Abstract
Aspergillus nidulans undergoes polarized hyphal growth during the majority of its life cycle. Regulatory mechanisms for hyphal polarity have been intensively investigated in a variety of filamentous fungi. Two important cellular processes, which have received recent attention, include protein myristoylation and endocytosis. It is clear that protein myristoylation is essential for polarity establishment because germinating A. nidulans conidia lost polarity in the presence of cerulenin, a lipid metabolism inhibitor and in an N-myristoyl transferase mutant background. Only 41 predicted proteins encoded by A. nidulans posses an N-myristoylation motif, one of which is ADP ribosylation factor B (ArfB). Disruption of ArfB leads to failure of polarity establishment and maintenance during early morphogenesis and in a delay in endocytosis. Therefore, ArfB connects N-myristoylation and endocytosis to polarized growth. Exocytotic vesicle trafficking through the Spitzenkörper may also require Arf proteins in their role in vesicle formation. Taken together, ArfB is one of the important key components for the fungal hyphal growth.
Keywords: ArfB, endocytosis, N-myristoylation, polarized growth, protein lipidation
Filamentous fungi serve as a model system for polarized growth since they display an extensive hyphal growth phase during the majority of their life cycle. For these organisms, conidia (asexual spores) initially establish polarity to send a germ tube during germination. Polarization of the cell is maintained as hyphae elongate at their apex to make a complex mycelium (reviewed in ref. 1). In this commentary, we describe how vesicle trafficking to the Spitzenkörper, endocytosis, and N-myristoylation are involved in fungal hyphal polarity in a model organism, Aspergillus nidulans and discuss the role of ArfB in linking these mechanisms to this developmental pattern together.
N-myristoyl transferase (NMT) is essential for hyphal polarity maintenance in A. nidulans.2 The temperature sensitive swoF1 mutant displayed a loss of polarity and grew isotropically at its apex immediately after germ tube emergence. In contrast wild-type is able to maintain polarized growth of the hyphal apex. N-myristoylation is a form of protein lipidation. Examples of protein lipidation include protein (iso) prenylation, protein palmitoylation, glycosylphosphatidylinositol (GPI) lipid anchoring, and protein N-myristoylation.3,4 Here we show for the first time that cerulenin—a lipid metabolism inhibitor—leads to a loss of cell polarity in A. nidulans (Fig. 1). This result coupled with the swoF1 mutant phenotype further demonstrates the importance of protein lipidation in polarized growth.
Figure 1.
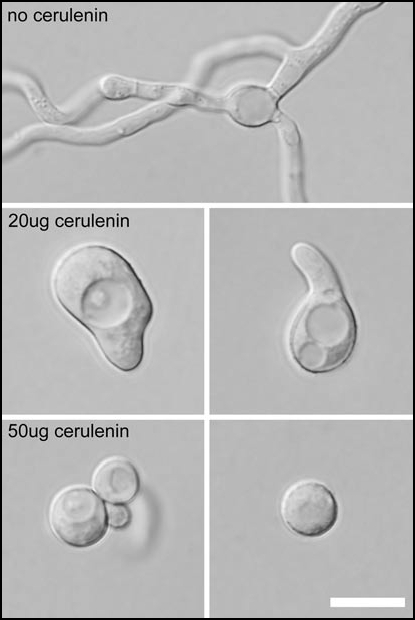
Effect of lipid metabolism inhibitor (cerulenin) on the polarity establishment in A. nidulans. Wild-type conidia grow isotropically until the first mitotic division (not shown) and then send a polarized germ-tube to establish the filamentous hyphae characteristic of A. nidulans. In the presence of 20 µg/ml of cerulenin, conidia exhibit an extended isotropic growth because of a delay in polarity establishment. In 50 µg ml of cerulenin, conidia exhibit isotropic growth but do not extend a germ tube. Scale = 10 µm. Objective numerical aperture = 1.4 with oil.
In the A. nidulans proteome, 41 proteins are predicted to be myristoylated, including a proteasome subunit and three ADP ribosylation factors (ArfA, ArfB and ArlA).5 We found that 20S proteasome activity is negatively regulated by N-myristoylation and the polarity phenotype of the swoF1 mutant phenotype was partially bypassed by a mutation in a proteasome α subunit or by exposure to MG132, a proteasome inhibitor.5 We also demonstrated that ArfA functions in polarized growth through a secretion pathway.6
Filamentous fungi have a unique apical body termed a Spitzenkörper, which is located at growing apices and is thought to control apical growth.7,8 The Spitzenkörper corresponds with a postulated secretory vesicle supply center (VSC) thought to be necessary to explain hyphal tip growth.7,9 ADP ribosylation factors are of particular interest in formation of the Spitzenkörper since these Ras related small GTPases are involved in vesicle assembly and trafficking.10 Arf proteins are also likely to play an important role in endocytosis. Relative to exocytosis, endocytosis has been underrepresented in research directed at polarized morphogenesis of filamentous fungi. Recently, an important role for endocytosis in regulating cell shape during polarized development is becoming clear. For example, in S. cerevisiae, the endocytic system uptakes chitin synthases on plasma membrane for recycling.11 Regulation of cell polarity by endocytosis is proposed in a mathematical model in the yeast.12 In filamentous fungi, FM4-64, an endocytotic marker dye is frequently used to stain the cell membranes and later the Spitzenkörper.13,14 Recently in A. nidulans, it was suggested that hyphal growth is mediated through the combined processes with endocytosis and exocytosis.15 Supporting this idea, is the proposal that maintenance of polarized growth might be achieved through endocytotic recycling of key components at the polar apex.16 One possibility for these components is the recently described cell end markers TeaA and TeaR17 which could be recycled through endocytosis.
Another important finding is that ArfB is involved in polarity establishment and maintenance.18 Disruption of arfB resulted in extended isotropic growth of conidia and abnormal hyphal growth characteristic of polarity establishment and polarity maintenance disruption. The arfB::Tn mutant was also found to be delayed for endocytic uptake of the marker dye FM4-64. The localization of ArfB::GFP was also found to be dependent on the myristoylation of its N-terminus. Therefore ArfB is one likely target to explain the cell polarity phenotype in the swoF1 N-myristoyl transferase mutant. It is noteworthy that ArfB localization was not altered in a fimbrin (fimA::Tn) mutant with endocytosis defects (data not shown), which indicates that ArfB is not dependant on actin/fimbrin patches for localization. These patches are sites of endocytosis as described in S. cerevisiae.19
Here we show that ArfB connects endocytosis and protein lipidation to hyphal polarity (Fig. 2). Therefore one might speculate that the mutant phenotype of the swoF1 mutant resulted at least in part from mislocalization of ArfB. The role of ArfB in vesicle assembly is yet to be determined. ArfB is a small GTPase protein that will cooperate with accessory proteins including a GTPase exchange factor (GEF), GTPase activation protein (GAP), and other auxiliary factors, which remain to be identified.
Figure 2.
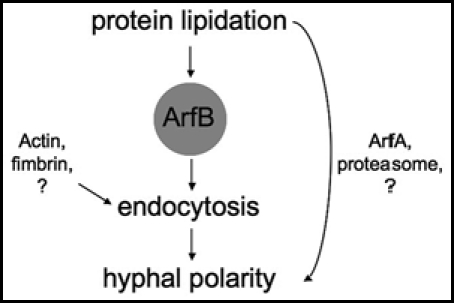
Model for the positions of ArfB, endocytosis and N-myristoylation in support of hyphal polarity in A. nidulans. ArfB mediates polarized growth by functioning in endocytosis. N-myristoylaiton of ArfB is critical for the proper localization of ArfB. Thus ArfB links endocytosis and N-myristoylation to hyphal tip growth of A. nidulans.
Acknowledgements
Soo Chan Lee was supported by a Nicholson fellowship and by a research assistantship from the Texas A&M University (TAMU) Department of Plant Pathology and Microbiology. This project was supported with startup funds to Brian D. Shaw from the TAMU Department of Plant Pathology and Microbiology, the TAMU College of Agriculture and Life Science, and the Texas AgriLife Research.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/6628
References
- 1.Harris SD. Cell polarity in filamentous fungi: Shaping the mold. Int Rev Cytol. 2006;251:41–77. doi: 10.1016/S0074-7696(06)51002-2. [DOI] [PubMed] [Google Scholar]
- 2.Shaw BD, Momany C, Momany M. Aspergillus nidulans swoF encodes an N-myristoyl transferase. Eukaryot Cell. 2002;1:241–248. doi: 10.1128/EC.1.2.241-248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casey P. Protein lipidation in cell signaling. Science. 1995;268:221–225. doi: 10.1126/science.7716512. [DOI] [PubMed] [Google Scholar]
- 4.Bhatnagar R, Gordon J. Understanding covalent modifications of proteins by lipids: where cell biology and biophysics mingle. Trends Cell Biol. 1997;7:14–20. doi: 10.1016/S0962-8924(97)10044-7. [DOI] [PubMed] [Google Scholar]
- 5.Lee SC, Shaw BD. A novel interaction between N-myristoylation and the 26S proteasome during cell morphogenesis. Mol Microbiol. 2007;63:1039–1053. doi: 10.1111/j.1365-2958.2006.05575.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee SC, Shaw BD. Localization and function of ADP ribosylation factor A in Aspergillus nidulans. FEMS Microbiology Letters. 2008;283:216–222. doi: 10.1111/j.1574-6968.2008.01174.x. [DOI] [PubMed] [Google Scholar]
- 7.Harris SD, Read ND, Roberson RW, Shaw B, Seiler S, Plamann M, Momany M. Polarisome meets spitzenkorper: Microscopy, genetics and genomics converge. Eukaryot Cell. 2005;4:225–229. doi: 10.1128/EC.4.2.225-229.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girbardt M. Der spitzenkörper von Polystictus versicolor. Planta. 1957;50:47–59. (Ger). [Google Scholar]
- 9.Grove SN, Bracker CE. Protoplasmic organization of hyphal tips among fungi: Vesicles and Spitzenkörper. J Bacteriol. 1970;104:989–1009. doi: 10.1128/jb.104.2.989-1009.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stearns T, Kahn RA, Botstein D, Hoyt MA. ADP ribosylation factor is an essential protein in Saccharomyces cerevisiae and is encoded by two genes. Mol Cell Biol. 1990;10:6690–6699. doi: 10.1128/mcb.10.12.6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziman M, Chuang JS, Schekman RW. Chs1p and Chs3p, two proteins involved in chitin synthesis, populate a compartment of the Saccharomyces cerevisiae endocytic pathway. Mol Biol Cell. 1996;7:1909–1919. doi: 10.1091/mbc.7.12.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marco E, Wedlich-Soldner R, Li R, Altschuler SJ, Wu LF. Endocytosis optimizes the dynamic localization of membrane proteins that regulate cortical polarity. Cell. 2007;129:411–422. doi: 10.1016/j.cell.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penalva MA. Tracing the endocytic pathway of Aspergillus nidulans with FM4-64. Fungal Genet Biol. 2005;42:963–975. doi: 10.1016/j.fgb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Fischer-Parton S, Parton RM, Hickey PC, Dijksterhuis J, Atkinson HA, Read ND. Confocal microscopy of FM4-64 as a tool for analysing endocytosis and vesicle trafficking in living fungal hyphae. J Microsc. 2000;198:246–259. doi: 10.1046/j.1365-2818.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 15.Taheri-Talesh N, Horio T, Araujo-Bazan L, Dou X, Espeso EA, Penalva MA, Osmani SA, Oakley BR. The Tip Growth Apparatus of Aspergillus nidulans. Mol Biol Cell. 2008;19:1439–1449. doi: 10.1091/mbc.E07-05-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Upadhyay S, Shaw BD. The role of actin, fimbrin and endocytosis in growth of hyphae in Aspergillus nidulans. Mol Microbiol. 2008;68:690–705. doi: 10.1111/j.1365-2958.2008.06178.x. [DOI] [PubMed] [Google Scholar]
- 17.Takeshita N, Higashitsuji Y, Konzack S, Fischer R. Apical Sterol-rich membranes are essential for localizing cell end markers that determine growth directionality in the filamentous fungus Aspergillus nidulans. Mol Biol Cell. 2008;19:339–351. doi: 10.1091/mbc.E07-06-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SC, Schmidtke SN, Dangott LJ, Shaw BD. Aspergillus nidulans ArfB is linked to endocytosis and polarized growth. Eukaryot Cell. 2008;7:1278–1288. doi: 10.1128/EC.00039-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaksonen M, Sun Y, Drubin DG. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell. 2003;115:475–487. doi: 10.1016/s0092-8674(03)00883-3. [DOI] [PubMed] [Google Scholar]


