoterpenoids (59%), abundant hexose-rich nectar, and a pale dull coloration (reflectance < 25%) (Fig. 1B). The hawkmoth, Manduca sexta (Sphingidae), is an important pollinator for D. wrightii and has an innate attraction to D. wrightii's floral odor. M. sexta hawkmoths also visit A. palmeri flowers, but only when D. wrightii is not locally abundant. Moths learn to utilize A. palmeri's abundant nectar resources through olfactory-mediated exploration and learning.4 The ability by pollinators to shift from using one nectar resource to another allows populations to exist when a preferred resource is scarce while likely permitting the maintenance of plant-pollinator associations.
Figure 1.
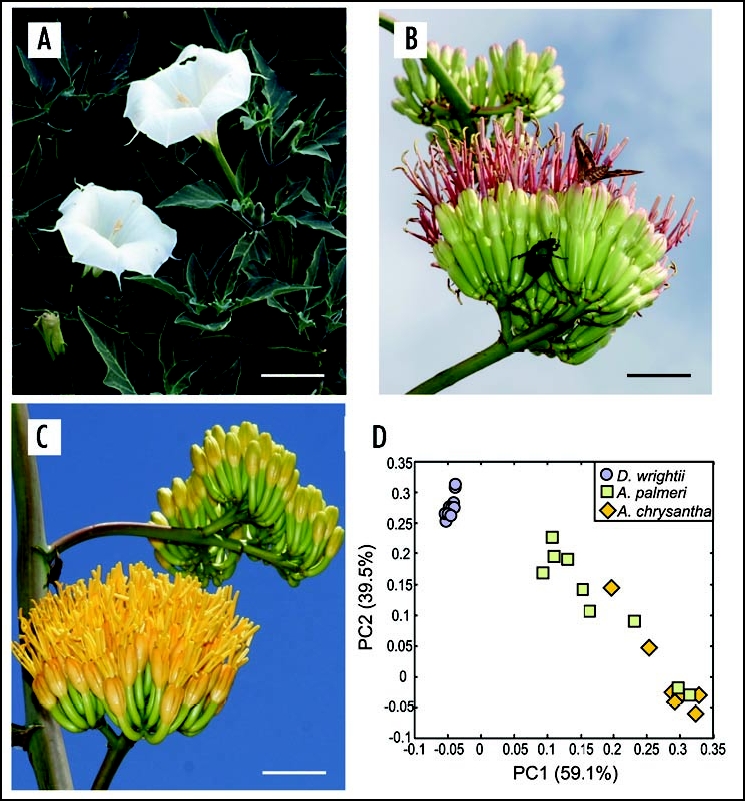
Contrast in floral characterisitics between D. wrightii, A. palmeri and A. chrysantha plants. The three plants are visited by nocturnal hawkmoths, but the floral traits in each plant differ from one another. (A) D. wrightii flowers. Note the bright floral reflectance of the corollas that produces a contrast with the dark foliage background. (B) A. palmeri umbel. Each umbel contains 8–20 flowers. Hyles lineata (Sphingidae), and Cotinus mutabilis (Scarabaeidae), are feeding from the umbel. (C) A. chrysantha umbel. The morphology of A. chrysantha closely resembles that of A. palmeri, but note the bright yellow corollas. (D) Principal-components analysis of the floral odor from these three plants. The D. wrightii flowers (blue circles) group together. In contrast, the agaves appear more variable. The odor of A. palmeri flowers (green squares) appears to more closely resemble that of D. wrightii due to the presence of monoterpenes and benzenoids in its headspace, but some A. palmeri flowers are also grouped in the same PCA space of A. chrysantha (yellow diamonds) due to their aliphatic esters. Scale bar = 5 cm. Picture images courtesy of Charles Hedgecock RBP, FBCA (ARL Division of Neurobiology, University of Arizona) and T. Beth Kinsey.
From an evolutionary perspective, behavioral plasticity is likely an adaptation in response to a variable floral environment. Pollinators capable of collecting floral resources from a wide variety of plants, including those morphologically adapted to different pollinator taxa, are more likely to persist within a fluctuating environment compared with those specialized to a single, or few, partner species.8,9 Thus it is not surprising that generalized, or mixed pollination systems, are more common than previously believed.10
Floral Traits of Two Agave Species in the Sonoran Desert
In the Sonoran Desert agaves are regarded as “keystone species” during summer months by providing copious amounts of nectar and pollen for a wide range of insects and vertebrates pollinators.11–14 Two agaves in particular, Agave palmeri and Agave chrysantha, represent a unique system to explore the evolution of floral traits under selection by a diverse pollinator assemblage. While the evolutionary relationship between Agave chrysantha and A. palmeri has yet to be fully resolved using molecular techniques, the occurrence of natural hybrids and morphological studies suggest that they are closely related.15 Agave chrysantha is endemic to central and southern Arizona, and is believed to be a relatively young species, whereas Agave palmeri is believed to be older and has a wider range that extends into Mexico.15,16 The flowers of both agaves superficially resemble one another, possibly reflecting their shared evolutionary history: both produce abundant nectar resources (>100 µl/day), emit odor composed of monoterpenes and aliphatic compounds known to attract diverse insect species, and have floral morphologies that permit nectar access by insects, bats and birds. However, their flowers' differ from one another in several ways. For instance, corollas of A. chrysantha are bright yellow-orange rather than the pale cream color of A. palmeri (Fig. 1C), which suggests that the former are visually more adapted to diurnal pollinators, and the latter to nocturnal pollinators.16 A. chrysantha flowers produce nectar that has more than 63-times the sucrose content of A. palmeri (7.56 mg/flower versus 0.12 mg/flower, respectively), which suggest that A. chrysantha's nectar is under greater selection by insect pollinators.
While differing in their visual display and nectar chemistry, examination of their scent profiles by principal components analysis (PCA) revealed substantial overlap in the two agave species (Fig. 1D). For instance, A. palmeri and A. chrysantha share similar percentages of chemical odorant classes, including low levels of sulfur compounds (<1%)—a common marker of bat-adapted flowers—esters (ca. 53% and 77%, respectively), and terpenoids (ca. 38% and 15%, respectively). The contrast in floral color and nectar chemistry suggest that these two agaves are adapted to different pollinator assemblages, but the similarity in their floral odors likely reflects their shared evolutionary history.
A Unique Study System for Evaluating Pollinator-Mediated Floral Evolution
Assuming that A. chrysantha recently evolved from A. palmeri,15 the differences in floral color, nectar and odor chemistry between the two species are likely due to differences in visitation by migratory bats, in particular the migratory Lesser Long-nosed Bat (Leptonycteris curasoae). Recent work12,14,17 suggests that the relationship between Lesser Long-nosed Bats and their nectar plants varies latitudinally, with bats and plants mutually dependent upon each other in central Mexico, but plants are less depended upon bats at the northern end of their range (i.e., Arizona, USA), presumably because bat migrations are variable from year to year. Given that the range of the Lesser Long-nosed Bat entirely overlaps with the distribution of A. palmeri in southern Arizona, but only overlaps the southern most portion of A. chrysantha's range,14 it is likely that A. chrysantha evolved in the absence of bat pollination. Coincidentally, the region where A. chrysantha and Lesser Long-nosed Bat's ranges overlap is where A. chrysantha hybridizes with A. palmeri, suggesting that bat-mediated gene flow, or the lack thereof, is central to the evolution of A. chrysantha's floral characters.
The differences in floral traits likely reflect variation in the effective pollinators of these two agave species. Sulfur compounds are important mediators of bat attraction to flowers18 and it has been suggested that shifts from bat to moth pollination reflects the reduction of sulfur compounds and the presence of terpenoids and benzenoids in a floral headspace. For instance, flowers of the plant Browneopsis disepala (Leguminosae) are visited by both moths and bats and displays a mixed pollination syndrome with a bat pollinated floral display and an odor profile that is dominated by aliphatics and monoterpenes but lacks the sulfur compounds.7 These agaves represent additional examples of mixed pollination systems, wherein the odor profile and visual displays of A. palmeri and A. chrysantha indicate that one is more bat-adapted than the other. Because hawkmoths are common nocturnal visitors of these agave species,14–16 the agaves, together with D. wrightii which is specialized for hawkmoth pollination, present an excellent system to examine the relationship between floral advertisements and rewards with their primary nocturnal visitors.
Addendum to: Riffell JA, Alarcón R, Abrell L, Davidowitz G, Bronstein JL, Hildebrand JG. Behavioral consequences of innate preferences and olfactory learning in hawkmoth-flower interactions. Proc Natl Acad Sci USA. 2008;105:3404–3409.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/6350
References
- 1.Faegri K, van der Pijl L. The principles of pollination ecology. Oxford, UK: Pergamon Press; 1979. [Google Scholar]
- 2.Grant V. Behavior of hawkmoths on flowers of Datura metaloides. Bot Gazette. 1983;144:439–449. [Google Scholar]
- 3.Raguso RA, Henzel C, Buchman SL, Nabhan GP. Trumpet flowers of the Sonoran desert: floral biology of peniocereus cacti and sacred datura. I J Plant Sci. 2003;164:877–892. [Google Scholar]
- 4.Riffell JA, Alarcon R, Abrell L, Davidowitz G, Bronstein JL, Hildebrand JG. Behavioral consequences of innate preferences and olfactory learning in hawkmoth-flower interactions. Proc Natl Acad Sci USA. 2008;105:3404–3409. doi: 10.1073/pnas.0709811105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bestmann HJ, Winkler L, von Helversen O. Headspace analysis of volatile flower scent constituents of bat-pollinated plants. Phytochemistry. 1997;46:1169–1172. [PubMed] [Google Scholar]
- 6.Dobson HEM. Relationship between floral fragrance composition and type of pollinator. In: Dudareva N, Pichersky E, editors. Biology of floral scent. Boca Raton, FL: CRC Press; 2006. pp. 147–198. [Google Scholar]
- 7.Knudsen JT, Tollsten L. Floral scent in bat-pollinated plants: a case for convergent evolution. Bot J Linn Soc. 1995;119:45–57. [Google Scholar]
- 8.Memmott J, Waser NM, Price MV. Tolerance of pollination networks to species extinctions. Proc R Soc B. 2004;271:2605–2611. doi: 10.1098/rspb.2004.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waser NM, Chittka L, Price MV, Williams NM, Ollerton J. Generalization in pollination systems, and why it matters. Ecology. 1996;77:1043–1060. [Google Scholar]
- 10.Waser NM. Specialization and generalization in plant-pollinator interactions: A historical perspective. In: Waser NM, Ollerton J, editors. Plant-pollinator interactions: From specialization to generalization. Chicago, IL: University of Chicago Press; 2006. pp. 3–17. [Google Scholar]
- 11.Molina-Freaner F, Eguiarte LE. The pollination biology of two paniculate agaves (Agavaceae) from northwestern Mexico: contrasting roles of bats as pollinators. Am J Bot. 2003;90:1016–1024. doi: 10.3732/ajb.90.7.1016. [DOI] [PubMed] [Google Scholar]
- 12.Scott PE. Timing of Agave palmeri flowering and nectar-feeding bat visitation in the Peloncillos and Chiricahua mountains. Southwestern Nat. 2004;49:425–434. [Google Scholar]
- 13.Silva-Montellano A, Eguiarte LE. Geographic patterns in the reproductive ecology of Agave lechuguilla (Agavaceae) in the Chihuahuan desert I. Floral characteristics, visitors and fecundity. Am J Bot. 2003;90:377–387. doi: 10.3732/ajb.90.3.377. [DOI] [PubMed] [Google Scholar]
- 14.Slauson LA. Pollination biology of two chiropterophilous agaves in Arizona. Am J Bot. 2000;87:825–836. [PubMed] [Google Scholar]
- 15.Gentry HS. The agaves of continental North America. Tucson, AZ: University of Arizona Press; 1982. [Google Scholar]
- 16.Slauson LA. Factors affecting the distribution, pollination ecology and evolution of Agave chrysantha Peebles and Agave palmeri Engelm. (Agavaceae) In: DeBano LF, editor. Biodiversity and the Management of the Madrean Archipelago: The Sky Islands of Southwestern USA and Northwestern Mexico. USDA Forest Service; 1995. pp. 194–205. [Google Scholar]
- 17.Fleming TH. Nectar corridors: migration and the annual cycle of lesser long-nosed bats. In: Nabhan GP, editor. Conserving Migratory Pollinators and Nectar Corridors in Western North America. Tucson, AZ: University of Arizona Press; 2004. pp. 23–42. [Google Scholar]
- 18.von Helversen O, Winkler L, Bestmann HJ. Sulphur-containing “perfumes” attract flower-visiting bats. J Comp Physiol A. 2000;186:143–153. doi: 10.1007/s003590050014. [DOI] [PubMed] [Google Scholar]


