Abstract
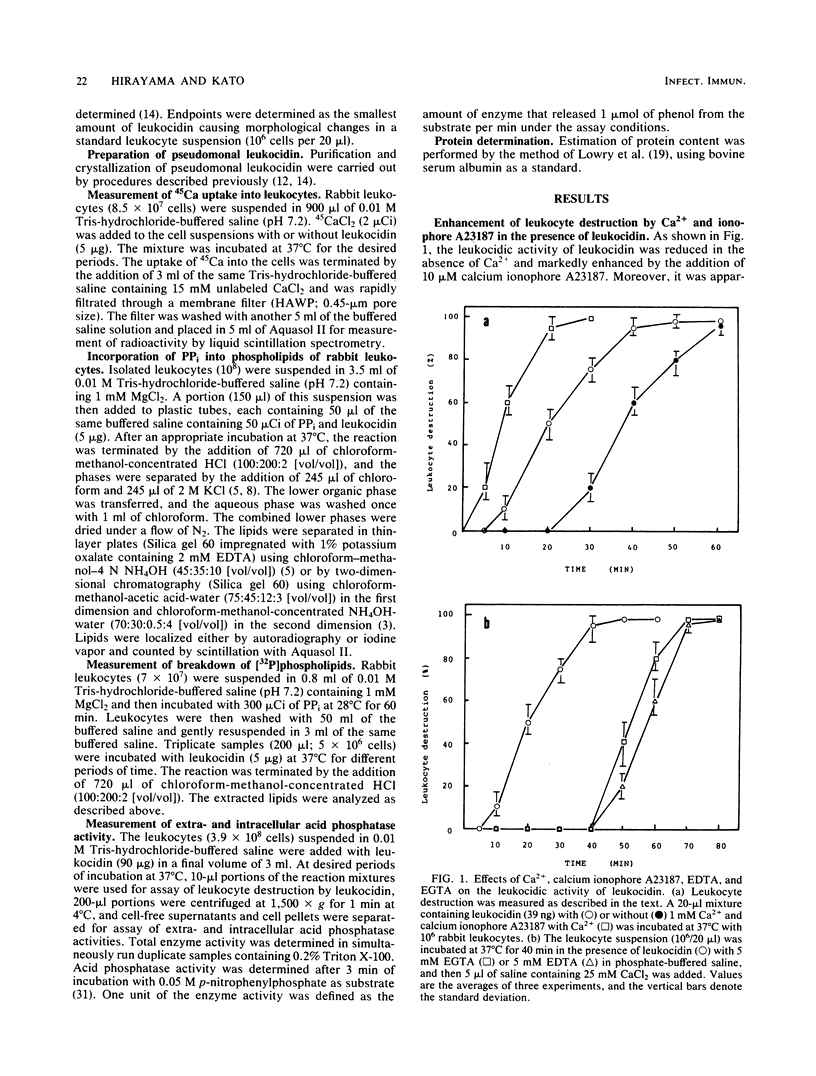
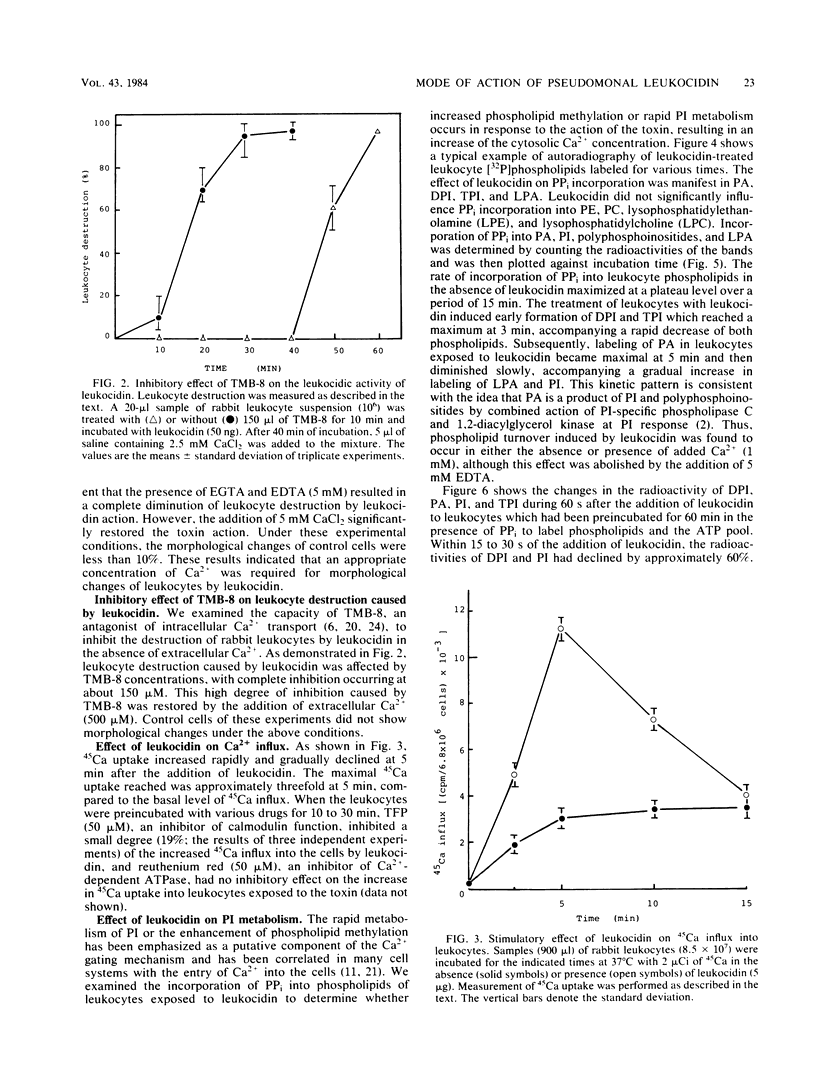
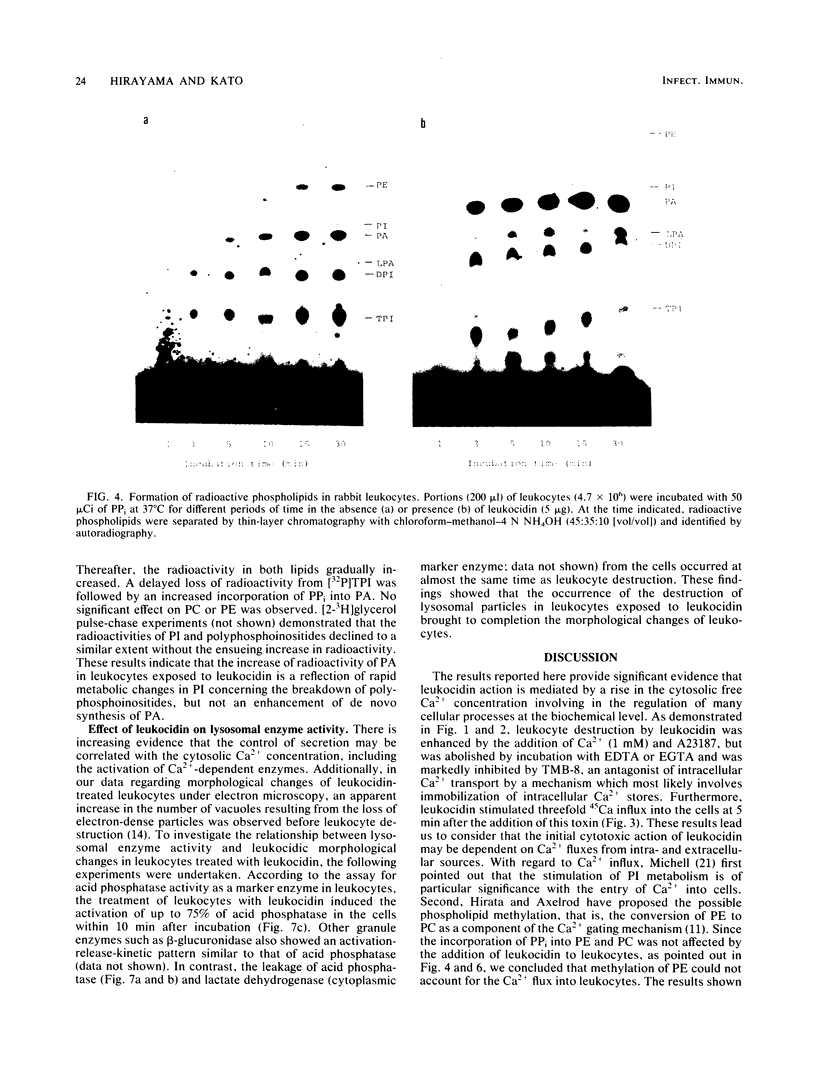
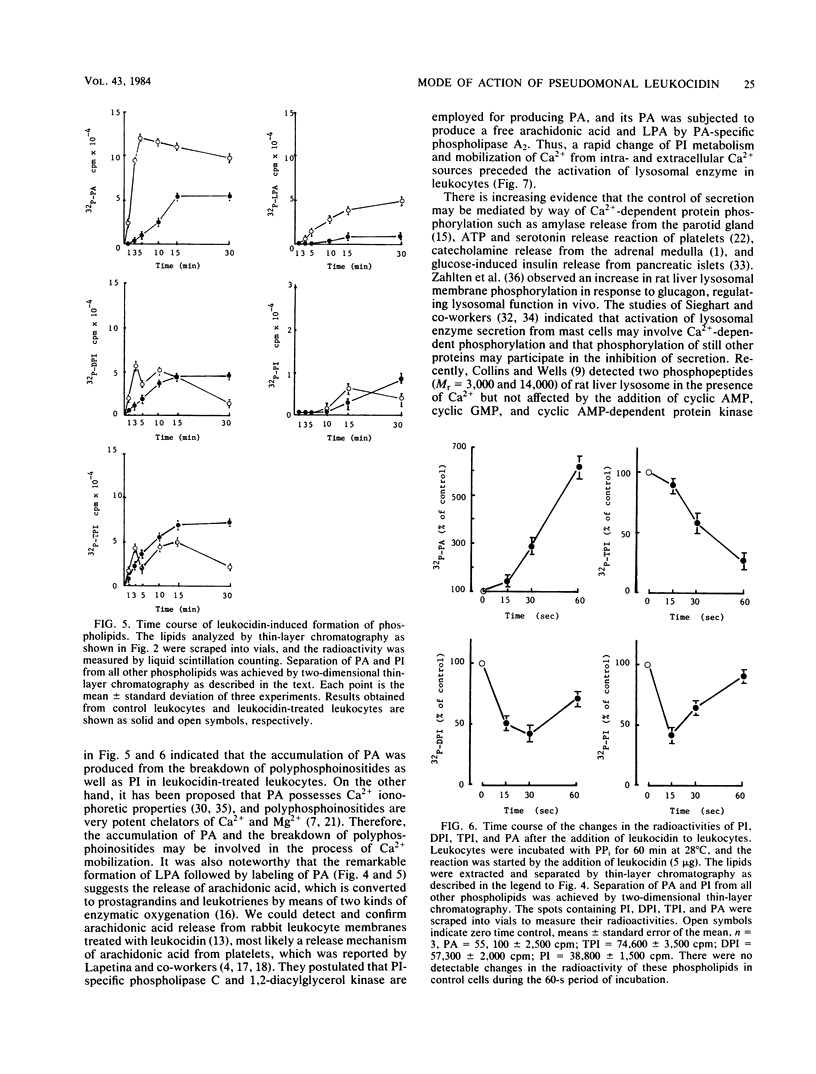
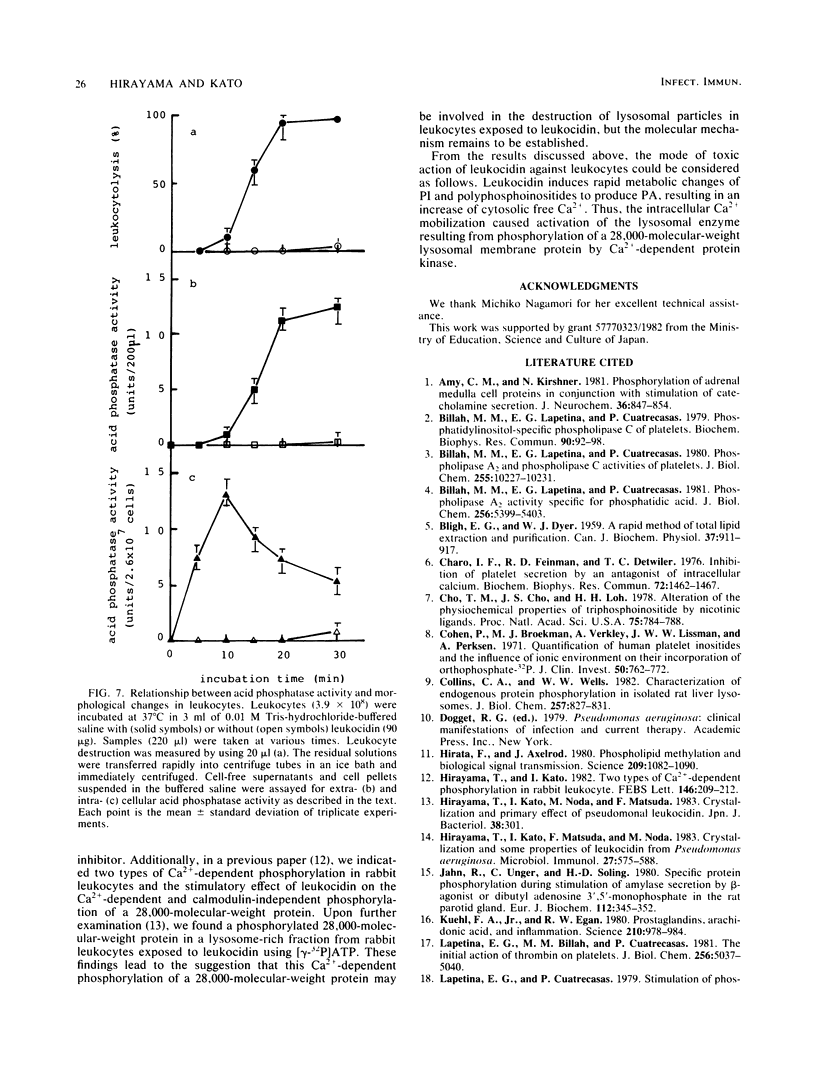
The cytotoxic action of leukocidin from Pseudomonas aeruginosa was supported by the following observations. (i) The destruction of rabbit leukocytes by the toxin was reduced in the absence of Ca2+ and stimulated by the addition of calcium ionophore A23187 but inhibited by EDTA, EGTA, and TMB-8, an antagonist of intracellular Ca2+ transport. (ii) Uptake of 45Ca into leukocytes exposed to the toxin was enhanced about threefold the rate of uptake into untreated cells. The increased 45Ca uptake into the cells was slightly inhibited by trifluoperazine, an inhibitor of Ca2+-calmodulin activity, but not by ruthenium red. (iii) Pseudomonal leukocidin enhanced rapidly the labeling of phosphatidylinositol, polyphosphoinositides, phosphatidic acid, and lysophosphatidic acid from [32P]phosphate. The time course experiments of the labeling and breakdown of these phospholipids suggested that the initial action of this toxin was to stimulate phosphatidic acid production, presumably causing a rapid metabolic change of phosphatidylinositol correlating with the activities of phosphatidylinositol-specific phospholipase C and 1,2-diacylglycerol kinase. It was considered that a rapid formation of phosphatidic acid and degradation of polyphosphoinositides might be related to a Ca2+ movement from extra- and intracellular space. (iv) In leukocytes exposed to the toxin, acid phosphatase activity as a marker enzyme of lysosome was activated up to 75% of the lysosomal enzyme before cell destruction. The leakage of lysosomal enzyme from the cells occurred at the almost same time as leukocyte destruction. The mode of cytotoxic action of pseudomonal leukocidin is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amy C. M., Kirshner N. Phosphorylation of adrenal medulla cell proteins in conjunction with stimulation of catecholamine secretion. J Neurochem. 1981 Mar;36(3):847–854. doi: 10.1111/j.1471-4159.1981.tb01671.x. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G., Cuatrecasas P. Phosphatidylinositol-specific phospholipase-C of platelets: association with 1,2-diacyglycerol-kinase and inhibition by cyclic-AMP. Biochem Biophys Res Commun. 1979 Sep 12;90(1):92–98. doi: 10.1016/0006-291x(79)91594-8. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G., Cuatrecasas P. Phospholipase A2 activity specific for phosphatidic acid. A possible mechanism for the production of arachidonic acid in platelets. J Biol Chem. 1981 Jun 10;256(11):5399–5403. [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G., Cuatrecasas P. Phospholipase A2 and phospholipase C activities of platelets. Differential substrate specificity, Ca2+ requirement, pH dependence, and cellular localization. J Biol Chem. 1980 Nov 10;255(21):10227–10231. [PubMed] [Google Scholar]
- Charo I. F., Feinman R. D., Detwiler T. C. Inhibition of platelet secretion by an antagonist of intracellular calcium. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1462–1467. doi: 10.1016/s0006-291x(76)80178-7. [DOI] [PubMed] [Google Scholar]
- Chiou C. Y., Malagodi M. H. Studies on the mechanism of action of a new Ca-2+ antagonist, 8-(N,N-diethylamino)octyl 3,4,5-trimethoxybenzoate hydrochloride in smooth and skeletal muscles. Br J Pharmacol. 1975 Feb;53(2):279–285. doi: 10.1111/j.1476-5381.1975.tb07359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho T. M., Cho J. S., Loh H. H. Alteration of the physicochemical properties of triphosphoinositide by nicotinic ligands. Proc Natl Acad Sci U S A. 1978 Feb;75(2):784–788. doi: 10.1073/pnas.75.2.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Broekman M. J., Verkley A., Lisman J. W., Derksen A. Quantification of human platelet inositides and the influence of ionic environment on their incorporation of orthophosphate-32P. J Clin Invest. 1971 Apr;50(4):762–772. doi: 10.1172/JCI106547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C. A., Wells W. W. Characterization of endogenous protein phosphorylation in isolated rat liver lysosomes. J Biol Chem. 1982 Jan 25;257(2):827–831. [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Phospholipid methylation and biological signal transmission. Science. 1980 Sep 5;209(4461):1082–1090. doi: 10.1126/science.6157192. [DOI] [PubMed] [Google Scholar]
- Hirayama T., Kato I., Matsuda F., Noda M. Crystallization and some properties of leukocidin from Pseudomonas aeruginosa. Microbiol Immunol. 1983;27(7):575–588. doi: 10.1111/j.1348-0421.1983.tb00619.x. [DOI] [PubMed] [Google Scholar]
- Hirayama T., Kato I. Two types of Ca2+-dependent phosphorylation in rabbit leukocytes. FEBS Lett. 1982 Sep 6;146(1):209–212. doi: 10.1016/0014-5793(82)80737-0. [DOI] [PubMed] [Google Scholar]
- Jahn R., Unger C., Söling H. D. Specific protein phosphorylation during stimulation of amylase secretion by beta-agonists or dibutyryl adenosine 3',5'-monophosphate in the rat parotid gland. Eur J Biochem. 1980 Nov;112(2):345–352. doi: 10.1111/j.1432-1033.1980.tb07211.x. [DOI] [PubMed] [Google Scholar]
- Kuehl F. A., Jr, Egan R. W. Prostaglandins, arachidonic acid, and inflammation. Science. 1980 Nov 28;210(4473):978–984. doi: 10.1126/science.6254151. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lapetina E. G., Billah M. M., Cuatrecasas P. The initial action of thrombin on platelets. Conversion of phosphatidylinositol to phosphatidic acid preceding the production of arachidonic acid. J Biol Chem. 1981 May 25;256(10):5037–5040. [PubMed] [Google Scholar]
- Lapetina E. G., Cuatrecasas P. Stimulation of phosphatidic acid production in platelets precedes the formation of arachidonate and parallels the release of serotonin. Biochim Biophys Acta. 1979 May 25;573(2):394–402. doi: 10.1016/0005-2760(79)90072-9. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Nishikawa M., Tanaka T., Hidaka H. Ca2+-calmodulin-dependent phosphorylation and platelet secretion. Nature. 1980 Oct 30;287(5785):863–865. doi: 10.1038/287863a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Hirayama T., Kato I., Matsuda F. Crystallization and properties of staphylococcal leukocidin. Biochim Biophys Acta. 1980 Nov 17;633(1):33–44. doi: 10.1016/0304-4165(80)90035-5. [DOI] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S., Deykin D. The activation by Ca2+ of platelet phospholipase A2. Effects of dibutyryl cyclic adenosine monophosphate and 8-(N,N-diethylamino)-octyl-3,4,5-trimethoxybenzoate. Biochim Biophys Acta. 1978 Nov 1;543(4):409–422. doi: 10.1016/0304-4165(78)90296-9. [DOI] [PubMed] [Google Scholar]
- SHIBKO S., TAPPEL A. L. RAT-KIDNEY LYSOSOMES: ISOLATION AND PROPERTIES. Biochem J. 1965 Jun;95:731–741. doi: 10.1042/bj0950731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharmann W. Cytotoxic effects of leukocidin from Pseudomonas aeruginosa on polymorphonuclear leukocytes from cattle. Infect Immun. 1976 Mar;13(3):836–843. doi: 10.1128/iai.13.3.836-843.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharmann W. Formation and isolation of leucocidin from Pseudomonas aeruginosa. J Gen Microbiol. 1976 Apr;93(2):283–291. doi: 10.1099/00221287-93-2-283. [DOI] [PubMed] [Google Scholar]
- Scharmann W. Interaction of purified leukocidin from Pseudomonas aeruginosa with Bovine polymorphonuclear leukocytes. Infect Immun. 1976 Apr;13(4):1046–1053. doi: 10.1128/iai.13.4.1046-1053.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharmann W. Purification and characterization of leucocidin from Pseudomonas aeruginosa. J Gen Microbiol. 1976 Apr;93(2):292–302. doi: 10.1099/00221287-93-2-292. [DOI] [PubMed] [Google Scholar]
- Serhan C., Anderson P., Goodman E., Dunham P., Weissmann G. Phosphatidate and oxidized fatty acids are calcium ionophores. Studies employing arsenazo III in liposomes. J Biol Chem. 1981 Mar 25;256(6):2736–2741. [PubMed] [Google Scholar]
- Sieghart W., Theoharides T. C., Alper S. L., Douglas W. W., Greengard P. Calcium-dependent protein phosphorylation during secretion by exocytosis in the mast cell. Nature. 1978 Sep 28;275(5678):329–331. doi: 10.1038/275329a0. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Oka H., Yasuda H., Ikeda M., Cheng P. Y., Oda T. Effect of glucose on protein phosphorylation in rat pancreatic islets. Biochem Biophys Res Commun. 1981 Apr 15;99(3):987–993. doi: 10.1016/0006-291x(81)91259-6. [DOI] [PubMed] [Google Scholar]
- Theoharides T. C., Sieghart W., Greengard P., Douglas W. W. Antiallergic drug cromolyn may inhibit histamine secretion by regulating phosphorylation of a mast cell protein. Science. 1980 Jan 4;207(4426):80–82. doi: 10.1126/science.6153130. [DOI] [PubMed] [Google Scholar]
- Tyson C. A., Vande Zande H., Green D. E. Phospholipids as ionophores. J Biol Chem. 1976 Mar 10;251(5):1326–1332. [PubMed] [Google Scholar]
- Zahlten R. N., Hochberg A. A., Stratman F. W., Lardy H. A. Glucagon-stimulated phosphorylation of mitochondrial and lysosomal membranes of rat liver in vivo. Proc Natl Acad Sci U S A. 1972 Apr;69(4):800–804. doi: 10.1073/pnas.69.4.800. [DOI] [PMC free article] [PubMed] [Google Scholar]