Abstract
Stem cell establishment and maintenance are essential for the largely post-embryonic developmental patterning that occurs in higher plants. Plant embryos establish two stem cell populations at the shoot and root meristems which then function to continuously generate shoot and root tissues, respectively. Research has uncovered entirely separate sets of regulators for the shoot and root stem cells, raising questions about the origin of the later-evolving root meristem and the relationship between the two meristems. We have recently demonstrated that the related Arabidopsis phosphatases POL and PLL1 are essential for both shoot and root stem cell maintenance and that they act in each population by promoting expression of related WOX transcription factors. Furthermore, pol pll1 mutant embryos exhibit defects in key asymmetric divisions in the early embryo. We hypothesize that the primary functions of POL and PLL1 are to establish and/or maintain stem cell polarity. Here we show additional data linking POL/PLL1 function to the proper polarization and localization of auxin signaling during embryogenesis.
Keywords: meristem, quiescent center, shoot, root, CLAVATA
Signaling by the CLAVATA (CLV) pathway is critical to stem cell homeostasis at the shoot meristem (and the highly related flower meristem) in Arabidopsis (Fig. 1).1,2 Key components of CLV signaling include the receptor-kinase, CLV1, the receptor-like protein, CLV2, and the putative ligand, CLV3. CLV signaling is essential to limit the expression of the transcription factor WUSCHEL (WUS), which establishes the organizing center (OC) at the shoot meristem. The size of the OC determines the size of the stem cell population and the ability of the stem cell daughters to differentiate. In clv mutants, WUS expression expands, leading to defects in differentiation and to an expansion of the stem cell population up to 1000-fold, depending on the particular clv allele. Two related protein phosphatases, POL and PLL1, act downstream of CLV1 to promote WUS expression.3–5 Genetic analysis revealed that CLV signaling represses WUS by negatively regulating POL and PLL1. As a result, pol pll1 mutants lack shoot stem cells and WUS expression.
Figure 1.
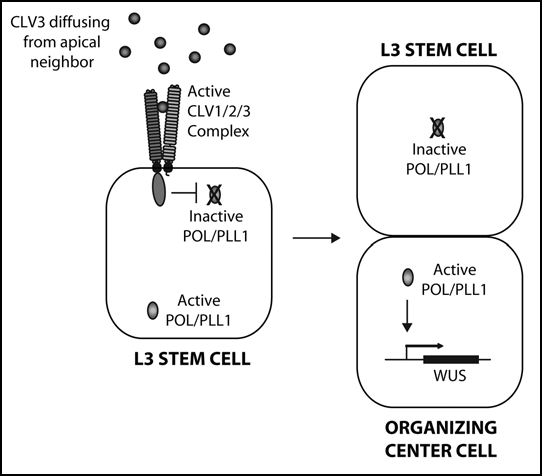
(At left) A model for CLV pathway signaling at the shoot meristem. Apically-generated CLV3 signal primarily activates CLV1 on the apical face of L3 stem cells within the shoot meristem. CLV1 in turn negatively regulates POL/PLL1 primarily on the apical portion of the cell. Upon periclinal division, the asymmetric distribution of POL/PLL1 drives apical/basal fates, including the activation of WUS in the basal daughter.
While the shoot meristem stem cells give rise to a pool of differentiating cells that are competent to organize distinct organ primordia, the root meristem, which evolved later in plant evolution, develops in a significantly different fashion.6,7 At the root, a highly organized set of stem cell initials gives rise continuously to differentiating cells whose fate is highly limited by positional information. This set of stem cell initials is arrayed around a Quiescent Center (QC). The QC acts to maintain the adjacent stem cell population in a manner that is similar in some ways to how the OC functions at the shoot meristem. Despite the functional similarities between the OC and the QC separate regulatory factors have been identified for each. While CLV, POL, WUS and other factors are critical at the OC, the intersection of different regulatory factors, including SCR, SHR, PLT1 and PLT2, with polar auxin transport down the root is required to establish and maintain the QC.8 The first real evidence of a regulatory link between the OC and QC came with the characterization of the WUS homolog, WOX5, which plays a role at the root meristem.9 Specifically, WOX5 is important for specification of a subset of the root stem cell initials and is expressed in the QC as it develops.
While analyzing the role of POL/PLL1 in shoot stem cell maintenance, we observed that pol pll1 mutants are seedling lethal because of defects in the basal portion of the embryo. We recently reported a detailed analysis of this defect, leading to the observation of a conserved mechanism of shoot and root stem cell regulation.10 Specifically, dramatic defects were identified in the central axis of pol pll1 embryos, including the loss of the vascular axis and the entire root meristem, including the QC and initials. We traced these defects back to a loss of asymmetry during divisions of the procambial and hypophyseal cells early in embryo development. Normally, these cells divide in a highly asymmetric fashion, both morphologically and developmentally.11 The procambium gives rise to the central vascular axis as well as the vascular initials adjacent to the QC, while the hypohyseal cell gives rise to the QC and other initials. We showed that in pol pll1 mutants neither the procambial nor hypohyseal cells or their progeny have evidence of asymmetry in terms of cell morphology, cell division patterns, or marker gene expression. Critically, WOX5 expression in the QC is lost from pol pll1 embryos upon division of the hypohyseal cell. Actively dividing putative stem cells were restored through ectopic expression of WOX5 in pol pll1 mutants, a result highly analogous to the ability of ectopic WUS expression to restore shoot stem cells to pol pll1 mutants.4 This provides evidence for POL/PLL1 dependent pathways at both the shoot and root meristems. Thus these disparate stem cell populations are functionally and perhaps evolutionarily related.
Since the primary defect in the pol pll1 embryo appears to be the loss of asymmetry of the hypohyseal and procambial cells, we re-examined our models for CLV/POL function in the shoot meristem. We propose a new model in which CLV signaling acts to indirectly regulate WUS by polarizing L3 stem cells prior to their division (Fig. 1). Here, CLV3, which is largely expressed by cells on the apical side of the L3 stem cells, acts as the polarizing signal detected by CLV1 and relayed within the cell by POL/PLL1. Following periclinal division of an L3 cell, WUS is then indirectly regulated by the asymmetry of the division. Consistent with this model is data showing that WOX genes are often expressed in asymmetrically-dividing cells and maintain expression in only one daughter.12,13
Our recent studies also showed a connection between the role of POL/PLL1 in specifying cell polarity and the most important and well-studied asymmetrically-localized proteins in plants, the auxin efflux carrier PIN proteins. PIN proteins are readily observed accumulating in a highly polarized fashion on cell membranes, and this asymmetry is critical for the proteins to regulate long-distance, polarized auxin transport.14 In pol pll1 mutant embryos PIN1 protein accumulation is highly reduced among the daughters of the procambial and hypohyseal cells, and the PIN protein does not undergo polarized distribution in these cells.10 One possibility is that POL/PLL1 act upstream to establish the cell polarity/identity of these cells and that PIN1 localization is dependent on this polarity. A rigorous examination of this issue has not been undertaken to date.
Here we report on an additional auxin connection, the relationship between POL/PLL1 and MONOPTEROUS (MP) expression. MP is an auxin response transcription factor that is critical for auxin signaling and gene regulation.15,16 It has been well-studied in many developmental contexts and is required for the formation of the basal vasculature and the root meristem. In our recent study of pol pll1 embryos, one of the most fascinating markers altered in the mutant was the enhancer-trap line J1721 from the Haseloff collection (http://www.plantsci.cam.ac.uk/Haseloff/Home.html). In wild-type, this marker is active throughout the early embryo, but becomes progressively restricted to the vasculature as development proceeds (Fig. 2B–G). In pol pll1 embryos, J1721 does not undergo this restriction but instead remains more broadly and highly expressed (Fig. 2H). In our original study, we concluded that J1721 might be a marker for differentiation during embryo development, but we now report that the J1721 enhancer trap contains an insertion upstream of MP (Fig. 2A). This enhancer-trap line likely reports MP expression, given the similarities between J1721 activity and previous analysis of MP expression.15,17
Figure 2.
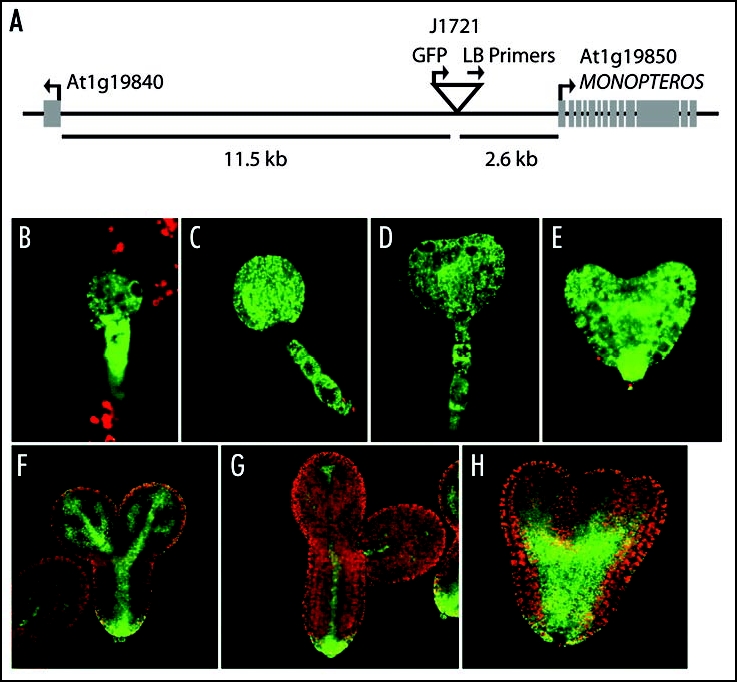
J1721 is located proximal to MP. (A). TAIL-PCR20 products, obtained from J1721 seedlings using the degenerate primers, AGWGNAGWANCAWAGG and NGTCGASWGANAWGAA (where W = A or T, S = C or G, and N = A or C or G or T) in combination with the T-DNA left border primers: (Round 1) TTGATTTATAAGGGATTTTGCCGATTTCGG, (Round 2) AACTCTCTCAGGGCCAGGCG and (Round 3) CCACCCCAGTACATTAAAAACGTC, were sequenced to determine the insert location. The location of the T-DNA insertion is represented by an upside down triangle. Arrows indicate the directionality of genes and primers. The distance of the T-DNA from the start codons of the surrounding genes are as marked. (B–G). J1721 enhancer trap expression in green in various stages of wild-type sibling embryos from pol/pol pll1/+ parents. (H) J1721 expression in a pol pll1 embryo—note the broader and more intense signal.
The failure of pol pll1 mutants to restrict J1721/MP expression and our previous studies showing that PIN1 localization and expression are defective in the double mutants, lead us to the hypothesis that the defects in the double mutant may directly or indirectly cause improper polar auxin transport. Furthermore, recent studies have shown that other members of the WOX family of transcription factors regulate PIN1 transcription during very early embryo development, suggesting that the WOX transcriptional machinery is required for the establishment of localized auxin response.18 Also, consistent with POL/PLL1 having a role in regulating polar auxin transport are studies by us and other groups which show that MP, PIN1, POL and PLL1 are all required for proper leaf vasculature development.3,19 Whether the effects of POL/PLL1 on auxin signaling are related to the regulation of cell polarity, cell specification, or another process is still unclear.
Further studies are needed to better understand the connections between POL/WOX dependent signaling and the regulation of polar auxin transport. Our previous studies showing that pol pll1 mutants are still sensitive to auxin suggest that the relationship between these pathways is indirect but recent data from multiple labs suggests that there is a more direct link between the polarity set up by POL/PLL1 dependent pathways and polar auxin transport.
Acknowledgements
This work was supported by National Institutes of Health grant (R01GM62962) to Steven E. Clark.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/6841
References
- 1.Doerner P. Plant meristems: a merry-go-round of signals. Curr Biol. 2003;13:368–374. doi: 10.1016/s0960-9822(03)00280-x. [DOI] [PubMed] [Google Scholar]
- 2.Williams L, Fletcher JC. Stem cell regulation in the Arabidopsis shoot apical meristem. Curr Opin Plant Biol. 2005;8:582–586. doi: 10.1016/j.pbi.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Song SK, Clark SE. POL and related phosphatases are dosage-sensitive regulators of meristem and organ development in Arabidopsis. Dev Biol. 2005;285:272–284. doi: 10.1016/j.ydbio.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Song SK, Lee MM, Clark SE. POL and PLL1 phosphatases are CLAVATA1 signaling intermediates required for Arabidopsis shoot and floral stem cells. Development. 2006;133:4691–4698. doi: 10.1242/dev.02652. [DOI] [PubMed] [Google Scholar]
- 5.Yu LP, Miller AK, Clark SE. POLTERGEIST encodes a protein phosphatase 2C that regulates CLAVATA pathways controlling stem cell identity at Arabidopsis shoot and flower meristems. Curr Biol. 2003;13:179–188. doi: 10.1016/s0960-9822(03)00042-3. [DOI] [PubMed] [Google Scholar]
- 6.Friedman WE, Moore RC, Purugganan MD. The evolution of plant development. Am J Bot. 2004;9:1726–1741. doi: 10.3732/ajb.91.10.1726. [DOI] [PubMed] [Google Scholar]
- 7.Scheres B, Benfey PN, Dolan L. Root Development. In: Somerville C, Meyerowitz EM, editors. The Arabidopsis Book. Rockville, MD: American Society of Plant Biologists; 2002. [Google Scholar]
- 8.Scheres B. Stem-cell niches: nursery rhymes across kingdoms. Nat Rec Mol Cell Biol. 2007;8:345–354. doi: 10.1038/nrm2164. [DOI] [PubMed] [Google Scholar]
- 9.Sarkar AK, Luijten M, Miyashima S, Lenhard M, Hashimoto T, Nakajima K, Scheres B, Heidstra R, Laux T. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature. 2007;446:811–814. doi: 10.1038/nature05703. [DOI] [PubMed] [Google Scholar]
- 10.Song SK, Hofhuis H, Lee MM, Clark SE. Key divisions in the early Arabidopsis embryo require POL and PLL1 phosphatases to establish the root stem cell organizer and vascular axis. Dev Cell. 2008;15:98–109. doi: 10.1016/j.devcel.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jürgens G. Apical-basal pattern formation in Arabidopsis embryogenesis. EMBO J. 2001;20:3609–3616. doi: 10.1093/emboj/20.14.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haecker A, Gross-Hardt R, Geiges B, Sarkar A, Breuninger H, Herrmann M, Laux T. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development. 2004;131:657–668. doi: 10.1242/dev.00963. [DOI] [PubMed] [Google Scholar]
- 13.Ten Hove CA, Heidstra R. Who begets whom? Plant cell fate determination by asymmetric cell division. Curr Opin Plant Biol. 2008;11:34–41. doi: 10.1016/j.pbi.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Vieten A, Sauer M, Brewer PB, Friml J. Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci. 2007;12:160–168. doi: 10.1016/j.tplants.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Hardtke CS, Berleth T. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 1998;17:1405–1411. doi: 10.1093/emboj/17.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guilfoyle TJ, Hagen G. Auxin response factors. Curr Opin Plant Biol. 2007;10:453–460. doi: 10.1016/j.pbi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Hamann T, Benkova E, Baurle I, Kientz M, Jurgens G. The Arabidopsis BODENLOS gene encodes an auxin response protein inhibiting MONOPTEROS-mediated embryo patterning. Genes Dev. 2002;16:1610–1615. doi: 10.1101/gad.229402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breuninger H, Rikirsch E, Hermann M, Ueda M, Laux T. Differential expression of WOX genes mediates apical-basal axis formation in the Arabidopsis embryo. Dev Cell. 2008;14:867–876. doi: 10.1016/j.devcel.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Wenzel CL, Schuetz M, Yu Q, Mattsson J. Dynamics of MONOPTEROS and PINFORMED1 expression during leaf vein pattern formation in Arabidopsis thaliana. Plant J. 2007;49:387–398. doi: 10.1111/j.1365-313X.2006.02977.x. [DOI] [PubMed] [Google Scholar]
- 20.Liu YG, Mitsukawa N, Oosumi T, Whittier RF. Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J. 1995;8:457–463. doi: 10.1046/j.1365-313x.1995.08030457.x. [DOI] [PubMed] [Google Scholar]


