Abstract
The last universal common ancestor (LUCA) might have been either prokaryotic- or eukaryotic-like. Nevertheless, the universally distributed components suggest rather LUCA consistent with the pre-cell theory of Kandler. The hypotheses for the origin of eukaryotes are briefly summarized. The models under which prokaryotes or their chimeras were direct ancestors of eukaryotes are criticized. It is proposed that the pre-karyote (a host entity for α-proteobacteria) was a remnant of pre-cellular world, and was unlucky to have evolved fusion prohibiting cell surface, and thus could have evolved sex. The DNA damage checkpoint pathway could have represented the only pre-karyotic checkpoint control allowing division only when DNA was completely replicated without mistakes. The fusion of two partially diploid (in S-phase blocked) pre-karyotes might have represented another repair strategy. After completing replication of both haploid sets, DNA damage checkpoint would allow two subsequent rounds of fission. Alternatively, pre-karyote might have possessed two membranes inherited from LUCA. Under this hypothesis symbiotic α-proteobacterial ancestors of mitochondria might have ancestrally been selfish parasites of pre-karyote intermembrane space whose infection might have been analogous to infection of G--bacterial periplasm by Bdellovibrio sp. It is suggested that eukaryotic plasma membrane might be derived from pre-karyote outer membrane and nuclear/ER membrane might be derived from pre-karyote inner membrane. Thus the nucleoplasm might be derived from pre-karyote cytoplasm and eukaryotic cytoplasm might be homologous to pre-karyote periplasm.
Key words: archaea, Bdellovibrio, endomembranes, evolution, ER, LACA, LECA, LUCA, meiosis, mitochondria, nucleus, phagocytosis, prokaryote
Introduction
The last eukaryotic common ancestor (LECA) is nowadays assumed to have been a complete eukaryotic cell. It possessed nucleus, endoplasmic reticulum (ER), Golgi apparatus (GA), complex cytoskeletal network based on actin and tubulin, and mitochondria. It was sexual, flagellated, it underwent mitosis and meiosis, and it was phagotrophic. Though not all eukaryotes share all these characteristics, most eukaryotes share most of them. Moreover, each of these characteristic features is such a complex one that the probability of their appearance in the evolution of eukaryotes more than once is close to zero. In addition, complex eukaryotic defining features are never found in prokaryotes, i.e., archaea and bacteria. The determination of the precise order of the origins of eukaryotic cell structures and eukaryote-specific cellular processes in the onset of the appearance of LECA has been a major challenge for evolutionists dealing with the origin(s) of cells. The precise order of the appearance of these features depends a lot on the philosopher's understanding of the nature of the universal ancestor, i.e., what the last universal common ancestor (LUCA) means for him (or her). Furthermore, the hypotheses for the origins of two other domains, Bacteria and Archaea,1 and the order of the appearance of three domains of life, also depend on the meaning of LUCA. In this paper we critically summarize various views of LUCA and various hypotheses for the origin of eukaryotes. In the end we present a novel hypothesis for the origin of eukaryotes.
The Nature of LUCA
Three different views of LUCA could be easily imagined: (1) LUCA was a prokaryote (i.e., bacterium or archeon), (2) LUCA was rather eukaryotic-like than prokaryotic-like; (3) LUCA was neither prokaryotic- nor eukaryotic-like.
LUCA might have been a prokaryote.
LUCA might have been either bacterium or archeon. However, bacterial membrane lipids are fatty acid esters linked to sn-glycerol-3-phosphate and archaeal membrane lipids are isoprenoid ethers built on sn-glycerol-1-phosphate. Assuming LUCA being identical to LBCA (last bacterial common ancestor) would require replacement of bacterial lipids by archaeal lipids in LACA (last archaeal common ancestor), while assuming LUCA being identical to LACA would require replacement of archaeal lipids by bacterial lipids in LBCA as well as in LECA, because eukaryotes also possess bacterial membrane lipids. This replacement of membrane lipids would most likely require a transient stage with a hybrid membrane(s) composed of a mixture of bacterial and archaeal lipids. It has been suggested on the basis of lipid incompatibilities in cases of chiral discrimination that such a hybrid membrane is less stable than bacterial or archaeal one, and thus would be selectively disfavoured.2,3 Therefore lipid replacement does not seem to be likely. If it had even ever happened, it might have happened once rather than twice. As the scenario suggesting LUCA being an archeon would require two lipid replacements (in LBCA as well as in LECA), the scenario suggesting LUCA being a bacterium seems to be more likely, thought it is also problematic.
In addition, two kinds of bacteria exist: G--bacteria bounded by two membranes and thin cell wall between them, and G+-bacteria possessing only one membrane surrounded by thick cell wall. The hypothesis of Cavalier-Smith4 suggests that LUCA was a well-developed G--bacterium, while G+-bacteria evolved afterwards via a loss of outer membrane. On the other hand there exist another evolutionary model under which LUCA was well-developed G+-bacterium,5 and G--bacteria might have evolved by de novo synthesis of outer membrane to resist aggressive viral infections.6
LUCA might have been eukaryotic-like.
Eukaryotic-like LUCA has been suggested, for example by Forterre and Philippe.7 Nevertheless, this scenario might be even less likely than previous one. It would not only require the lipid replacement in LACA, but it would also have to explain the origin of complex eukaryotic-like LUCA at the first place, and it would have to propose high levels of reductive evolution leading to the origins of LACA and LBCA afterwards. However, this does not mean that at least some features of LUCA might not have been eukaryotic-like rather than prokaryotic-like. For example, Woese has suggested that LUCA might have possessed linear chromosomes,8 and Wächtershäuser2,3 has proposed that LUCA practiced frequent ‘quasi-sexual’ fusions and fissions. In addition, some common prokaryotic features including e.g., the prokaryotic textbook prototype parsimoniously organized circular (however, see ref. 9), single-copy (however, see ref. 10) membrane-attached genomes might be a convergence of Bacteria and Archaea caused by similar selective pressures for fast and precise DNA replication and reproduction.11
LUCA without membrane(s)?
Under the scenario suggested by Martin and Russel12 and Koonin and Martin13 LUCA did not possess lipid membrane, instead it was living in some kind of inorganic compartment. In this view LBCA and LACA independently escaped from the inorganic compartment when they were enclosed in bacterial membrane lipids and archaeal membrane lipids, respectively. This scenario was suggested mainly to explain the great biochemical and biophysical differences between bacterial and archaeal membranes. However, the enclosure of genes and catalysts by membrane lipids as a ‘replicating whole’ is the greatest problem of cell origin and assuming happening this twice is highly ‘unparsimonious’ scenario.14,15 Moreover, the universal conservation of components of pathways that translocate proteins through and mediate insertion of proteins into plasma membrane (or ER membrane in eukaryotes), in particular SecY, SRP54, FtsY/SRα and signal peptidase, as well as universal conservation of F0F1-ATPase, suggest that LUCA was bounded by membrane lipids.14
LUCA as a pre-cell.
In fact, there exists no direct evidence that LUCA was either prokaryotic- or eukaryotic-like.16 All we can be sure about is that LUCA possessed all components, which are universally distributed among all three domains and all organisms. These include ribosomes,8,17,18 lipid membrane(s) with membrane secretory and insertion apparatus as well as ATP-ase;2,3,14 and LUCA most likely possessed DNA.2,3,8,17 Otherwise, we can think about the nature of LUCA quite freely.
Lipid-bounded LUCA. As suggested by universally distributed components of membrane secretory and insertion machinery14 and distribution of enzymes involved in lipid synthesis19 LUCA was certainly bounded by membrane lipids. Wächtershäuser2,20,21 suggested that LUCA possessed membrane composed of mixture of archaeal and bacterial lipids. This view has been compellingly supported by the phylogenetic study of enzymes involved in lipid synthesis, and it has been suggested that the synthesis of lipids in LUCA was enzymatic but probably non-stereospecific.19 As hybrid membranes composed of mixture of bacterial and archaeal lipids are less stable than membranes composed only of bacterial or archaeal lipids, it is reasonable that evolution could have proceeded from the membranes of mixed composition to membranes composed of only bacterial or only archaeal lipids with higher probability than in the opposite direction.2,3
Wächtershäuser2,3,20,21 proposes that LUCA could have practiced frequent promiscuous ‘quasi-sexual’ fusions and fissions. Under fusions and fissions population of LUCA might have segregated into subpopulations with pre-dominantly bacterial (subpopulations of type B) or archaeal (subpopulations of type A) lipids strictly by physical and chemical forces.2,3,20,21 However, this does not mean that LUCA of type B could not have transformed into type A or vice verse. It would depend on with which types and how frequently it would fuse. The origin of Bacteria might have been nothing else than the emergence of an enzyme for the stereospecific formation of glycerol-3-phosphate units22 and the emergence of fusion-prohibiting cell wall23–26 in a subpopulation of LUCA B. Similarly, the origin of Archaea might have been a consequence of the emergence of an enzyme for the stereospecific formation of glycerol-1-phosphate units22 and the emergence of fusion prohibiting cell wall23–26 in a subpopulation of LUCA A.
Translation and secretion. LUCA had certainly the ability to translate RNAs into polypeptides as suggested by the universal presence of ribosomes with their central rRNA components.8,17,27 Thirty-three ribosomal proteins and more than 30 other enzymes associated with translation are universally present in all living organisms and thus are traceable to LUCA.18 Universal presence of some enzymes linking translation and secretion14,18 further suggests that LUCA had the ability of co-translational secretion of proteins through plasma membrane. Membrane lipids, ribosomes and co-translational secretion thus suggest quite complex LUCA reminding in these aspects any modern cell, however, in other aspects LUCA did not have to be such complex and modern anyway, and there is virtually no evidence that it was.
Genome structure and replication. Compelling evidence that LUCA almost certainly possessed DNA is that DNA-dependent RNA-polymerase β and β′ subunits are universal in distribution,17 although other components of machinery for transcription are not. This suggests that the transcription apparatus is less conserved than translational and must have underwent dramatic changes in the course of the appearance and/or the evolution of the ancestors of each domain. The universal distribution of RecA/Rad51 homologs28 can be also taken as evidence in favor of DNA-bearing LUCA.
It is striking that some enzymes (but only minority) involved in DNA replication are orthologous in Archaea and Eukarya, but these seem to have no homologs in Bacteria.17,29,30 DNA replication mechanism thus must have evolved two17,29,30 or three30,31 times, and taking multiple exchanges of DNA-polymerases between viruses and cells32 into play, it could have evolved in fact many times. As ‘evolution of genome structure goes hand in hand with that of corresponding mechanism to replicate it’,8 this fairly challenges the view that prokaryotic prototype single copy circular parsimoniously organized genomes were the ancestral form.
Wächtershäuser2,3,33 reconstructed ancestral gene cluster of about 40 genes (whose products are involved mainly in translation, SecY and SecE in secretion, NusG in antitermination of transcription, and three are RNA-polymerase subunits) that is shared between Bacteria and Archaea and thus was most likely present in LUCA. He suggests that such cluster encoded by DNA circular multicopy plasmids would have been ideal for rolling circle transcription without termination leaving large polycistronic transcripts.2,3
It has been suggested that DNA viruses even pre-date LUCA and that many enzymes of DNA metabolisms may be of viral origin.29–31 Even a symbiotic RNA virus encoding reverse transcriptase might have provided a simple mechanism of replication of LUCA's circular multicopy plasmids in form of reverse transcription of polycistronic transcripts and subsequent circularization via recA/rad51-homolog-mediated mechanism.
Wächtershäuser2,3 argues that multicopy linear chromosomes (plasmids) suggested as LUCA's genome by Woese (1998)8 would not be stable. However, various types of telomeres different from conventional eukaryotic type are present in various extant prokaryotes, eukaryotes as well as eukaryotic organelles.34 Thus multicopy linear plasmids with telomeres as a genome of LUCA can not be absolutely ruled out. Alternatively, various types of multicopy DNA plasmid-like genomes (linear, circular or mixed) and various DNA replication strategies might have co-existed within the subpopulations of LUCA depending on viral symbionts and other mobile elements that these subpopulations possessed and exchanged.
Metabolism. As LUCA probably possessed neither universal mechanism of DNA replication nor conserved genome structure, it probably did not possess conserved operational energetic metabolic properties. Operational metabolic genes are transferred horizontally easier than informational ones also in extant organisms.35 Thus LUCA might have occupied variety of habitats.2,36 Some subpopulations might have been autotrophic, some heterotrophic, some saprotrophic, some might have been sulfur-reducing, some sulfur-oxidizing, some might have been H2-producers, some H2-consumers etc.2,36
Universally distributed components suggest LUCA similar to views of Kandler and Woese. Taken together, LUCA was a self-replicating entity, with most basal attributes of cells (self-replicating entity with membrane lipids, ribosomes, genome and metabolism); however, it seems that it was not able to limit the frequent horizontal exchange of genetic information. This does not mean that vertical inheritance did not exist in the period of LUCA. If it did not, then there would be no conserved cellular components such as ribosomes. It only means that horizontal transfer was more frequent in the era of LUCA than nowadays. LUCA as described here seems to be consistent with the pre-cell theory of Kandler.23,24 Under Kandler's view, pre-cells, though forming coherent population, were not genetically identical, and subpopulations of pre-cells of various phenotypes inhabited various habitats.23,24 This contrasts a little bit the view of Woese8 under which LUCA was rather a communal network of diverse ‘progenotes’. Otherwise, both views are quite complementary and consistent with LUCA as described above. In addition, Woese8,17,27 described evolution from pre-cellular conglomerate seriously affected by horizontal gene transfer to cellular population that was not as much. Woese8,17 has named this transition milestone the ‘Darwinian Threshold’ and has defined it as ‘the stage in the cellular organization where the organismal genealogical trace (recorded in common histories of the genes of an organism) goes from being completely ephemeral to being increasingly permanent’. Taking into account the similarities of informational systems of Archaea and Eukarya as well as phylogenies of informational genes revealing their sisterhood, LBCA arose (crossed the Darwinian Threshold) prior to LACA.8,17,27
Hypotheses for the Origin of Eukaryotes and their Criticism
Various models for the origin of eukaryotes exist and it is difficult to create certain categories and place these hypotheses within them. For our purposes, we decided to classify the hypotheses for the origin of eukaryotes into two basic categories: (1) hypotheses proposing that prokaryotes or their chimeras were direct ancestors of eukaryotes; (2) hypotheses proposing that an entity different from bacteria and archaea was involved in the origin of eukaryotes.
Hypotheses proposing prokaryotic origin of eukaryotes and their pitfalls.
These hypotheses can be classified into four categories: (1) the hypotheses suggesting the true fusion of two (or more) prokaryotes [i.e., fusion of archaeal cell(s) and bacterial cell(s)]; (2) hypotheses suggesting a symbiotic association of two prokaryotes (i.e., an archaeal host and an α-proteobacterial ancestor of mitochondria); (3) hypotheses suggesting a symbiotic association of three prokaryotic species [i.e., symbiosis of archaea and α-proteobacteria in bacterial cell(s), or symbiosis of spirochetes with an archeon prior to the α-proteobacterial origin of mitochondria]; (4) hypothesis suggesting G+-bacterial ancestry of Eukarya and Archaea.
Fusion model for the origin of eukaryotes. Rivera and Lake37 propose a true chimera model under which a true fusion of bacterial and archaeal cells was the origin for eukaryotes. This model is based on ‘BLASTological’ molecular data,35 suggesting that eukaryotic informational genes (e.g., those whose products are involved in translation) are more closely related to archaeal than to bacterial genes, while operational (metabolic) genes are generally more closely related to bacterial than to archaeal genes.38 This fusion model would require almost certainly lethal interdomain hybridization. Rivera and Lake37 suggest that a ‘ring of life’ should reflect the relationship between domains rather than the tree of life. In fact, their ‘ring of life’ can be an artifact of their analysis that can be easily substituted and reinterpreted by means of prokaryote-to-eukaryote serial horizontal gene transfer.39,40
Metabolic symbiosis of an α-proteobacterium in an archeon. Martin and Müller41 propose that eukaryotic cells arose as a symbiosis of hydrogen-producing α-proteobacteria in methanogenic hydrogen-consuming archaea (Hydrogen hypothesis). In contrast, Searcy42 suggests that eukaryotes originated as a result of a symbiosis of sulfur-oxidizing α-proteobacteria in sulfur-reducing archaea (Sulfur syntrophy hypothesis).
In our opinion, it would be hard to guess the ancestral nutritional mode of both host and the symbiont due to rampant lateral transfer of operational metabolic genes from prokaryotes to eukaryotes. It seems that about 75% of genes of Saccharomyces cerevisiae (most of which are operational metabolic genes) is of bacterial origin.43 In addition, e.g., three enzymes involved in sulfite reduction (pathway localized in different compartments in different organisms) have multiple origins in opistokonts, different isoforms are present in streptophytes and chlorophytes, and even evidence for a eukaryote-to-prokaryote lateral transfer has been observed.44
The well-supported grouping of mitochondria (bounded by two membranes) with α-proteobacteria (bounded by two membranes as well) in phylogenies leaves no doubt that mitochondria were ancestrally α-proteobacteria.43,45,46 On the other hand, the phylogenies of informational genes group Eukarya with Archaea and do not place them within their extant diversity; symbiosis of bacteria in archaea has not been observed so far; and the absence of eukaryotes without mitochondria (or mitochondrial remnants) can not be taken as evidence for an archaeal host (reviewed in ref. 47).
In addition, Bell48 has suggested, that the nucleus is derived from an endosymbiotic large pox-like virus in an archaeal host bearing α-proteobacterial ancestors of mitochondria. Although this scenario is interesting, most of the criticism of the model suggesting a symbiosis of α-proteobacteria in archaea is relevant also to this model.
Symbiosis of three prokaryotic species. Moreira and López-García49 propose that a complicated metabolic symbiotic association of α-proteobacterial methanotrophs, δ-proteobacterial sulfate-reducers and hydrogen- and acetate-consuming methanogenic archaea led to the appearance of the first eukaryotic cell (Syntrophy hypothesis). Due to rampant lateral transfer of metabolic genes in eukaryotes (see above) we are even more skeptic considering this model then the previous one. In addition, the model of Hoorike et al.50,51 suggests a symbiosis of archaea, γ-proteobacteria and α-proteobacteria, while not defining metabolic properties of partners.
Under the models of Moreira and López-García49 and Hoorike et al.50,51 the nucleus is derived from a symbiosis of an archeon in δ- and γ-proteobacterial cytoplasm, respectively. However, these models could hardly explain the origin of nuclear membrane topology and the replacement of archaeal membrane of an archeon/nucleus by bacterial one.
In addition, Margulis et al.52 still curiously insist on the model under which a symbiosis of a spirochete (under their scenario the ancestor of eukaryotic cilium and mitotic apparatus) in wall-less Thermoplasma-like archeon predated α-proteobacterial symbiosis, despite the fact that tubulin genes were not discovered in spirochetes so far.53
G+-bacterial origin of Eukarya and Archaea. Cavalier-Smith4,54 suggests that LUCA was a G--bacterium and G+-bacteria arose afterwards via the loss of outer membrane. The common ancestor of Archaea and Eukarya (the Neomuran) evolved throughout a drastic modification of cell surface of a G+-bacterium.4,54 While LACA replaced bacterial lipids by those of archaeal type as an adaptation to thermophily, LECA lost cell wall and evolved phagocytosis.55 Under this model phagocytosis was a pre-requisite to evolve eukaryotic endomembrane system.55
The model of Cavalier-Smith seems to be consistent with de Duve56 who recently wrote: ‘The cytomembrane network most likely originated from infoldings of plasma membrane of some wall-less ancestral cell, probably related to eubacteria, which likewise possess ester phospholipids.’ The invagination model for the origin of the nucleus suggests that a fusion of either endocytic or phagocytic vesicules derived from plasma membrane around the genetic material of LECA resulted in nuclear/ER membrane topology.55–59 Jékely57–59 adopts most of the ideas of Cavalier-Smith,55 but he suggests that plasma membrane tubularization rather than phagocytosis, was the pre-requisite to evolve endomembranes. Jékely57 analysed the phylogeny of small GTP-ases (key regulators of vesicular transport and dynamics) and he suggested that exocytosis pre-dated endocytosis in protoeukaryote evolution. Under his model the ancestor of eukaryotes secreted the digestive enzymes outside via exocytosis and took the digested particles in via endocytosis.58 The model suggests that endocytosis was a pre-requisite to evolve phagocytosis.58 Nevertheless, the loss of cell wall is also very important for this model. Jékely58 in his response to Reviewer's report I (E. Koonin) wrote: “It would be hard to argue that a cell with a rigid cell wall developed phagotrophy and endomembrane dynamics. The loss of cell wall is ‘must’ in any scenario.” Jékely means by ‘any scenario’ any scenario proposing prokaryotic origin of eukaryotes.
Both Cavalier-Smith55 and Jékely57,58 see the main advantage of plasma membrane invagination in feeding on ‘food’ either digested from inside or from outside, respectively. These models (as their authors themselves confess) would certainly require the loss of cell wall of the hypothetical prokaryotic ancestor of eukaryotes. However, cell wall is required to maintain the cell shape of prokaryotes and protects them from external dangers. Moreover, the prokaryote without cell wall would have to have plasma membrane invaginated under these scenarios, what would increase even more the contact of the lucky survivor without cell wall with external environment as well as with all prokaryotic and viral parasites in the microbial community. Thus this scenario seems to be problematic.
Furthermore, in our opinion, the model suggesting G+-bacterial origin of Eukarya and Archaea ignores phylogenies of informational genes. If G+-bacteria were the ancestors of Archaea and Eukarya, then the phylogenies of informational genes should place archaeo-eukaryotic branch within G+-bacteria. However, the unrooted phylogenetic trees of informational genes place archaeo-eukaryotic branch as a sister branch to all bacteria.
Common pitfalls of all prokaryote-to-eukaryote transition models. All prokaryote-to-eukaryote transition models: (1) assume that prokaryotes (i.e., bacteria, archaea and/or prokaryotic chimeras, hybrids and/or associations) were direct ancestors of eukaryotes for what no direct evidence exists;16 (2) do not explain precisely how exactly eukaryotic specific proteins60 and features with no prokaryotic counterparts arose;31 (3) have to suggest that either LECA or LACA underwent through selectively costly stage with membrane(s) composed of a mixture of archaeal and bacterial lipids, which is (are) less stable than membranes composed of only archaeal or bacterial lipids;2,3 (4) have to propose dramatic and unrealistic change in rates of protein evolution in only one (two or three) prokaryotic species,31 while no such drastic change in rates of evolution has been detected by recent analysis of protein families;61 (5) ignore to explain the origin of meiosis and sex.62
An additional pitfall of models proposing that an archaeal cell was involved in the origin of eukaryotes (all models mentioned above except for G+-bacterial origin of Eukarya and Archaea) is that they do not explain why phylogenies of informational genes place Eukarya as a sister group to Archaea, and not within the diversity of modern archaea.31,47
The criticism of prokaryote-to-eukaryote transition models has been covered by excellent papers of Forterre,31 Poole and Penny,47 and Wächtershäuser,2 which we would recommend to an interested reader. In the following section we criticize these models for not explaining the origin of meiosis and sex as this is perhaps the least discussed pitfall of all prokaryote-to-eukaryote transition models.
The failure of prokaryote-to-eukaryote transition models to explain the origin of sex. The occurrence of sex, despite its high costs, is often stated as the ‘paradox of sex’. The costs of sex (reviewed in refs. 63 and 64) include the break-up of co-adapted gene combinations,65 the two-fold ‘cost of producing males’,66 the two-fold ‘cost of meiosis’,67 the risk of fusion, the risk of infection, the time and energy costs while looking for the partner etc. The hypotheses for the origin of eukaryotes that derive them from prokaryotes (or they conglomerates or chimeras) have to propose that sexual reproduction specific to Eukarya evolved from less risky and less costly prokaryotic asexual one. Moreover, while prokaryotes undergo true Darwinian evolution under which mutations either increase or decrease the fitness of prokaryotic carrier and either let it survive (and make its clone) or die, the break-up of co-evolved genetic combinations with each meiotic cycle does not allow to understand the evolution of eukaryotes under pure Darwinian mode of thinking, and the evolution of eukaryotes could be better understood in terms of ‘selfish genes’68 and/or ‘frozen plasticity’.69,70 If we consider that the era of LUCA was dominated by horizontal gene transfer which was mediated by viruses and other mobile elements, then it was in fact the era of ‘selfish genes’. Furthermore, if LUCA practiced promiscuous fusions and fissions, the evolution of eukaryotic sexual life cycle from prokaryotic asexual one would be an unlikely step back.
Not only would be the ‘prokaryotic’ origin of sex switch back from Darwinian evolution to Dawkinsian, but it would be switch back also in that it would require a cell wall loss to allow fusions. Moreover, true sex and primordial two-step meiosis might have helped to uncouple and thereby emasculate ‘acquired’ parasitic genomes (such as viruses, transposons and other mobile elements).71 In addition, it has been already suggested that primary significance of sex was not to promote evolutionary change but to limit it.28,69,70 Thus sex and meiosis might be viewed not only as another solution of elimination of risks of fusions, but also as a solution of elimination of risks of horizontal gene transfer.
In addition, the followers of prokaryote-to-eukaryote transition models López-García and Moreira,72 Martin and Koonin,73 and Koonin74 have recently suggested, following the original ideas of Cavalier-Smith75 and Doolittle76 that a massive intron spread was the main driving force leading to the appearance of the nucleus to separate slow splicing from fast translation to avoid the synthesis of aberrant proteins. However, Poole77 has argued that also the massive intron proliferation would most likely require sexual life cycle.
Models proposing that a lineage different from bacteria and archaea was involved in the origin of eukaryotes and their criticism.
Some models suggest that at least one entity different from typical prokaryotes was involved in the origin of eukaryotes. Three most common models of this type exist: (1) symbioses of prokaryotes in RNA-based host; (2) eukaryotic-like host (sharing a more recent ancestor with Archaea than with Bacteria) for α-proteobacterial ancestors of mitochondria; (3) symbiosis of a pre-cell in a host entity creating an ancestor of eukaryotes possessing outer membrane and inner membrane system prior to the origin of mitochondria.
Symbioses of prokaryotes in RNA-based host. Some authors suggest a symbioses of archaeal and bacterial cells in RNA-based entity (member of extinct perhaps pre-cellular lineage) to create the first eukaryote.60,78,79 Under these scenarios, the nucleus is derived from a symbiotic archaeal cell. Though these scenarios explain the origin of eukaryotic signature proteins, we have argued (see above) that LUCA probably possessed DNA. RNA-based entity would be probably out-competed by fast reproducing bacteria and archaea. This scenario does not explain the origin of nuclear/ER membrane topology and suffers from the problem of passing through a stage with less stable hybrid membranes composed of a mixture of bacterial and archaeal lipids.
Eukaryotic-like host for α-proteobacterial ancestors of mitochondria. We have criticized the models under which prokaryotes were direct ancestors of eukaryotes (see above). Assuming that the host for α-proteobacteria was neither an archeon nor a bacterium nor a chimera of an archeon and a bacterium, but rather a member of the third line of descent sharing a common more recent ancestor with Archaea than with Bacteria,47 makes the phagocytic models for the origin of eukaryotic endomembranes more plausible. Such a scenario would not require the loss of prokaryotic cell wall to evolve phagocytosis, because one could simply assume that protoeukaryotic host for α-proteobacteria had never possessed it, and thus could, unlike prokaryotes, have evolved phagocytosis as well as sex. There seems to be no need to propose that the origin of mitochondria pre-dated the origin of many eukaryotic specific features and the absence of mitochondria-, mitosome- or hydrogenosome-less eukaryotes can not be taken as evidence for this.47 Although the view of phagotrophic protoeukaryotic host is probably the best what we have with respect to the origin of mitochondria as well as endomembrane topology, there exists a number of examples of prokaryotes living and/or parasiting within host cells that do not need to be phagocytosized to get inside. Thus phagocytosis might not be viewed as absolute pre-requisite for the acquisition of mitochondria (see comment on Poole and Penny47 of Davidov and Jurkevitch80).
Symbiosis of a pre-cell in a host entity to create an ancestor of eukaryotes possessing two membranes prior to the origin of mitochondria. The scenario of Baluška et al.81 suggests that the nucleus is a remnant of a ‘tubulin-based guest proto-cell’ in an ‘actin-based host proto-cell’. Although this model does not explain the origin of nuclear/ER topology, novel interesting points of this hypothesis are that: (1) it proposes that different pre-cells might have exhibited very different phenotypes, and that (2) there might have existed an ancestor of eukaryotes possessing outer membrane, inner membrane system, and eukaryotic-like cytoskeleton prior to the α-proteobacterial symbiosis.
Wächtershäuser2,3 suggests a symbiosis of a pre-cellular symbiont of B-type in a bacterial cell to create eukaryotic nucleus. Though proto-cellular/pre-cellular origin of the nucleus is probably more likely than archaeal, the scenario does also not explain the origin of nuclear/ER membrane topology. Nevertheless, this hypothesis also proposes that LECA might have possessed outer membrane and inner membrane system prior to the α-proteobacterial symbiosis.
Novel Hypothesis for the Origin of Eukaryotes
Sexual host for α-proteobacteria.
The important consequence of independent origins (reachings of ‘Darwinian Thresholds’) by LBCA and afterwards by LACA mediated by the emergence of stable cell surface (a switch to stereospecific lipid synthesis and the emergence of cell walls) is the elimination of risks of fusion. Nevertheless, after the origins of Bacteria and Archaea, some pre-cellular populations of both types A and B could have survived and co-existed with them. Though most of these subpopulations (in fact A completely) were probably soon out-competed by faster and precisely reproducing Darwinian entities (bacteria and archaea), one subpopulation of B type might have survived. This might have been the ancestor of the pre-karyote—the host cell for α-proteobacterial ancestors of mitochondria. Under our hypothesis ‘the pre-karyote was unlucky to have evolved fusion-prohibiting cell surface’, but it could have evolved different strategies to eliminate all risks of fusion in form of different regulation of cell cycle, regulation of fusion process, and revolution in repair processes with evolution of true sex as an outcome.62
The ancestral DNA damage checkpoint. Krylov et al.82 have published the phylogenetic analysis of cell cycle kinases. In their study meiotic kinase Ime2 branched earlier than cyclin dependent kinase (Cdc28/Cdc2/Cdk1), while the earliest branch was represented by DNA damage checkpoint kinase Chk1. One of the suggestions of Krylov et al.,82 was that Chk1 might have been the only basal regulator of ancient eukaryotic cell cycle at the level of DNA damage checkpoint and only duplication and diversification of Chk1 (stepwise addition of other kinases) might have led to diversification of both mitosis and meiosis. We propose that the serial duplication of ancestral gene encoding DNA damage kinase,82 as well as the origins of many ancient eukaryotic paralogs,83 as well as proliferation of introns and other selfish elements77 would themselves require sexual cycle. On the basis of the basal position of DNA damage kinase we have recently suggested that DNA damage checkpoint might have been the only ancient checkpoint regulating the ancient pre-karyotic cell cycle and life cycle, and we have suggested how it might have looked like.62
A model for the pre-karyote cell cycle and life cycle. It is noteworthy that recombination is the process essential for restarting DNA synthesis when the replication fork is stalled or broken in prokaryotes as well as eukaryotes,84–86 though bacteria lack a physiological mechanism to stop DNA replication when their DNA is damaged (they have neither DNA damage checkpoint nor any other checkpoints).86
We have suggested that the ancestral role of pre-karyotic DNA damage checkpoint might have been to monitor the quality and quantity of DNA synthesis and to allow the pre-karyte to divide only when its DNA was completely replicated without mistakes, what would most likely mean that pre-karyotic cell cycle would have consisted only of S phase and M phase.62 Under our hypothesis DNA damage checkpoint could have stopped replication of pre-karyote's genome when the DNA was damaged (partially diploid stage) to allow the DNA repair systems to fix it (see Fig. 1). After repairing DNA and completing replication cell division would be allowed. However, if the DNA damage was huge and partially diploid (in S phase blocked) damaged pre-karyote would not be able to repair its DNA and restart DNA synthesis by itself, the last chance to survive might have been the fusion with the partner (another partially diploid prekaryote) (Fig 1). After repairing the damage, restarting the DNA synthesis via recombination with the DNA of the partner, replication of both haploid sets could have been completed, and the result would have been a tetraploid entity. Now the cell division would be allowed resulting in two diploid entities that would be allowed to divide again (Fig. 1).
Figure 1.

Hypothetical pre-karyote cell cycle and life cycle controlled by ancient DNA damage checkpoint. The haploid pre-karyote is assumed to start DNA replication (A). If there was no DNA damage, DNA replication would be completed (B) resulting in diploid entity (C), that would be allowed to divide to produce two haploid pre-karyotes (D). If there were DNA damage (E) resulting in broken replication forks, DNA damage checkpoint would stop replication to allow the DNA repair and the restart of DNA synthesis via recombiantion with the matrix DNA (F). After completing the DNA replication (G), diploid pre-karyote (C) would be allowed to divide and the result would be two haploid entities (D). If the DNA damage were huge and pre-karyote would not be able to repair it and restart the DNA synthesis by itself (H), it could fuse with the partner (perhaps damaged too) (I). After the repair of the damage and the restart of DNA synthesis via recombination with the DNA of the partner (J), replication of both haploid sets would result in a tetraploid entity (K). Now the cell division would be allowed (L) resulting in two diploid entities. However, these would have no need to start replication again, instead both would be allowed to divide again (M and C) and the result would be four haploid entities (N and D).
Testability of our hypothesis for the origin of meiosis and sex. Our proposed model could be tested by two approaches: (1) phylogenetic analyses of proteins involved in regulation of mitosis and meiosis; and (2) theoretical simulations. The phylogenetic analysis of Krylov et al.,82 as well as some theoretical tests seem to support our model. The proposed scheme62 seems to be consistent with the repair hypothesis, suggesting that sex evolved to repair errors (e.g., double strand DNA breaks).87,88 The model seems to be compatible also with theoretical model of Michod89 showing that beginning with an ancestral state in which cells are asexual haploids, the sexual life cycle would emerge before asexual diploidy as a response to increasing DNA damage. In addition, theoretical model of Archetti90 showing that sexual process resolves the intragenomic conflict between asexual diploid reproduction and recombination (which would unmask deleterious mutations in asexual diploid population) is also consistent with our hypothesis.
Two-membrane-bounded host for α-proteobacteria and the origin of compartments of eukaryotic cells.
LUCA might have been bounded by two membranes. We have recently suggested that LUCA was bounded by two membranes composed of a mixture of bacterial and archaeal lipids mainly from three reasons:15 (1) there exist both two-membrane-bounded bacteria (G--bacteria) and two-membrane-bounded archaea, e.g., Ignicoccus sp. (see Rachel et al.91 and Nather and Rachel92); (2) LUCA with a single membrane composed of a mixture of bacterial and archaeal lipids would not be as stable as if it was bounded by two membranes;15 and (3) four hypothetical scenarios of the origins of two-membrane-bounded LUCA exist (Fig. 2). These four scenarios are based on Obcell hypothesis94,95 proposing the existence of obcells prior to the origin of cells. Obcells might have been membrane vesicles with the ancestral ribosomes bound to the vesicles from outside. Such obcells could have originated in an inorganic compartment similar to that described by Martin and Russel12 and Koonin and Martin.13 The scenarios for the internalization of ribosomes and for the origin of two-membrane-bounded LUCA include: (a) the fusion occurring at the orifice of a single gastruloid membrane vesicle;93 (b) the fusion of two cup-shaped membrane vesicles;94,95 (c) the fusion of more membrane vesicles;15 and (d) vesicular budding analogous to endocytic invagination96 (Fig. 2). It would be hard to imagine the enclosure of hydrophilic cytoplasm by one membrane due to hydrophobic interactions that force lipids in hydrophilic environment to aggregate and to form membrane vesicles. Thus the entry of hydrophobic cytoplasm into vesicle through hydrophobic lipid layer seems problematic. Nevertheless, Griffiths96 suggests that single-membrane-bounded pre-cells might have originated via vesicular budding analogous to endocytic invagination and subsequent escape from the maternal vesicle (subsequent loss of maternal outer membrane).
Figure 2.
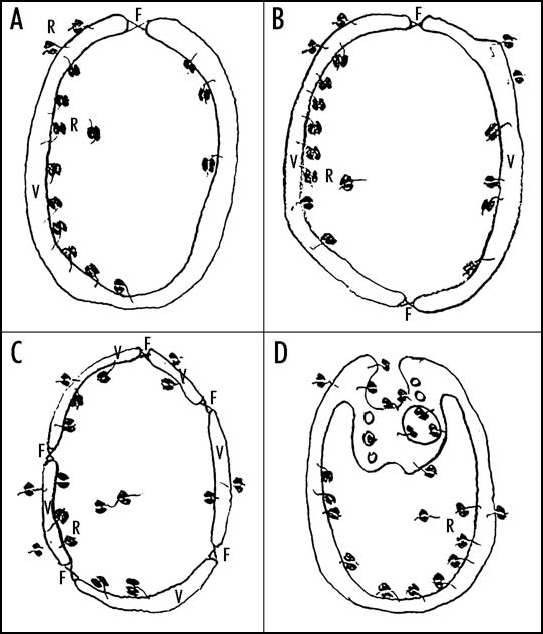
The hypothetical scenarios for the origin of two-membrane-bounded LUCA. (A) The fusion occurring at the orifice of a single gastruloid membrane vesicle. (B) The fusion of two cup-shaped membrane vesicles. (C) The fusion of more membrane vesicles. (D) Vesicular budding analogous to endocytic invagination. Abbreviations: V, membrane vesicle; F, the place of fusion; R, ribosomes.
Two-membrane-bounded LECA, infection of its periplasm by α-proteobacteria, and the origin of nuclear/ER membrane topology. Under our view the pre-karyotic host for α-proteobacteria ancestrally possessed two membranes inherited from LUCA.15 We have recently suggested that the pre-karyote periplasm (intermembrane space) might have been dominated by the protein ancestral to actin, which could have helped the wall-less pre-karyote to maintain the cell shape97 and might have been a pre-requisite to evolve endomembrane system. Symbiotic α-proteobacterial ancestors of mitochondria might have ancestrally been selfish parasites of pre-karyote's intermembrane space whose infection might have reminded infection of G--bacterial periplasm by Bdellovibrio sp. (see Saier98) in many aspects.15 Bdellovibrio-like α-proteobacterial predators have been recently discovered, and it has been suggested that this might have implications for the origin of mitochondria.99
In our view selfish α-proteobacterial parasites had the ability to disrupt pre-karyotes' membranes.15 Under this selective pressure the pre-karyote evolved various mechanisms to resist the aggressive infection of α-proteobacterial parasite. Some of these were: (1) remodeling of the secondary and tertiary pre-karyote inner membrane structures via (perhaps actin-mediated) reticularization, invagination, vesicular budding and vesicular fusion such as to allow the repair of the damaged membranes; (2) insertion of ADP/ATP translocase into mitochondrial inner membrane such as to allow the efflux of ATP from the symbiont, which attenuated the symbiont aggressiveness; and (3) the replacement of ribosomes into the pre-karyote intermembrane space such as to allow: (a) the escape of host RNAs, proteins and metabolism from symbiont degradative enzymes secreted into pre-karyote cytoplasm, (b) physical separation of splicing (which is slow) and translation (which is fast), and (c) the usage of intermembrane space pool of ATP.15
Thus the evolution of nuclear compartment, nuclear/ER membrane topology and nuclear pore complex might have been driven by the conflict between selfish intermembrane space symbiont and the immunity response of the host, which resulted in obligatory cooperation. It has been shown for example that a pore-forming toxin produced by Aeromonas hydrophila causes vacuolation of ER membranes.100 In fact, similar membrane vacuoles (or vesicles) might have been formed by pre-karyote inner membrane derivatives while α-proteobacteria perhaps had the ability to insert toxins into pre-karyote inner membrane, and to secrete degradative enzymes into pre-karyote cytoplasm. These vesicles derived from disrupted pre-karyote inner membrane might have fused (perhaps to restore essential compartmentalization) in a manner similar to that proposed by model under which endocytic, phagocytic vesicles or ER-derived vesicles fused around the genetic material what would result in exactly the same nuclear/ER membrane topology (Fig. 3). Phagocytosis might have evolved later perhaps ancestrally serving as another mechanism of protection against aggressive bacterial parasites.
Figure 3.

Hypothetical scenario for the origin of eukaryotic endomembranes. The pre-karyote is assumed to have been bounded by two membranes, inner (IM) and outer (OM). α-proteobacterial ancestors (A) of mitochondria (M) are proposed to have been parasites of pre-karyote periplasm (P). Endoplasmic reticulum (ER), nuclear membrane (N = nucleus) and Golgi apparatus (GA) are assumed to be derived from pre-karyote inner mebrane, while eukaryotic plasma membrane (PM) is assumed to be derived from pre-karyote outer membrane. It is suggested that ribosomes (R) were removed to pre-karyote periplasm (nowadays eukaryotic cytoplasm).
Testability of our hypothesis for the origin of eukaryotic endomembranes. It has been suggested that the physical and chemical properties of membranes might be more useful for the rooting of the tree of life and for the understanding of the origins of domains than hypothetical and controversial selective advantages postulated by different transition analyses.16 Here we suggest that the scenario of two-membrane-bounded LUCA and LECA can be tested in physical and chemical context. The experiments with membrane lipids and fusing membrane vesicles could tell us whether scenarios proposed here are plausible.
Some common features of all cells might be informative of the origin of eukaryotic membranes. For example, co-translational secretion of proteins into ER is analogous to secretion of proteins through bacterial and archaeal plasma membrane, but such a secretory apparatus is found neither in eukaryotic plasma membrane nor in the outer membrane of two-membrane-bounded prokaryotes. This indicates that eukaryotic nuclear/ER membrane is homologous to archaeal and bacterial plasma membrane (inner membrane if considering two-membrane-bounded prokaryotes) and inner membrane of LUCA, while eukaryotic plasma membrane is homologous to outer membrane of LUCA and to outer membrane of two-mebrane-bounded prokaryotes.
The localization of proteins and RNAs might be informative of the origin of eukaryotic nucleoplasm. Eukaryotic nucleolus with 109 unique eukaryotic protein domains101 is still the place of rRNA synthesis and assembly of ribosomal subunits, some components of signal recognition particle (SRP) (involved in secretory process) are still present in the nucleolus,102 and there have been reports about translation still occurring in the eukaryotic nucleus.103–105 From these perspectives, it seems reasonable that eukaryotic nucleoplasm is derived from pre-karyotic cytoplasm.
Conclusion
The origin of cells and domains is highly controversial issue. Different authors suggest different hypotheses at the same time. The different views of LUCA and different hypothesis for the origin of eukaryotes depend on author's interpretation of current scientific knowledge. Christian de Duve106 recently wrote: ‘The only scientific valid hypothesis is to assume that natural phenomena have natural explanations, that can be elucidated by research.’ Each hypothesis mentioned in this paper was suggested in this belief. Though the history of life on our planet is unique, reproduced nowhere else in the universe,107 nor in the laboratory, the reliability of the hypotheses mentioned here can be tested. For example, some scenarios seem to be obviously more likely in the lipidological context than the others.
We conclude that eukaryotic defining features such as telomeres, many RNA-based systems not found in prokaryotes (including e.g., telomerase or splicosomal RNAs),108 perhaps also some types of introns, parasitic repetitive sequences, fusion process, and a number of unique signature proteins might trace back to the Woesean era occupied by the pre-cellular ancestor of LECA longer than by pre-cellular ancestors of LBCA and LACA. In addition, Eukarya evolved enormous complexity in relatively short evolutionary time, while Bacteria and Archaea are relatively simple, though they exist longer. This high evolvability suggests that Eukarya are still not as far from Woesean era as Bacteria and Archaea.
In conclusion, we do not see any reason why the uptake of α-proteobacteria should pre-date sex. Furthermore, sex (fusion of pre-karyote outer membranes) and subsequent two-step division might have been themselves important for the establishment of α-proteobacterial symbionts within the whole population of pre-karyotes.62 This hypothesis could be tested by theoretical models considering the spread of bacterial symbionts in fussing (sexual) and non-fusing (asexual) populations.
Acknowledgements
We thank the Ministry of Education of the Slovak Republic (grant VEGA 1/3249/06, to Juraj Krajčovič) and Comenius University (grant UK/98/2006, grant UK/144/2007 to Matej Vesteg) for support. We wish to thank anonymous reviewers for their useful comments that contributed to the final version of the manuscript. We thank František Baluška (Institute of Cellular and Molecular Botany, University of Bonn, Bonn, Germany) for initiating us to write this manuscript.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/6349
References
- 1.Woese CR, Kandler O, Wheelis ML. Toward a natural system of organisms: Proposal for the domains Archaea, Bacteria and Eukarya. Proc Natl Acad Sci USA. 1990;87:4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wächtershäuser G. From pre-cells to Eukarya—a tale of two lipids. Mol Microbiol. 2003;47:13–22. doi: 10.1046/j.1365-2958.2003.03267.x. [DOI] [PubMed] [Google Scholar]
- 3.Wächtershäuser G. From volcanic origins of chemoautotrophic life to Bacteria, Archaea and Eukarya. Phil Trans R Soc B. 2006;361:1787–1880. doi: 10.1098/rstb.2006.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavalier-Smith T. The Neomuran origin of archaebacteria, the negibacterial root of the universal tree and bacterial megaclassification. Int J Syst Evol Microbiol. 2002;52:7–76. doi: 10.1099/00207713-52-1-7. [DOI] [PubMed] [Google Scholar]
- 5.Koch AL. Were Gram-positive rods the first bacteria? Trends Microbiol. 2003;11:166–170. doi: 10.1016/s0966-842x(03)00063-5. [DOI] [PubMed] [Google Scholar]
- 6.Jalasvuori M, Bamford JKH. Structural co-evolution of viruses and cells in the primordial world. Orig Life Evol Biosph. 2008;38:165–181. doi: 10.1007/s11084-008-9121-x. [DOI] [PubMed] [Google Scholar]
- 7.Forterre P, Philippe H. Where is the root of the universal tree of life? BioEssays. 1999;21:871–879. doi: 10.1002/(SICI)1521-1878(199910)21:10<871::AID-BIES10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 8.Woese CR. The universal ancestor. Proc Natl Acad Sci USA. 1998;95:6854–6859. doi: 10.1073/pnas.95.12.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bendich AJ. The form of chromosomal DNA molecules in bacterial cells. Biochimie. 2001;83:177–186. doi: 10.1016/s0300-9084(00)01209-8. [DOI] [PubMed] [Google Scholar]
- 10.Akerlund T, Nordstrom K, Bernander R. Analysis of cell size and DNA content of exponentially growing and stationary-phase batch cultures of Escherichia coli. J Bacteriol. 1995;177:6791–6797. doi: 10.1128/jb.177.23.6791-6797.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penny D, Poole A. The nature of the last universal common ancestor. Curr Op Gen Dev. 1999;9:672–677. doi: 10.1016/s0959-437x(99)00020-9. [DOI] [PubMed] [Google Scholar]
- 12.Martin W, Russel MJ. On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Phil Trans R Soc Lond B. 2003;358:59–85. doi: 10.1098/rstb.2002.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koonin EV, Martin W. On the origin of genomes within inorganic compartments. Trends Genet. 2005;21:647–654. doi: 10.1016/j.tig.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jékely G. Did the last common ancestor have a biological membrane? Biol Direct. 2006:35. doi: 10.1186/1745-6150-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vesteg M, Krajcovic J, Ebringer L. On the origin of eukaryotes and their endomembranes. Riv Biol. 2006;99:499–519. [PubMed] [Google Scholar]
- 16.Bapteste E, Brochier C. On the conceptual difficulties in rooting the tree of life. Trends Microbiol. 2004;12:9–13. doi: 10.1016/j.tim.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Woese CR. On the evolution of cells. Proc Natl Acad Sci USA. 2002;99:8742–8747. doi: 10.1073/pnas.132266999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anantharaman V, Koonin EV, Aravind L. Comparative genomics and evolution of proteins involved in RNA metabolism. Nucleic Acids Res. 2002;30:1427–1464. doi: 10.1093/nar/30.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peretó J, López-García P, Moreira D. Ancestral lipid biosynthesis and early membrane evolution. Trends Biochem Sci. 2004;29:469–477. doi: 10.1016/j.tibs.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Wächtershäuser G. Before enzymes and templates: Theory of surface metabolism. Microbiol Rev. 1988;52:452–484. doi: 10.1128/mr.52.4.452-484.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wächtershäuser G. Groundworks for an evolutionary biochemistry—the iron-sulfur world. Prog Biophys Mol Biol. 1992;58:85–201. doi: 10.1016/0079-6107(92)90022-x. [DOI] [PubMed] [Google Scholar]
- 22.Koga Y, Kyuragi T, Nishihara M, Sone N. Did archaeal and bacterial cells arise independently from non-cellular precursors? A hypothesis stating that the advent of membrane phospholipids with enantiomeric glycerophosphate backbones caused the separation of the two lines of descent. J Mol Evol. 1998;46:54–63. doi: 10.1007/pl00006283. [DOI] [PubMed] [Google Scholar]
- 23.Kandler O. The early diversification of life. In: Bengtson S, editor. Nobel Symposium No. 84. Early Life on Earth. New York: Columbia University Press; 1994. pp. 152–160. [Google Scholar]
- 24.Kandler O. Cell wall biochemistry in Archaea and its phylogenetic implications. J Biol Phys. 1994;20:165–169. [Google Scholar]
- 25.Woese CR. The primary lines of descent and the universal ancestor. In: Bendall DS, editor. Evolution from Molecules to Men. Cambridge: Cambridge University Press; 1983. pp. 209–233. [Google Scholar]
- 26.Kandler O. The early diversification of life and the origin of the three domains: a proposal. In: Wiegel J, Adams MWW, editors. Thermophiles: the Keys to Molecular Evolution and the Origin of Life. London: Taylor & Francis; 1998. pp. 19–28. [Google Scholar]
- 27.Woese CR. Interpreting the universal phylogenetic tree. Proc Natl Acad Sci USA. 2000;97:8392–8396. doi: 10.1073/pnas.97.15.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavalier-Smith T. Origin of the machinery of recombination and sex. Heredity. 2002;88:125–141. doi: 10.1038/sj.hdy.6800034. [DOI] [PubMed] [Google Scholar]
- 29.Forterre P. The origin of DNA genomes and DNA replication proteins. Curr Opin Microbiol. 2002;5:525–532. doi: 10.1016/s1369-5274(02)00360-0. [DOI] [PubMed] [Google Scholar]
- 30.Forterre P. The two ages of the RNA world, and transition to the DNA world: a story of viruses and cells. Biochimie. 2005;87:793–803. doi: 10.1016/j.biochi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 31.Forterre P. Three RNA cells for ribosomal lineages and three DNA viruses to replicate their genomes: a hypothesis for the origin of cellular domain. Proc Natl Acad Sci USA. 2006;103:3669–3674. doi: 10.1073/pnas.0510333103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filée J, Forterre P, Sen-Lin T, Laurent J. Evolution of DNA-polymerase families: evidence for multiple gene exchange between cellular and viral proteins. J Mol Evol. 2002;54:763–773. doi: 10.1007/s00239-001-0078-x. [DOI] [PubMed] [Google Scholar]
- 33.Wächtershäuser G. Towards a reconstruction of ancestral genomes by gene cluster alignment. System Appl Microbiol. 1998;21:473–477. [Google Scholar]
- 34.Nosek J, Kosa P, Tomáška L. On the origin of telomeres: a glimpse at the pre-telomerase world. BioEssays. 2006;28:182–190. doi: 10.1002/bies.20355. [DOI] [PubMed] [Google Scholar]
- 35.Kurland CG, Canback B, Berg OG. Horizontal gene transfer: A critical view. Proc Natl Acad Sci USA. 2003;100:9658–9662. doi: 10.1073/pnas.1632870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurland CG, Collins LJ, Penny D. Genomics and the irreducible nature of eukaryote cells. Science. 2006;312:1011–1014. doi: 10.1126/science.1121674. [DOI] [PubMed] [Google Scholar]
- 37.Rivera MC, Lake JA. The ring of life provides evidence for a genome fusion origin of eukaryotes. Nature. 2004;431:152–155. doi: 10.1038/nature02848. [DOI] [PubMed] [Google Scholar]
- 38.Brown JR, Doolittle WF. Archaea and the prokaryote-to-eukaryote transition. Microbiol Mol Biol Rev. 1997;61:456–502. doi: 10.1128/mmbr.61.4.456-502.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bapteste E, Walsh DA. Does the ‘ring of life’ ring true? Trends Microbiol. 2005;13:256–261. doi: 10.1016/j.tim.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Lester L, Meade A, Pagel M. The slow road to the eukaryotic genome. BioEssays. 2006;28:57–64. doi: 10.1002/bies.20344. [DOI] [PubMed] [Google Scholar]
- 41.Martin W, Müller M. The Hydrogen hypothesis for the first eukaryote. Nature. 1998;392:37–41. doi: 10.1038/32096. [DOI] [PubMed] [Google Scholar]
- 42.Searcy DG. Metabolic integration during the evolutionary origin of mitochondria. Cell Res. 2003;13:229–238. doi: 10.1038/sj.cr.7290168. [DOI] [PubMed] [Google Scholar]
- 43.Esser C, Ahmadinejad N, Wiegand C, Route C, Sebastiani F, Gelius-Dietrich G, Hentze K, Kretschmann E, Richly E, Leister D, Bryant D, Steel MA, Lockhart PJ, Penny D, Martin W. A genome phylogeny for mitochondria among α-proteobacteria and predominantly eubacterial ancestry of yeast nuclear genes. Mol Biol Evol. 2004;21:1643–1660. doi: 10.1093/molbev/msh160. [DOI] [PubMed] [Google Scholar]
- 44.Patron NJ, Durnford DG, Kopriva S. Sulfate assimilation in eukaryotes: fusions, relocations and lateral transfers. BMC Evol Biol. 2008;8:39. doi: 10.1186/1471-2148-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andersson SGE, Zomorodipour A, Andersson JO, Sicheritz-Ponten T, Alsmark UCM, Podowski RM, Naslund AK, Eriksson AS, Winkler HH, Kurland CG. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature. 1998;396:133–140. doi: 10.1038/24094. [DOI] [PubMed] [Google Scholar]
- 46.Andersson SGE, Karlberg O, Canback B, Kurland CG. On the Origin of mitochondria: a genomic perspective. Phil Trans R Soc London B. 2003;358:165–179. doi: 10.1098/rstb.2002.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poole AM, Penny D. Evaluating hypotheses for the origin of eukaryotes. BioEssays. 2006;29:74–84. doi: 10.1002/bies.20516. [DOI] [PubMed] [Google Scholar]
- 48.Bell PJL. Viral eukaryogenesis: Was the ancestor of the nucleus a complex DNA virus? J Mol Evol. 2001;53:251–256. doi: 10.1007/s002390010215. [DOI] [PubMed] [Google Scholar]
- 49.Moreira D, López-García P. Symbiosis between methanogenic archaea and δ-proteobacteria as the origin of eukaryotes: the Syntrophic hypothesis. J Mol Evol. 1998;47:517–530. doi: 10.1007/pl00006408. [DOI] [PubMed] [Google Scholar]
- 50.Hoorike T, Hamada K, Kanaya S, Shinozawa T. Origin of nucleic acid cell nuclei by symbiosis of archaea in bacteria is revealed by homology-hit analysis. Nat Cell Biol. 2001;3:210–214. doi: 10.1038/35055129. [DOI] [PubMed] [Google Scholar]
- 51.Hoorike T, Hamada K, Miyata D, Shinozawa T. The origin of eukaryotes is suggested as the symbiosis of Pyrococcus into γ-proteobacteria by phylogenetic tree based on gene content. J Mol Evol. 2004;59:606–619. doi: 10.1007/s00239-004-2652-5. [DOI] [PubMed] [Google Scholar]
- 52.Margulis L, Chapman M, Guerrero R, Hall J. The last eukaryotic common ancestor (LECA): Acquisition of cytoskeletal motility from aerotolerant spirochetes in the Proterozoic Eon. Proc Natl Acad Sci USA. 2006;103:13080–13085. doi: 10.1073/pnas.0604985103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Satir P, Guerra C, Bell AJ. Evolution and the persistence of the cilium. Cell Motil Cytoskeleton. 2007;64:906–913. doi: 10.1002/cm.20238. [DOI] [PubMed] [Google Scholar]
- 54.Cavalier-Smith T. Rooting the tree of life by transition analyses. Biol Direct. 2006;1:19. doi: 10.1186/1745-6150-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cavalier-Smith T. The phagotrophic origin of eukaryotes and the phylogenetic classification of protozoa. Int J Syst Evol Microbiol. 2002;52:297–354. doi: 10.1099/00207713-52-2-297. [DOI] [PubMed] [Google Scholar]
- 56.de Duve C. Origin of eukaryotes: a reapparaisal. Nat Rev Genet. 2007;8:395–403. doi: 10.1038/nrg2071. [DOI] [PubMed] [Google Scholar]
- 57.Jékely G. Small GTPases and the evolution of the eukaryotic cell. BioEssays. 2003;25:1129–1138. doi: 10.1002/bies.10353. [DOI] [PubMed] [Google Scholar]
- 58.Jékely G. Origin of phagotrophic eukaryotes as social cheaters in microbial biofilms. Biol Direct. 2007;2:3. doi: 10.1186/1745-6150-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jékely G. Origin of eukaryotic endomembranes—a critical evaluation of different model scenarios. Adv Exp Med Biol. 2007;607:38–51. doi: 10.1007/978-0-387-74021-8_3. [DOI] [PubMed] [Google Scholar]
- 60.Hartman H, Fedorov A. The origin of the eukaryotic cell: A genomic investigation. Proc Natl Acad Sci USA. 2002;99:1420–1425. doi: 10.1073/pnas.032658599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sales-Pardo M, Chan AOB, Amaral LAN, Guimerà R. Evolution of protein families: Is it possible to distinguish between domains of life? Gene. 2007;402:81–93. doi: 10.1016/j.gene.2007.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vesteg M, Krajcovic J. On the origin of meiosis and sex. Riv Biol. 2007;100:147–161. [PubMed] [Google Scholar]
- 63.Rice WR. Experimental tests of the adaptive significance of sexual recombination. Nat Rev Genet. 2002;3:241–251. doi: 10.1038/nrg760. [DOI] [PubMed] [Google Scholar]
- 64.Otto SP, Lenormand T. Resolving the paradox of sex and recombination. Nat Rev Genet. 2002;3:252–261. doi: 10.1038/nrg761. [DOI] [PubMed] [Google Scholar]
- 65.Fisher RA. The genetical theory of natural selection. Oxford: Oxford University Press; 1930. [Google Scholar]
- 66.Maynard Smith J. The evolution of sex. Cambridge: Cambridge University Press; 1978. [Google Scholar]
- 67.Williams GC. Sex and evolution. New Jersey: Princeston University Press; 1975. [Google Scholar]
- 68.Dawkins R. The selfish gene. Oxford: Oxford University Press; 1976. [Google Scholar]
- 69.Flegr J. On the “origin” of natural selection by means of speciation. Riv Biol. 1998;91:291–304. [Google Scholar]
- 70.Flegr J. Zamrzlá evoluce aneb je to jinak pane Darwin. Praha: Academia; 2006. (published in Czech; English translation of the title: Frozen evolution or it is not like that Mr. Darwin). [Google Scholar]
- 71.Sterrer W. On the origin of sex as vaccination. J Theor Biol. 2002;216:387–396. doi: 10.1006/jtbi.2002.3008. [DOI] [PubMed] [Google Scholar]
- 72.López-García P, Moreira D. Selective forces for the origin of the eukaryotic nucleus. BioEssays. 2006;28:525–533. doi: 10.1002/bies.20413. [DOI] [PubMed] [Google Scholar]
- 73.Martin W, Koonin EV. Introns and the origin of nucleus-cytosol compartmentalization. Nature. 2006;440:41–45. doi: 10.1038/nature04531. [DOI] [PubMed] [Google Scholar]
- 74.Koonin EV. The origin of introns and their role in eukaryogenesis: a compromise solution to the introns-early versus introns-late debate? Biol Direct. 2006:22. doi: 10.1186/1745-6150-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cavalier-Smith T. Intron phylogeny: A new hypothesis. Trends Genet. 1991;7:145–148. [PubMed] [Google Scholar]
- 76.Doolittle WF. The origin of introns. Curr Biol. 1991;1:145–146. doi: 10.1016/0960-9822(91)90214-h. [DOI] [PubMed] [Google Scholar]
- 77.Poole AM. Did Group II Intron proliferation in an endosymbiont-bearing archaeon create eukaryotes? Biol Direct. 2006:36. doi: 10.1186/1745-6150-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Doolittle RF. Searching for the common ancestor. Res Microbiol. 2000;151:85–89. doi: 10.1016/s0923-2508(00)00124-8. [DOI] [PubMed] [Google Scholar]
- 79.Hartman H, Favaretto P, Smith TF. The archaeal origins of the eukaryotic translational system. Archaea. 2006;2:1–9. doi: 10.1155/2006/431618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Davidov Y, Jurkevitch E. Comments on Poole and Penny's essay “Evaluating hypotheses for the origin of eukaryotes.”. BioEssays. 2007;29:74–84. doi: 10.1002/bies.20516. [DOI] [PubMed] [Google Scholar]
- 81.Baluška F, Volkmann D, Barlow PW. Eukaryotic cells and their cell bodies: cell theory revised. Ann Bot. 2004;94:9–32. doi: 10.1093/aob/mch109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krylov DM, Nasmyth K, Koonin EV. Evolution of eukaryotic cell cycle regulation: Stepwise addition of regulatory kinases and late advent of CDKs. Curr Biol. 2003;13:173–177. doi: 10.1016/s0960-9822(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 83.Makarova KS, Wolf YI, Kekhedov SL, Mirkin BG, Koonin EV. Ancestral paralogs and pseudoparalogs and their role in the emergence of the eukaryotic cell. Nucl Acids Res. 2005;33:4626–4638. doi: 10.1093/nar/gki775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haber JE. DNA recombination: the replication connection. TIBS. 1999;24:271–275. doi: 10.1016/s0968-0004(99)01413-9. [DOI] [PubMed] [Google Scholar]
- 85.Haber JE. Recombination: a frank view of changes and vice versa. Curr Opin Cell Biol. 2000;12:286–292. doi: 10.1016/s0955-0674(00)00090-9. [DOI] [PubMed] [Google Scholar]
- 86.Kuzminov A. Recombinational repair of DNA damage in Escherichia coli and bacteriophage λ. Microbiol Mol Biol Rev. 1999;63:751–813. doi: 10.1128/mmbr.63.4.751-813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bernstein H, Byers GS, Michod RE. Evolution of sexual reproduction: importance of DNA repair, complementation and variation. Am Nat. 1981;117:537–549. [Google Scholar]
- 88.Maynard Smith J, Szathmáry E. The major evolutionary transitions. Oxford: W. II. Freeman; 1995. [DOI] [PubMed] [Google Scholar]
- 89.Michod RE. Origin of sex for error repair III. Selfish sex. Theor Popul Biol. 1998;53:60–74. doi: 10.1006/tpbi.1997.1341. [DOI] [PubMed] [Google Scholar]
- 90.Archetti M. A Selfish origin for recombination. J Theor Biol. 2003;223:335–346. doi: 10.1016/s0022-5193(03)00102-4. [DOI] [PubMed] [Google Scholar]
- 91.Rachel R, Wyschkony I, Riehl S, Huber H. The ultrastructure of Ignicoccus: Evidence for a novel outer membrane and for intracellular vesicle budding in an archaeon. Archaea. 2002;1:9–18. doi: 10.1155/2002/307480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nather DJ, Rachel R. The outer membrane of the hyperthermophilic Archaeon Ignicoccus: dynamics, ultrastructure and composition. Biochem Soc Trans. 2004;32:199–203. doi: 10.1042/bst0320199. [DOI] [PubMed] [Google Scholar]
- 93.Blobel G. Intracellular topogenesis. Proc Natl Acad Sci USA. 1980;77:1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cavalier-Smith T. The origin of eukaryotic and archaebacterial cells. Ann New York Acad Sci. 1987;503:17–54. doi: 10.1111/j.1749-6632.1987.tb40596.x. [DOI] [PubMed] [Google Scholar]
- 95.Cavalier-Smith T. Obcells as protoorganisms: Membrane heredity, lithophosphorylation, and the origins of genetic code, the first cells, and photosynthesis. J Mol Evol. 2001;53:555–595. doi: 10.1007/s002390010245. [DOI] [PubMed] [Google Scholar]
- 96.Griffiths G. Cell evolution and the problem of membrane topology. Nat Rev Mol Cell Biol. 2007;8:1018–1024. doi: 10.1038/nrm2287. [DOI] [PubMed] [Google Scholar]
- 97.Vesteg M, Krajcovic J. On the origin of eukaryotic cytoskeleton. Riv Biol. 2008;101:47–56. [PubMed] [Google Scholar]
- 98.Saier MH., Jr Protein uptake into E. coli during Bdellovibrio infection. A process of reverse secretion? FEBS Lett. 1994;337:14–17. doi: 10.1016/0014-5793(94)80620-9. [DOI] [PubMed] [Google Scholar]
- 99.Davidov Y, Huchon D, Koval SF, Jurkevitch E. A new α-proteobacterial clade of Bdellovibrio-like predators: implications for the mitochondrial endosymbiotic theory. Environ Microbiol. 2006;8:2179–2188. doi: 10.1111/j.1462-2920.2006.01101.x. [DOI] [PubMed] [Google Scholar]
- 100.Abrami L, Fivaz M, Glauser PE, Parton RG, van der Goot FG. A pore-forming toxin interacts with a GPI-anchored protein and causes vacuolation of the endoplasmic reticulum. J Cell Biol. 1998;140:525–540. doi: 10.1083/jcb.140.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Staub E, Fiziev P, Rosenthal A, Hinzmann B. Insights into the evolution of the nucleolus by an analysis of its protein domain repertoire. BioEssays. 2004;26:567–581. doi: 10.1002/bies.20032. [DOI] [PubMed] [Google Scholar]
- 102.Politz JC, Yarovoi S, Kilroy SM, Gowda K, Zwieb C, Pederson T. Signal recognition particle components in the nucleolus. Proc Natl Acad Sci USA. 2000;97:55–60. doi: 10.1073/pnas.97.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iborra FJ, Jackson DA, Cook PR. Coupled transcription and translation within nuclei of mammalian cells. Science. 2001;293:1139–1142. doi: 10.1126/science.1061216. [DOI] [PubMed] [Google Scholar]
- 104.Hentze MW. Believe it or not—translation in the nucleus. Science. 2001;293:1058–1059. doi: 10.1126/science.1063290. [DOI] [PubMed] [Google Scholar]
- 105.Pederson T. Is the nucleus in need of translation? Trends Cell Biol. 2001;11:395–397. doi: 10.1016/s0962-8924(01)02105-5. [DOI] [PubMed] [Google Scholar]
- 106.de Duve C. Thoughts of a senior scientist. Chance and necessity revisited. Cell Mol Life Sci. 2007;64:3149–3158. doi: 10.1007/s00018-007-7442-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.de Duve C. Chemistry and selection. Chem Biodiv. 2007;4:574–583. doi: 10.1002/cbdv.200790051. [DOI] [PubMed] [Google Scholar]
- 108.Poole A, Jeffares D, Penny D. Prokaryotes, the new kids on the block. Bioessays. 1999;21:880–889. doi: 10.1002/(SICI)1521-1878(199910)21:10<880::AID-BIES11>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]


