Abstract
During the apparently mindless act of localizing a tactile sensation, our brain must realign its initial spatial representation (somatotopicaly arranged) according to current body posture (arising from proprioception, vision and even audition).1–3 We have recently illustrated4 the temporal course of this recoding of tactile space from somatotopic to external coordinates using a crossmodal cueing psychophysical paradigm5,6 where behavioral reactions to visual targets are evaluated as a function of the location of irrelevant tactile cues. We found that the tactile events are initially represented in terms of a fleeting, non-conscious but nevertheless behaviorally consequential somatotopic format, which is quickly replaced by the representations referred to external spatial locations that prevail in our everyday experience. In this addendum, we test the intuition that frequent changes in body posture will make it harder to update the spatial remapping system and thus, produce stronger psychophysical correlates of the initial somatotopically-based spatial representations. Contrary to this expectation, however, we found no evidence for a modulation when preventing adaptation to a body posture.
Keywords: tactile localization, tactile remapping, frame of reference, spatial representations, crossmodal cueing, crossed-hands deficit
Background
In our cross-modal cueing paradigm4 human observers performed an elevation discrimination task (top vs. bottom) on visual targets (9 ms flash) near one of the two hands, after receiving a non-predictive tactile cue (9 ms tap) at the ring finger of the same- or the opposite-hand. Participants were simply asked to judge the elevation of the light flash as quickly as possible, irrespective of side of presentation and of the preceding tactile event, which was completely uninformative about the location or elevation of the upcoming target. We measured response times (RTs) to the visual targets as a function of the spatial validity of the tactile cue (same- vs. opposite side, in terms of external location) and the cue-target onset asynchrony (CTOA, ranging from 30 ms to 360 ms). This test was run under two postural conditions: with the hands placed straight in front of the body (20 cm inter-manual distance) so the somatotopic and external spatial maps were aligned (i.e., the left hand was placed in the left external space); or with the hands crossed over the body midline (same inter-manual distance) so the somatotopic and external maps were misaligned (i.e., the left hand was placed on the right side of space). The effects of tactile cue validity on visual processing allowed us to gauge the dominant tactile spatial frame of reference. In the crossed-hands condition, cueing effects (opposite minus same-side response latencies) reflected the use of an external reference frame at long CTOAs (beyond 200 ms), but at short intervals (below 100 ms) the cueing effects revealed the use of a somatotopically based map, so that reactions to the visual events were faster for opposite-side trials (i.e., after anatomically congruent but spatially incongruent touch).
Scope of the Present Study
The remapping from somatotopic to external coordinates seems to be carried out obligatorily every time a somatic event is presented, even if posture is kept constant. In fact, the dramatic impairments in tactile temporal order judgement tasks7–10 (TOJ) observed with the hands-crossed posture are explained because the remapping process of the second tactile event at one hand is initiated before the first stimulus (at the other hand) has been adjusted for the crossed position of the arms.9 Yet, a potentially important question is whether the tactile remapping system can be modulated to some degree by continued adaptation to a crossed-hands posture. Intuitively, if the remapping system is sufficiently flexible, one would expect to observe more effective remapping when maintaining a body posture over an extended period of time. If such an adaptation occurs, then previous hands-crossed experiments2,4,7–10 (using TOJ or the cueing paradigm described above) may underestimate the size of the somatotopically-based effects and perhaps their duration. Indeed, some studies11 show that the crossed-hands deficit on tactile TOJs decrease when observers perform the task during extended periods of time (over 2,000 trials, run over 12 sessions). We hypothesized that changing posture frequently during the experiment would reset the putative adaptation to the position of the hands and result in stronger evidence of the somatotopically based representations.
Methods
We ran a crossmodal cueing paradigm just as in Azañón and Soto-Faraco (2008)4, but only including CTOAs of 60 ms and 360 ms. The critical novelty was that the posture condition was either blocked or varied every few trials. In the blocked condition participants (n = 16; mean age 21 years; SD = 1.80) were asked to keep their arms crossed or uncrossed (order counterbalanced) during an entire block of 192 trials, for a total of 384 trials. In the interleaved condition, posture varied (from crossed to uncrossed and vice-versa) every 16, 32 or 48 trials (unpredictable run length, equivalent total number of trials).
Results and Discussion
In accord with previous findings,4 with hands-crossed reaction times to the visual targets at short (60 ms) CTOAs were faster in opposite-side (anatomically congruent but spatially incongruent) trials than in same-side trials (see Fig. 1). This pattern completely reversed at long CTOAs (360 ms) so that tactile cues produced a facilitation of targets presented at the same external location. With uncrossed hands, cueing effects for both CTOAs were positive, as expected (see Fig. 1). All these effects were, however, of comparable size for the interleaved and blocked conditions, for hands crossed (respectively, -6 vs. -8 cueing effect at 60 ms CTOA; +12 vs. +10 ms at 360 ms CTOA) and uncrossed (respectively, +7 vs. +11 cueing effect at 60 ms CTOA, +11 vs. +15 ms at 360 ms CTOA). An ANOVA on RT data revealed a significant three way interaction between posture, cue and CTOA [F(1,15) = 5.54, p < 0.033], thus confirming the reversal in cueing effect selectively for the crossed-hands, short CTOA, condition. Yet, critically, the ANOVA showed that the interleaved vs. blocked condition did not produce a significant main effect nor participated in any interaction (all Fs, p > 0.1).
Figure 1.
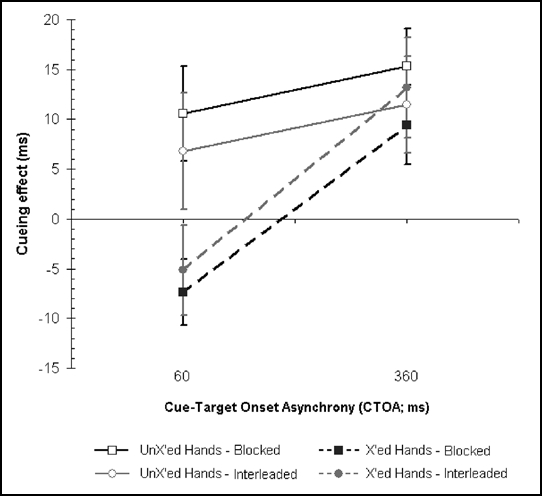
Experimental results. Mean spatial cueing effects (opposite minus same-side trials reaction times) as a function of CTOA and posture change condition. A positive cueing effect indicates faster performance for visual events in the same-side trials as compared to the opposite-side trials, as coded with respect to positions in external space. Black lines/symbols represent the results in the blocked posture condition and grey lines/symbols in the interleaved postures condition. The results for the uncrossed-hands conditions are represented by solid lines/open symbols and the results of crossed-hands conditions are represented by dashed lines/filled symbols. The error bars denote the SEM (standard error of the mean).
These results suggest that the remapping system underlying the recoding of tactile events from somatotopic to external coordinates is not easily modulated by adaptation to a given posture. Indeed, it seems that the remapping process follows the same path, trial after trial, independently of the frequency of posture updates throughout the experiment. Given that the spatial remapping processes underlying the cueing effects reported here and in our previous paper4 are thought to be supported by association, high-level, brain systems, the lack of short-term flexibility seen here is somehow intriguing.12 We believe that some postural adaptation in tactile processing could occur but, if present, short-term effects seem to be certainly weak and can only be seen under the most favorable conditions. For instance, Craig et al.11 showed practice effects in the tactile TOJ task with the crossed-hands posture, albeit in Carig's study performance levels were very poor to start with and thus, there was great room for improvement.
Acknowledgements
This research was supported by grants from the Spanish Ministerio de Educación y Ciencia (Spain; SEJ 2007-64103/PSIC and CDS00012) and by a fellowship Beca de Formación de Profesorado Universitario from the Spanish Ministerio de Educación y Ciencia to Elena Azañón.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/6724
References
- 1.Aglioti S, Smania N, Peru A. Frames of reference for mapping tactile stimuli in brain-damaged patients. J Cogn Neurosci. 1999;1:67–79. doi: 10.1162/089892999563256. [DOI] [PubMed] [Google Scholar]
- 2.Azañón E, Soto-Faraco S. Alleviating the ‘crossed-hands’ deficit by seeing uncrossed rubber hands. Exp Brain Res. 2007;4:537–548. doi: 10.1007/s00221-007-1011-3. [DOI] [PubMed] [Google Scholar]
- 3.Soto-Faraco S, Ronald A, Spence C. Tactile selective attention and body posture: assessing the multisensory contributions of vision and proprioception. Percept Psychophys. 2004;7:1077–1094. doi: 10.3758/bf03196837. [DOI] [PubMed] [Google Scholar]
- 4.Azañón E, Soto-Faraco S. Changing reference frames during the encoding of tactile events. Curr Biol. 2008;18:1044–1049. doi: 10.1016/j.cub.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 5.Kennett S, Eimer M, Spence C, Driver J. Tactile-visual links in exogenous spatial attention under different postures: convergent evidence from psychophysics and ERPs. J Cogn Neurosci. 2001;4:462–478. doi: 10.1162/08989290152001899. [DOI] [PubMed] [Google Scholar]
- 6.Kennett S, Spence C, Driver J. Visuo-tactile links in covert exogenous spatial attention remap across changes in unseen hand posture. Percept Psychophys. 2002;7:1083–1094. doi: 10.3758/bf03194758. [DOI] [PubMed] [Google Scholar]
- 7.Shore D, Spry E, Spence C. Confusing the mind by crossing the hands. Brain Res Cogn Brain Res. 2002;1:153–163. doi: 10.1016/s0926-6410(02)00070-8. [DOI] [PubMed] [Google Scholar]
- 8.Wada M, Yamamoto S, Kitazawa S. Effects of handedness on tactile temporal order judgment. Neuropsychologia. 2004;14:1887–1895. doi: 10.1016/j.neuropsychologia.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto S, Kitazawa S. Reversal of subjective temporal order due to arm crossing. Nat Neurosci. 2001;7:759–765. doi: 10.1038/89559. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto S, Moizumi S, Kitazawa S. Referral of tactile sensation to the tips of L-shaped sticks. J Neurophysiol. 2005;5:2856–2863. doi: 10.1152/jn.01015.2004. [DOI] [PubMed] [Google Scholar]
- 11.Craig JC, Belser N. The crossed-hands deficit in tactile temporal-order judgments: the effect of training. Perception. 2006;35:1561–1572. doi: 10.1068/p5481. [DOI] [PubMed] [Google Scholar]
- 12.Bavelier D, Neville H. Cross-modal plasticity: Where and How. Nat Rev Neurosci. 2002;3:443–452. doi: 10.1038/nrn848. [DOI] [PubMed] [Google Scholar]


