Abstract
It was recently discovered that the protozoan parasite, Toxoplasma gondii produces and uses the plant hormone, abscisic acid (ABA), for communication. Following intracellular replication, ABA production influences the timing of parasite egress from the host cell. This density-dependent signal may serve to coordinate exit from the host cell in a synchronous manner by triggering calcium-dependent activation of motility. In the absence of ABA production, parasites undergo differentiation to the semidormant, tissue cyst. The pathway for ABA production in T. gondii may be derived from a relict endosymbiont, acquired by ingestion of a red algal cell. Although the parasite has lost the capacity for photosynthesis, the plant-like nature of this signaling pathway may be exploited to develop new drugs. In support of this idea, an inhibitor of ABA biosynthesis protected mice against lethal infection with T. gondii. Here, we compare the role of ABA in parasites to its activities in plants, where it is know to control development and stress responses.
Key words: parasite, calcium, signaling, hormone, stress, chemotherapy
Toxoplasma gondii: Ubiquitous Protozoan Parasite with a Plant-like Endosymbiont
T. gondii is found in a wide range of warm-blood animals and infects about one third of world's human population.1 While normally benign, infections in immunocompromised patients can result in severe disease.1 This parasite has two main proliferative stages that contribute to infection in humans.2 Tachyzoites replicate rapidly within a segregated vacuole, rupture out of the cell, and reinvade new cells. In contrast, bradyzoites encyst and grow slowly, mainly in long-lived cells of the central nervous system and musculature. The encysted form can survive for a long time and poses a risk due to reactivation following host immuno-suppression. Once reactivated, parasites revert to the lytic phase characterized by rapid growth, egress and invasion of new host cells, thus causing considerable tissue damage.
T. gondii is a parasite in the phylum Apicomplexa, which includes a large number of obligate intracellular parasites. The causative agent of malaria, Plasmodium and a cause of serious gastrointestinal disease, Cryptosporidium, also belong to the Apicomplexa. Most apicomplexans contain a special organelle called the apicoplast, which was acquired by engulfment of an algal cell in a secondary endosymbiotic event.3,4 The original endosymbiont likely had four membranes; two of them derived from photosynthetic bacteria and two additional ones from the algal cell and progenitor host cell plasma membranes.5 This configuration persists in T. gondii (Fig. A), although some reports indicate only three membranes surround the malaria apicoplast.5 Debate about the origin of this endosymbiont was recently resolved by the discovery of a photosynthetic relative of the Apicomplexa and their sister taxa the dino a new flagellates. Prototrophic branch of the tree is represented by the marine alga called Chromera, which contains an endosymbiont of red algal origin that retains key features seen in Apicomplexa and dinoflagellates.6 Since its engulfment, the endosymbiont has undergone a variety of rearrangements in different derived lineages to shuffle most of the genes to the nucleus. In the process, many pathways have been modified or entirely lost, for example the absence of photosynthesis among all apicomplexans.5
Figure 1.
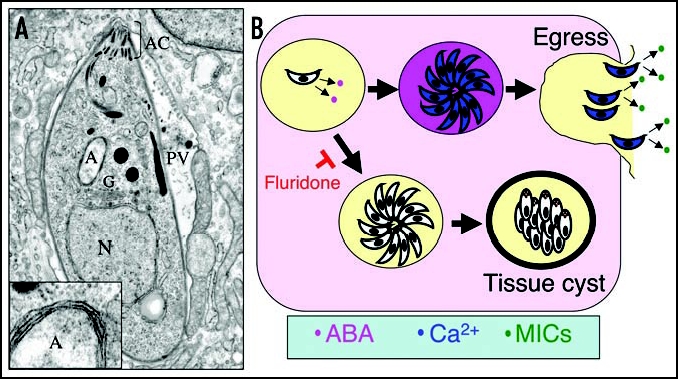
ABA production in T. gondii. (A) Cross-section through an intracellular T. gondii showing organellar structures. Insert illustrates four surrounding membranes from enlarged apicoplast. N, nucleus; A, apicoplast; AC, apical complex consisting of conoid and secretory organelles; PV, parasitophorous vacuole. (B) Model of ABA production by the parasite with in the PV. Accumulation of ABA (pink) leads to increases in intracellular calcium (blue) activating egress and triggering secretion of micronemes (MICs shown in green). Fluridone blocks ABA production, preventing egress and favoring differentiation of tissue cysts.
The presence of the apicoplast implies that T. gondii and related parasites have the potential to use plastid or algal-like pathways derived from the endosymbiont. Previous studies have highlighted the importance of various pathways for metabolism such a fatty acid FAS II-like pathway, ferridoxin-ferrodoxin-NADP+ reductase, heme biosysnthesis and a DOXP isoprenoid biosynthesis pathway.5 These have been suggested as important targets for development of new therapeutics, which is especially attractive given their bacterial or plastid-like nature. However, acquisition of primitive signaling pathways had not been considered previously. Thus, the discovery of the ABA response pathway in T. gondii is notable as it suggests early branching alga may have contained metabolites that control signaling between cells, and these pathways may have been inherited in parasites.
Calcium, Motility and Signaling in T. gondii
T. gondii uses intracellular calcium as a signal to control protein secretion and to activate motility, two events that are crucial to the parasite's intracellular existence.7 Apicomplexan parasites undergo actin-based gliding on substrates and this form of motility powers cell invasion.8 Intracellular calcium stores are required for activating increases in cytosolic calcium, which induces protein secretion and facilitates cell invasion.9 Intracellular calcium has also been implicated in controlling egress of the parasite from infected cells.10 Pharmocological evidence suggests the presence of both ryanodine-responsive and IP3-receptor-like channels, although the genes encoding these activities have not been identified in apicomplexan parasites.11 In searching the genome for calcium regulation pathways, it became clear that T. gondii lacks many animal-like pathways, yet has affinities with plants.12 For example, T. gondii lacks conventional IP3 channels but instead contains a gene that is similar to the two-pore calcium channel found in plants.2,3 Apicomplexans also contain a variety of calcium-dependent protein kinases, which are highly developed in plants but absent in animals.14
The second messenger cyclic ADP ribose (cADPR) evokes changes in cytosolic calcium in both plants15 and animals16 by stimulating calcium release from intracellular stores. T. gondii also uses the second messenger cADPR to stimulate release of internal calcium stores.17 The antagonist 8-Br-cADPR inhibits protein secretion and motility in the parasite,17 indicating this pathway is important for controlling cell invasion. Plants generate cADPR in response to the phytohormone ABA,18 leading to elevated cytosolic calcium that controls stomata closure in guard cells and induces gene expression.18 ABA has also been linked to generation of cADPR and induction of calcium signaling in other metazoans including hydra19 and sponges.20 Recently, human granulocytes (myeloid cells) were found to produce ABA, which triggers calcium increases by a cADPRdependent pathway.21 This prompted us to consider whether apicomplexan parasites might also contain such an ABA-dependent, cADPR pathway for controlling calcium release.
ABA Production and Signaling in T. gondii
Addition of exogenous ABA to extracellular T. gondii parasites strongly stimulated secretion of proteins from apical organelles called micronemes,22 a process necessary for parasite motility and cell invasion. Previous studies have shown that this pathway relies on intracellular calcium and is mediated by increases in cADPR.11,17 Addition of ABA also stimulated increases in cADPR and lead to a calcium-dependent induction of protein secretion.22 This activity was restricted to (± ABA) and was not stimulated by β-carotene, retinoic acid or (-) ABA.22
Further evidence that T. gondii contains ABA was provided by ELISA using an antibody specific to ABA and by mass spectrometric (MS) analysis.22 Levels of ABA reached a peak just prior to natural egress, when parasite-containing vacuoles reach their maximum size. MS/MS analysis revealed the characteristic fragments for both trans-ABA and cis-ABA in extracts of T. gondii that were subjected to HPLC purification and gas chromatography and tandem MS/MS analysis.22 The levels of ABA in parasites ranged from 0.1–0.2 µM during initial infection to ∼4 µM near the end of the infection cycle, when the density of parasites within the host cell is highest.
These levels are 10–40 times higher than that reported for human granulocytes,2 supporting the idea that ABA is produced by the parasite rather than concentrated from environmental sources. Remarkably, when exogenous ABA was added to late stage vacuoles, it induced premature egress, consistent with the idea that this signal mediates exit of the parasite from the host cell. Similar to plants, the production of ABA in T. gondii was inhibited by treatment with fluridone, which blocks ABA-biosynthesis at the step of phytoene desaturase24 (Fig. 2). Disruption of ABA production by fluridone inhibited the egress of T. gondii from the host cell. The fluridone block was rescued by addition of exogenous ABA, supporting the idea that this signal controls nature egress. Collectively, these studies indicate that T. gondii produces ABA, elevating the second messenger cADPR, and increasing intracellular calcium to control motility and cell egress (Fig. B).
Figure 2.
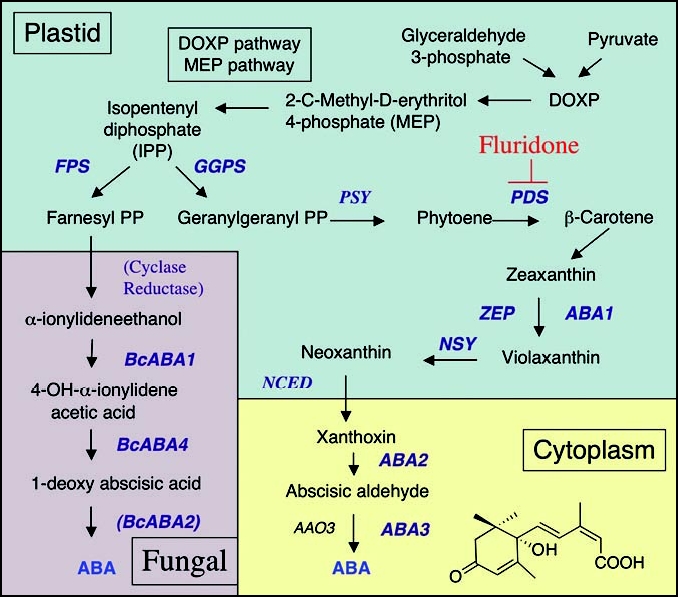
Biosynthetic pathways for ABA production in higher plants and fungi. This is an abbreviated scheme showing only the major steps in the pathways. For further details consult.24,25,28 DOXP, deoxy-D-xylulose-5-phosphate; FPS, farnesyl diposphate synthase; GGPS, geranylgeranyl diphosphate synthase; PSY, phytoene synthase; PDS, phytoene desaturase; ZEP, zeaxanthin epoxidase; NSY, neoxanthin synthase; NCED, 9-cis-epoxycarotenoid dioxygenase; ABA, abscisic acid.
In plants, ABA also controls development by acting to increase dormancy in seeds and to prevent premature germination of embryos.25 Transcript levels of ABA biosynthetic genes (see below) in plants are also upregulated during osmotic, cold and salt stress.25 Thus, ABA has been recognized as a stress hormone, able to increase plant tolerance to stressful conditions. A role in the stress response does not appear to be directly analogous in parasites. Instead repression of ABA synthesis by fluridone induced the differentiation to bradyzoites in vitro.22 Dormancy in T. gondii is normally a stress-induced pathway that can be triggered by nutrient limitation.26 Thus, differentiation to bradyzoites in the absence of ABA may simply reflect a default pathway for parasites remaining within a host cell that contains diminishing resources and which lacks a signal for egress. It is also possible that reduction in ABA synthesis may make parasites more susceptible to stressful conditions in the environment, thus triggering a differentiation process that would otherwise be blocked under high levels of ABA.
Fluridone was also effective in preventing lethal toxoplasmosis in the murine model, consistent with its ability to block ABA production, delay egress and thus prevent spread of infection.22 Previous studies demonstrate the related compound norflorizone is also effective at inhibiting malaria in vitro,27 although the biological role of ABA has not been studied in this system. Because fluridone is an effective herbicide that is relatively non-toxic to mammals, it may provide a good lead for the development of anti-parasitic compounds to combat infection.
Pathways for ABA Production in Plants, Fungi and Algae
The synthesis of ABA in higher plants occurs by an indirect pathway that is initiated by cleavage of carotenoids in the plastid and subsequent modification of the resulting xanthoxin products in the cytosol24 (summarized in Fig. 2). The pathway is known from a variety of metabolic studies as well as mutants that are disrupted in specific steps of synthesis, largely from studies in Arabidopsis, maize and tomato.24 In plastids, the β-carotene hydroxylated product zeaxanthin is converted to violaxanthin by a zeaxanthin epoxidase (ZEP), also known as ABA1 in Arabidopsis. Mutants in this step are defective in production of ABA, demonstrating that ABA is derived from C40 carotenoids. The first committed step in the pathway to ABA is catalyzed by 9-cis-epoxycarotenoid dioxygenase (NCED), which cleaves the C40 substrate 9-cis-epoxycarotenoid to yield the C15 intermediate xanthoxin. NCED is related to dioxygenases in mammalian cells that generate vitamin A (retinol) from β-carotene obtained in the diet. NCED activity is impaired in the viviparous 14 (vp14) mutant of maize, leading to premature seed germination.
Subsequent modification of xanthoxin by ABA2, a short-chain alcohol dehydrogenase/reductase, yields abscisic aldhehyde. This product is then converted to ABA, by the action of ABA aldehyde oxidases (AAO), which require the molybdenum cofactor (MoCo) sulfurase (ABA3)-activated MoCo factor for the catalytic activity. ABA is catabolized in plants to the inactive phaseic acid by the action of ABA 8′-hydroxylases. Fungi also produce ABA; however, they use a direct route from farnesyl pyrophosphate through a cyclized C15 intermediate known as α-ionylideneethanol28 (Fig. 2).
The initial steps in ABA synthesis in higher plants take place in the plastids, attesting to their origin from algal endosymbionts. While it is not yet clear if the downstream enzymes are conserved in algae, there is compelling evidence for ABA biosynthesis in these organisms. ABA has been reported in a wide variety of algae including green (chlorophyta), red (rhodophyta), brown (heterokontophyta) and several cyanobacteria, with intracellular concentrations ranging from 1∼40 µM.29 Radiolabeling studies indicate that ABA production by the green alga Dunaliella salina is enhanced by hypersalinity stress.30 Cyanobacteria have also been reported to produce ABA under stress conditions.31 Furthermore, ABA influences stress responses and morphogenesis in the green alga Haematococcus pluviali32 and induces differentiation and spore formation in the brown alga Laminaria japonica.31
While there is clear evidence for the production of ABA and physiological responses to this hormone in algae, the biosynthetic pathway is less well studied than in higher plants. Moreover, higher plants often contain gene families that encode ABA biosynthetic enzymes.24 This gene redundancy may partly contribute to the ‘leaky’ phenotypes of certain ABA-deficient mutants (i.e., vp14 in maize) that are only partially impaired in ABA production.24 Additionally although most plants contain only single ABA2 and ABA3 genes, residual levels of ABA in mutants of these genes may reflect alternative shunt pathways that are not fully defined. Hence, the lack of close similarity between ABA biosynthetic genes in higher plants and algae may reflect gene divergence since the common origin of plastids.33
Apicomplexans lack the mevalonate pathway but instead have a conserved DOXP-MEP pathway for synthesis of isoprenoids, that is thought to be derived from the plastid.34 However, searching the T. gondii genome database (http://toxodb.org) does not provide strong support for a conserved carotenoid or ABA biosynthetic pathway. For example, T. gondii appears to lack clear orthologues for several steps in carotenoid biosynthesis including phytoene synthase (PSY) and NCED, at least based on searches using genes from higher plants. Weak orthologues for phytoene desaturase (PDS) and zeta carotene desaturase (ZDS) are found, and this is consistent with the inhibition of ABA production following treatment with fluridone.22 T. gondii contains multiple candidate genes for biosynthesis of ABA (i.e., ABA1, ABA2 and ABA3). However, these genes have low homologies with higher plant genes, and do not allow unambiguous assignments of the enzymes in this pathway. This may reflect a divergence of the pathways in parasite and/or similarity to algal like pathways, rather than those found in higher plants. Future analysis of this pathway in algae and parasites should provide further insight into the biosynthesis pathway and the role of this hormone outside of higher plants.
Outlook
Many apicomplexans retain an endosymbiont derived from ingestion of a red algal cell, giving rise to the possibility that plastid-like pathways have been retained in these parasites. Although apicomplexans have lost the means for photosynthesis, they appear to retain a primitive pathway for production of plant hormones such as ABA. Our studies reveal that T. gondii produces ABA, which likely originates from the DOXP isoprenoid pathway via carotenoid intermediates. However, apicomplexans appear to lack highly conserved enzymes found in this pathway in higher plants. Whether they contain a primitive or divergent pathway, or one that resembles the direct pathway found in fungi is presently unclear. In this regard, Chromera, an ancestrally related photosynthetic alveolate.6 may shed light on the pathways for carotenoid biosynthesis and downstream metabolites that existed in the ancestral algal cell that gave rise to the apicoplast.
In higher plants, the indirect pathway for ABA production begins in the plastid and is completed in the cytosol.24 However, it is not known where ABA is synthesized, stored and accumulated in parasite-infected cells. Figure 1 shows ABA released into the vacuole; however, it is also possible it accumulates within the parasite or in the host cell. Since ABA is a weak acid (pKa 4.7) it tends to concentrate in compartments with neutral or basic pH and does not readily pass membranes. Accumulation in separate compartments may trigger different responses depending on the localization of specific mechanisms for detection.
Finally, it is not known how ABA is sensed in parasites, In this regard, the conservation of several recently described plant receptors for ABA in apicomplexan parasites is intriguing. The RNA-binding protein FCA is an ABA receptor that controls translational repression and flowering responses in plants.35 The second of these is a G-protein-coupled receptor that functions as a plasma membrane receptor to control ABA responses in Arabidopsis.36 Both of these receptors have putative orthologues with high BLAST hits in apicomplexan parasites (http:://ApiDB.org). Downstream of such receptors, it is also not known if ABA induces transcriptional changes in parasites, similar to those described in plants, where this is a predominant mode of action.24,25 Thus, while our initial studies implicate ABA in calcium-triggered egress, it is likely this metabolite has many other influences on parasite biology that are yet to be discovered.
Acknowledgements
We are grateful to Boris Striepen, Sylvia Moreno, Geoff McFadden and Jan Zeevaart for helpful discussions. Work in the author's laboratories was supported by the NIH (L. David Sibley), AHA (Eduardo Chini), NSF (Liming Xiong) and MEXT (Kisaburo Nagamune).
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/6106
References
- 1.Peterson E. In: Toxoplasma: Molecular and Cellular Biology. Ajioka JW, Soldati D, editors. Norfolk, UK: Horizon Bioscience; 2007. pp. 37–58. [Google Scholar]
- 2.Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev. 1998;11:267–299. doi: 10.1128/cmr.11.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Köhler S, Delwiche CF, Denny PW, Tilney LG, Webster P, Wilson RJ, Palmer JD, Roos DS. A plastid of probable green algal origin in Apicomplexan parasites. Science. 1997;275:485–489. doi: 10.1126/science.275.5305.1485. [DOI] [PubMed] [Google Scholar]
- 4.McFadden GI, Reith ME, Munholland J, Lang-Unnasch N. Plastid in human parasites. Nature. 1996;381:482. doi: 10.1038/381482a0. [DOI] [PubMed] [Google Scholar]
- 5.Waller RF, McFadden GI. The apicoplast: a review of the derived plastid of apicomplexan parasites. Curr Issues Mol Biol. 2005;7:57–79. [PubMed] [Google Scholar]
- 6.Moore RB, Oborník M, Janouskovec J, Chrudimský T, Vancovα M, Green DH, Wright SW, Davies NW, Bolch CJ, Heimann K, Slapeta J, Hoegh Guldberg O, Logsdon JM, Carter DA. A photsynthetic alveolate closely related to apicomplexan parasites. Nature. 2008;45:959–963. doi: 10.1038/nature06635. [DOI] [PubMed] [Google Scholar]
- 7.Moreno SNJ, Docampo R. Calcium regulation in protozoan parasites. Curr Opin Microbiol. 2003;6:359–364. doi: 10.1016/s1369-5274(03)00091-2. [DOI] [PubMed] [Google Scholar]
- 8.Sibley LD. Invasion strategies of intracellular parasites. Science. 2004;304:248–253. doi: 10.1126/science.1094717. [DOI] [PubMed] [Google Scholar]
- 9.Lovett JL, Sibley LD. Intracellular calcium stores in Toxoplasma gondii govern invasion of host cells. J Cell Sci. 2003;6:3009–3016. doi: 10.1242/jcs.00596. [DOI] [PubMed] [Google Scholar]
- 10.Black MW, Arrizabalaga G, Boothroyd JC. Ionophore-resistant mutants of Toxoplasma gondii reveal host cell permeabilization as an early event in egress. Mol Cell Biol. 2000;20:9399–9408. doi: 10.1128/mcb.20.24.9399-9408.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovett JL, Marchesini N, Moreno SN, Sibley LD. Toxoplasma gondii microneme secretion involves intracellular Ca2+ release from IP3/ryanodine sensitive stores. J Biol Chem. 2002;277:25870–25876. doi: 10.1074/jbc.M202553200. [DOI] [PubMed] [Google Scholar]
- 12.Nagamune K, Sibley LD. Comparative genomic and phylogenetic analyses of calcium ATPases and calcium-regulated proteins in the Apicomplexa. Molec Biol Evol. 2006;23:613–627. doi: 10.1093/molbev/msl026. [DOI] [PubMed] [Google Scholar]
- 13.White PJ. Calcium channels in higher plants. Biochem Biophys Acta. 2000;465:171–189. doi: 10.1016/s0005-2736(00)00137-1. [DOI] [PubMed] [Google Scholar]
- 14.Harper JF, Harmon A. Plants, symbiosis and parasites: a calcium signalling connection. Nat Rev Mol Cell Biol. 2005;6:555–566. doi: 10.1038/nrm1679. [DOI] [PubMed] [Google Scholar]
- 15.Allen GJ, Muir SR, Sanders D. Release of Ca2+ from individual plant vacuoles by both IP3 and cyclic-ADP-ribose. Science. 1995;268:735–737. doi: 10.1126/science.7732384. [DOI] [PubMed] [Google Scholar]
- 16.Guse AH. Cyclic ADP-ribose: a novel Ca2+-mobilising second messenger. Cell Signal. 1999;11:309–316. doi: 10.1016/s0898-6568(99)00004-2. [DOI] [PubMed] [Google Scholar]
- 17.Chini EN, Nagamune K, Wetzel DM, Sibley LD. Evidence that the cADPR signaling pathway controls calcium-mediated secretion in Toxoplasma gondii. Biochem J. 2005;389:269–277. doi: 10.1042/BJ20041971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y, Kuzma J, Maréchal E, Graeff R, Lee HC, Foster R, Chua NH. Abscisic acid signaling through cyclic ADP ribose in plants. Science. 1997;278:2126–2130. doi: 10.1126/science.278.5346.2126. [DOI] [PubMed] [Google Scholar]
- 19.Puce S, Basile G, Bavestrello G, Bruzzone S, Cerrano C, Giovine M, Arillo A, Zocchi E. Abscisic acid signaling through cyclic ADP-ribose in hydroid regeneration. J Biol Chem. 2004;279:39783–39788. doi: 10.1074/jbc.M405348200. [DOI] [PubMed] [Google Scholar]
- 20.Zocchi E, Carpaneto A, Cerrano C, Bavestrello G, Giovine M, Bruzzone S, Guida L, Franco L, Usai C. The temperature-signaling cascade in sponges involves a heat-gated cation channel, abscisic acid and cyclic ADP ribose. Proc Natl Acad Sci USA. 2001;98:14859–14864. doi: 10.1073/pnas.261448698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruzzone S, Moreschi I, Usai C, Guida L, Damonte G, Salis A, Scarfì S, Millo E, De Flora A, Zocchi E. Abscisic acid is an endogenous cytokine in human granulocytes with cyclic ADP-ribose as second messenger. Proc Natl Acad Sci USA. 2007;104:5759–5764. doi: 10.1073/pnas.0609379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagamune K, Hicks LM, Fux B, Brossier F, Chini EN, Sibley LD. Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii. Nature. 2008;451:207–211. doi: 10.1038/nature06478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finkelstein R, Reeves W, Ariizumi T, Steber C. Molecular aspects of seed dormancy. Annu Rev Plant Biol. 2008 doi: 10.1146/annurev.arplant.59.032607.092740. PMID: 18257711. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz SH, Qin X, Zeevaart JAD. Elucidation of the indirect pathway of abscisic acid biosynthesis by mutants, genes, and enzymes. Plant Phyiol. 2003;131:1591–1601. doi: 10.1104/pp.102.017921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong L, Zhu JK. Regulation of abscisic acid biosynthesis. Plant Physiol. 2003;133:29–36. doi: 10.1104/pp.103.025395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss LM, Kim K. In: Toxoplasma gondii the model apicomplexan: perspectives and methods. Weiss LM, Kim K, editors. New York: Academic Press; 2007. pp. 341–366. [Google Scholar]
- 27.Leef JL, Carlson PS. Carotenoid synthesis inhibiting herbicides and fatty acid synthesis oxime herbicides as anti-apicomplexa protozoan parasite agents. Potomax Ltd. Prtn. 1999:1–8. [Google Scholar]
- 28.Oritani T, Kiyota H. Biosynthesis and metabolism of abscisic acid and related compounds. Nat Prod Rep. 2003;20:414–425. doi: 10.1039/b109859b. [DOI] [PubMed] [Google Scholar]
- 29.Hirsch R, Hartung W, Gimmler H. Abscisic acid content of algae under stress. Botanica Acta. 1989;102:326–334. [Google Scholar]
- 30.Cowan AK, Rose PD. Abscisic acid metabolism in salt stressed cells of Dunaliella salina. Plant Physiol. 1991;97:798–803. doi: 10.1104/pp.97.2.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marsalek B, Zahradnickova H, Hronkova M. Extracellular abscisic acid produced by cyanobacteria under salt stress. J Plant Physiol. 1992;139:506–508. [Google Scholar]
- 32.Kobayashi M, Hirai N, Kurimura Y, Ohigashi H, Tsuji Y. Abscisic acid-dependent algal morphogenesis in the unicellular green alga Haematococcus pluvialis. Plant Growth Reg. 1997;22:79–85. [Google Scholar]
- 33.McFadden GI, van Dooren GG. Evolution; Red algal genome affirms a common origin of all plastids. Curr Biol. 2004;14:514–516. doi: 10.1016/j.cub.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 34.Ralph SA, van Dooren GG, Waller RF, Crawford MJ, Fraunholz MJ, Foth BJ, Tonkin CJ, Roos DS, McFadden GI. Metabolic maps and functions of the Plasmodium falciparum apicoplast. Nature. 2004;2:203–206. doi: 10.1038/nrmicro843. [DOI] [PubMed] [Google Scholar]
- 35.Razem FA, El-Kereamy A, Abrams SR, Hill RD. The RNA-binding protein FCA is an abscisic acid receptor. Nature. 2006;439:290–294. doi: 10.1038/nature04373. [DOI] [PubMed] [Google Scholar]
- 36.Liu X, Yue Y, Li B, Nie Y, Li W, Wu WH, Ma L. A G protein-coupled receptor is a plasma membrane receptor for the plant hormone abscisic acid. Science. 2007;315:1712–1716. doi: 10.1126/science.1135882. [DOI] [PubMed] [Google Scholar]


