Abstract
Plant internal oxygen concentrations can drop well below ambient even when the plant grows under optimal conditions. Using pea (Pisum sativum) roots, we show how amenable respiration adapts to hypoxia to save oxygen when the oxygen availability decreases. The data cannot simply be explained by oxygen being limiting as substrate but indicate the existence of a regulatory mechanism, because the oxygen concentration at which the adaptive response is initiated is independent of the actual respiratory rate. Two phases can be discerned during the adaptive reaction: an initial linear decline of respiration is followed by a nonlinear inhibition in which the respiratory rate decreased progressively faster upon decreasing oxygen availability. In contrast to the cytochrome c pathway, the inhibition of the alternative oxidase pathway shows only the linear component of the adaptive response. Feeding pyruvate to the roots led to an increase of the oxygen consumption rate, which ultimately led to anoxia. The importance of balancing the in vivo pyruvate availability in the tissue was further investigated. Using various alcohol dehydrogenase knockout lines of Arabidopsis (Arabidopsis thaliana), it was shown that even under aerobic conditions, alcohol fermentation plays an important role in the control of the level of pyruvate in the tissue. Interestingly, alcohol fermentation appeared to be primarily induced by a drop in the energy status of the tissue rather than by a low oxygen concentration, indicating that sensing the energy status is an important component of optimizing plant metabolism to changes in the oxygen availability.
Plants are obligate aerobic organisms, with oxygen being an essential substrate for mitochondrial energy production. However, the poor distribution efficiency for oxygen through root, tuber, seed, or stem tissue of various species results in steep drops of the internal oxygen concentration, ranging from values near above zero to just below 40% of air saturation (e.g. Armstrong et al., 1994; Geigenberger et al., 2000; Rolletschek et al., 2002; van Dongen et al., 2003, 2004; Vigeolas et al., 2003).
These low levels of internal oxygen strongly affect plant metabolism. Several studies showed that energy-consuming metabolic pathways are adjusted to the actual oxygen availability (for review, see Geigenberger, 2003; Bailey-Serres and Voesenek, 2008). By saving energy, the plant decreases the demand for respiratory oxygen consumption that could help to postpone or even prevent the tissue from becoming anoxic. Indeed, it was observed that the metabolic flux through glycolysis slows down, respiratory oxygen consumption decreases, and adenylate levels drop in response to low internal oxygen (Geigenberger et al., 2000; Bologa et al., 2003). This inhibition of respiration is not easily explained by substrate limitation of cytochrome c oxidase (COX). First, the reduction of respiration becomes apparent already at oxygen concentrations around 20% of air saturation, whereas the Km value for oxygen of COX lies around 0.05% of air saturation (which equals 0.14 μm under standard conditions; Drew, 1997). Second, the inhibition of respiration could be clearly distinguished from the induction of fermentation, which did not occur until oxygen fell to levels close to zero (Geigenberger, 2003).
Based on these studies, it is reasonable to assume that a sensitive tuning mechanism must exist that allows the plant to regulate oxygen consumption while simultaneously preventing anoxia. However, hardly anything is known about the mechanism by which a plant induces adaptive responses to low oxygen (Bailey-Serres and Chang, 2005). Vice versa, comparably little is known about how metabolic activity affects the plant internal oxygen concentration. Previous studies focused on the effect that feeding of different sugars had on the respiration rate of tissue slices (Loef et al., 2001) or used transgenic approaches to stimulate metabolism by introducing a more energy-consuming route of Suc degradation via invertase in growing tubers (Bologa et al., 2003). In the latter case, respiration rates were increased and internal oxygen concentrations fell to very low levels that were close to zero. This shows that plant internal oxygen concentrations respond very sensitively to changes in metabolic activities. However, the underlying mechanism remains unclear.
Glycolysis is part of the central backbone of primary carbohydrate metabolism and respiration. Pyruvate serves as a key metabolite linking glycolysis in the cytosol with mitochondrial respiration. Under aerobic conditions, pyruvate is transported into mitochondria and is oxidized through the tricarboxylic acid (TCA) cycle into organic acids and NADH. Moreover, pyruvate has regulatory potential, as it was shown that alternative oxidase (AOX) becomes more active in the presence of α-keto acids such as pyruvate (Millar et al., 1993, 1996; Vanlerberghe et al., 1995; Vanlerberghe and McIntosh, 1997), thus affecting the efficiency of ATP production per unit of oxygen being respired. Under oxygen-limiting conditions, pyruvate can either be converted into ethanol by pyruvate decarboxylase (PDC) and alcohol dehydrogenase (ADH) or to lactate by lactate dehydrogenase. Pyruvate also serves as a precursor for the synthesis of Ala via the reversible reaction catalyzed by Ala amino transferase, which was shown to play a crucial role in the rapid conversion of Ala to pyruvate during recovery from low-oxygen stress (Miyashita et al., 2007). Furthermore, pyruvate is the substrate of acetolactate synthase, which is the first enzyme committed to the biosynthesis of the branched-chain amino acids Val, Leu, and Ile. Inhibition of acetolactate synthase by the herbicide imazethapyr induces aerobic fermentation in plants (Gaston et al., 2002). Despite its central role in energy metabolism under both oxygen-rich and oxygen-depleted conditions, no investigations were made, to our knowledge, until now on the impact pyruvate has on the plant internal oxygen concentration.
The aim of this study was to investigate the regulation of respiration and its relation to the plant internal oxygen concentration. To investigate this, we changed the oxygen concentration of the nutrient solution of hydroponically grown pea (Pisum sativum) plants and tested the influence of several sugars and organic acids. We measured root internal oxygen concentrations as well as the energy status of the tissue and related this to the rate of oxygen consumption by both the cytochrome c pathway and the AOX. The results are investigated in relation to the function and regulation of fermentative metabolism.
RESULTS
Root Oxygen Concentrations under Hypoxic Conditions and after Feeding Pyruvate
The root internal concentration of molecular oxygen was measured throughout a transect from the outside to the center using a small needle-type oxygen sensor that was injected into the tissue. Pea roots appeared hypoxic inside with lowest internal oxygen concentrations of only approximately 40% of normal air saturation (Fig. 1). As the actual amount of oxygen that can dissolve in an aqueous solution depends on many different factors, such as temperature and solute concentration, we describe the concentration of dissolved oxygen throughout this study in relative units that indicate the amount of oxygen as a percentage of the maximum amount of oxygen that the solution can contain when it is in equilibrium with normal air. As a reference value, Benson and Krause (1980) determined the oxygen concentration of 100% air-saturated fresh water at 25.0°C at 258.2 μm.
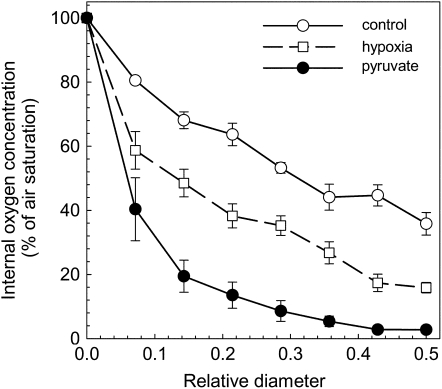
Figure 1.
Profile of the oxygen concentration within pea roots. The surface of the root is indicated by 0 and the center of the root is indicated by 0.5 of relative diameter. The measurements were performed on nontreated roots (control, white circles) after 1 d of incubation in a hypoxic nutrient solution (white squares) or after 1 d of incubation in a well-aerated nutrient solution supplemented with 8 mm pyruvate (black circles). Mean ± se (n = 7–15).
Under hypoxic conditions, when the oxygen concentration of the nutrient solution in which the roots were growing was reduced to 25% of the air saturated value, the plant internal oxygen concentration decreased 2 times only, from approximately 40% to approximately 20% (Fig. 1). This treatment had no effect on the survival of the pea plants. Apparently, a mechanism exists that allows the plant tissue to minimize the internal decrease of the oxygen concentration when the oxygen supply is reduced.
In contrast, plants supplied with 8 mm pyruvate in the nutrient solution showed a steeper internal oxygen gradient compared to control, and the center of the root became almost anoxic even though the nutrient solution was continuously aerated and the oxygen level of the solution was well above that of the hypoxic treatment (Figs. 1 and 2A). After a few days of pyruvate treatment, a reduction of the growth rate could be observed, and after 3 weeks of pyruvate treatment, the plants died. A similar effect was observed when 8 mm succinate was supplied to the nutrient solution. However, the addition of 8 mm Suc or Glc to the medium had no apparent effect on the internal oxygen concentration (Fig. 2A).
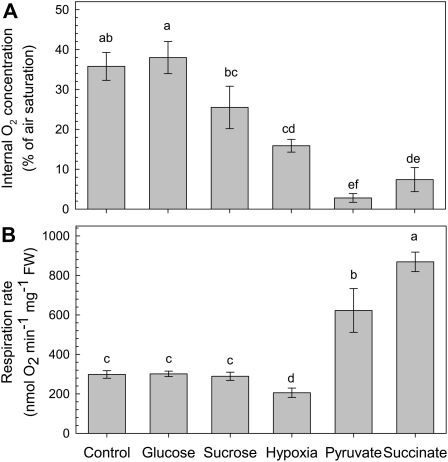
Figure 2.
Internal oxygen concentration (A) and respiratory rate (B) of pea roots that were grown in different nutrient solutions. The hydroponic nutrient solutions were well aerated and either used directly for the measurements (control) or supplemented with Suc, Glc, pyruvate, or succinate (final concentration 8 mm) 1 d before measuring. For the hypoxia treatment, the nutrient solution was aerated for 1 d with gas that consisted of 4% (v/v) O2, 0.035% CO2, and the rest volume N2. The values for the internal oxygen concentration represent the lowest oxygen concentration as measured in the middle of a root. Mean ± se (n = 3). Different letters mark mean values that are significantly different from each other (ANOVA; P < 0.05).
Apparently, pea roots are not able to control the regulation of the internal oxygen concentration when the TCA cycle is directly supplied with extra substrate by feeding the organic acids pyruvate or succinate, whereas the addition of similar amounts of sugars as substrates for glycolysis did not affect the internal oxygen concentration (Fig. 2).
Regulation of Respiration under Hypoxic Conditions and after Feeding Pyruvate
Oxygen consumption rates were measured with Clark-type oxygen electrodes on root pieces that were cut from plants that were pretreated for 1 d with 8 mm of Suc, Glc, pyruvate, or succinate (Fig. 2B). The addition of sugars had no stimulatory effect on respiration, whereas feeding pyruvate or succinate increased the oxygen consumption rate by 2- to 3-fold. Interestingly, the oxygen consumption rate under hypoxic conditions decreased significantly as compared to the control treatment. Hypoxic respiration was measured on root pieces that were incubated in a buffer with an oxygen concentration of 25% of air saturation (Fig. 2B). The inhibition of respiration at hypoxia is likely to be associated with an adaptive metabolic response in order to save oxygen when its availability becomes limited.
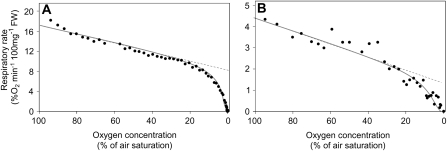
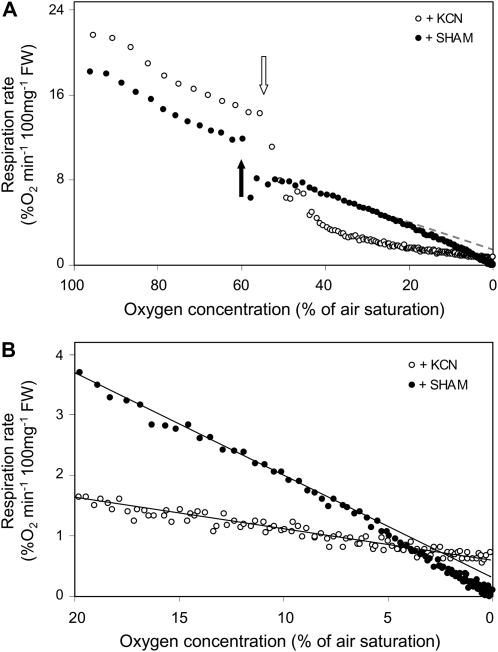
To evaluate the changes in respiration under hypoxic conditions more precisely, the oxygen consumption rate of root pieces was measured in a tightly closed Clark-type oxygen electrode, starting with a fully air-saturated buffer and lasting until all oxygen was consumed from the solution (Fig. 3). As the oxygen availability in the buffer decreases, the respiratory activity of the tissue slows down in a biphasic manner. Until an oxygen concentration of approximately 20% of air saturation is reached, the respiratory rate is decreasing slowly and the inhibition correlates in a linear manner to the oxygen concentration of the solution. However, when oxygen concentrations decrease below this threshold value, the decline of the oxygen consumption curves gets progressively steeper until the respiration rate reaches zero. It is noteworthy that this second phase does not show linear behavior.
Figure 3.
Rate of respiratory oxygen consumption as a function of the external oxygen concentration from roots of pea (A) and Arabidopsis (B). For the measurements, roots were kept in a hermetically closed vial in which the oxygen concentration was measured through time. Due to the respiratory activity of the tissue, oxygen depleted from the solution. Respiration rates were calculated from the raw data and plotted here against the external oxygen concentration. The graphs show the values of representative experiments that were repeated at least four times.
The inhibition of respiration as shown in Figure 3 is not due to cell death occurring during the course of the experiment, because identical oxygen consumption curves were observed repeatedly when the incubation solution was re-aerated after the oxygen concentration in the medium reached zero and the measurement was performed again (data not shown). It can also be excluded that the rate of oxygen diffusion through the tissue becomes limiting at low external oxygen concentrations in the medium, as similar oxygen consumption curves were observed for pea roots (diameter approximately 2 mm; Fig. 3A), Arabidopsis (Arabidopsis thaliana) roots (diameter approximately 200 μm; Fig. 3B), and Chlamydomonas cell culture (individual cell size of approximately 20 μm; data not shown). Apparently, the diffusion resistance for oxygen does not influence the adaptive response of respiration to hypoxia.
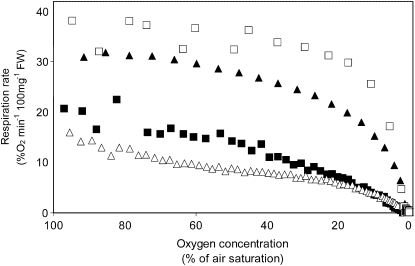
Feeding the organic acids pyruvate or succinate to the roots (Fig. 4) resulted in a strong increase of the oxygen consumption rate at all oxygen concentrations measured, but the shape of the curve remained similar to the control treatment, as described in Figure 3. Adding Suc or Glc to the medium had no effect on either the oxygen consumption rate or the shape of the curve.
Figure 4.
Rate of respiratory oxygen consumption as a function of the external oxygen concentration of pea roots incubated in different nutrient solutions for 1 d prior to the measurements. The concentration of the supplemented carbohydrates or organic acids was always 8 mm. □, Pyruvate; ▴, succinate; ▪, Suc; ▵, Glc. Respiration rates from roots that were not supplemented with any substrate were as shown in Figure 3. Also, the experimental details are as described for Figure 3. The graphs show the values of representative experiments that were repeated at least three times.
Our data show that the rate of respiration depends on the external oxygen concentration. The biphasic behavior of this hypoxic response indicates the existence of an adaptive mechanism that changes the extent to which respiration is inhibited as a function of the oxygen availability.
Regulation of AOX and COX under Hypoxia
To investigate how the oxygen availability affects the different terminal oxidases of the mitochondrial electron transport chain (AOX and COX), we determined the adaptive response to hypoxia in the presence of inhibitors for either COX or AOX. The addition of 20 mm salicylhydroxamic acid (SHAM) resulted in a slight decrease of respiration from about 12% oxygen min−1 100 mg fresh weight (FW) to 8% oxygen min−1 100 mg FW (Fig. 5). Subsequently, the rate of oxygen consumption continued to decline steadily until it reached zero (Fig. 5, A and B). A slight deviation between the measured data points and a theoretical straight line that fitted to the data obtained between an oxygen concentration of 40% and 20% of air saturation could be observed (Fig. 5, A and B). This is likely to be explained by the same mechanism that causes the decline in respiration at oxygen concentrations below 20% of air saturation as observed for roots that were not treated with SHAM. In either case, the cytochrome c pathway changes its activity in a hypoxia-dependent manner, ultimately reaching zero activity when oxygen depletes completely.
Figure 5.
Effect of respiratory inhibitors SHAM (black symbols) and KCN (white symbols) on the rate of respiratory oxygen consumption while the oxygen availability declines. A, The full profile of respiration from full saturation (100% of air saturation) of the incubation solution to full depletion (0% of air saturation). Inhibitors were added as indicated by the arrows in the figure. The gray dotted line fits to the linear part of the graph representing the cytochrome c pathway. B, The same data as in A, but scales on the horizontal and vertical axis are adjusted such that the low oxygen response can be observed more precisely. The experimental setup is as described for Figure 3. The graphs show the values of representative experiments that were repeated at least three times.
In contrast to this, inhibition of the cytochrome c pathway using 200 μm KCN resulted in an immediate steep drop of the respiratory oxygen consumption (Fig. 5; oxygen consumption rate before KCN addition, approximately 16% O2 min−1 100 mg FW; after KCN addition, 3% O2 min−1 100 mg FW). It took approximately 5 min before cyanide reached its maximal inhibitory effect. Subsequently, the rate of oxygen consumption decreased further in a remarkably linear dependency to the oxygen availability. It is striking that even at near-anoxic conditions, when hardly any free oxygen was available in the solution, the oxygen consumption rate still remained well above zero. Clearly, hypoxia does not affect the oxygen consumption rate by AOX to the same extent as it does for the cytochrome c pathway.
Induction of Fermentative Pathways under Hypoxia and after Feeding Pyruvate
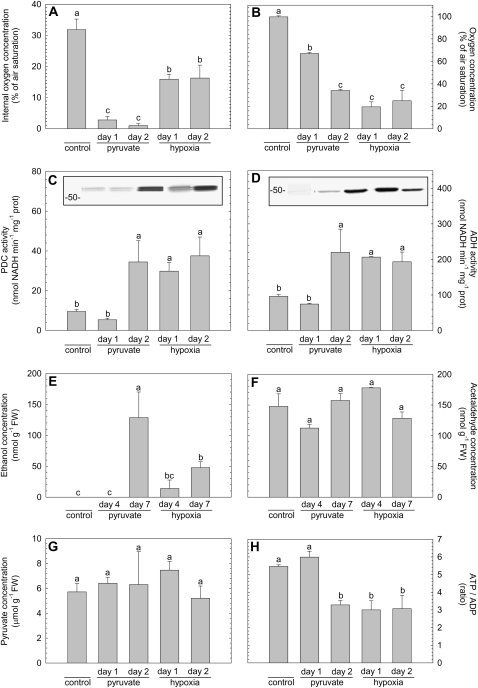
The induction of fermentative activity was investigated in pea roots in which the internal oxygen concentration was reduced either by treating the roots with a hypoxic nutrient solution (25% of air saturation) or via up-regulation of the respiratory oxygen consumption after feeding pyruvate for 1 or 2 d. Fermentative activity was evaluated by measuring the extractable enzyme activities of both PDC (Fig. 6C) and ADH (Fig. 6D) as well as detecting their protein abundance using western blotting and measuring subsequent ethanol and acetaldehyde (Fig. 6, E and F) accumulation in the roots. Lactate metabolism was not considered in our work, because earlier studies with pea concluded that both lactate dehydrogenase activity as well as lactate accumulation were not considerable parameters of fermentation in pea roots (Smith and ap Rees, 1979; Zabalza et al., 2005).
Figure 6.
Overview of the effect of hypoxia or pyruvate supplementation on various parameters that are suggested to be linked with the induction of fermentation in pea roots: internal oxygen concentration as measured in the middle of a root (A), oxygen concentration in the nutrient solution (B), PDC activity (C), ADH activity (D), the concentration of ethanol inside the root tissue (E), the concentration of acetaldehyde within the root tissue (F), the concentration of pyruvate within the root tissue (G), and the ratio of ATP to ADP as a measure for the energy status of the tissue (H). The insets in graphs C and D represent immunoblots showing the protein abundance of, respectively, PDC and ADH. Samples from the various treatments were taken 24 h (day 1) and 48 h (day 2) after addition of pyruvate to air-saturated nutrient solution (bars are labeled with pyruvate) or after switching to a nutrient solution with an oxygen concentration of 25% of air saturation (bars labeled with hypoxia). Control plants grew in air-saturated nutrient solution and samples from these plants were taken shortly before the experimental treatments started. Further control samples were taken during the course of the experiment, simultaneously with the samples taken from the various treatments. Because the control samples did not show any significant variation, the mean value of all control samples was calculated and given in the figure. The samples used for ethanol (A) or acetaldehyde (F) determination were taken 4 and 7 d after the pyruvate or hypoxic treatment started. The bars represent the average of at least three measurements ± se. Different letters mark mean values that are significantly different from each other (ANOVA; P < 0.05).
As expected, external hypoxia induced fermentative enzyme activity and protein abundance within 1 d and led to sustained levels after 2 d. Both PDC and ADH were induced more than 2 times compared to the control. The level of pyruvate (Fig. 6G) as well as acetaldehyde (Fig. 6F) in the root tissue did not change significantly in any of the treatments, but ethanol accumulation within the roots was detectable after 4 d of hypoxia and after 7 d the level of internal ethanol was significantly increased (Fig. 6E). It should be noted that ethanol is a highly volatile metabolite. Therefore, it is to be expected that the level of ethanol that can be detected within tissue is underestimating the real amount of ethanol being produced. Nevertheless, the increase in ethanol that was measured proves unequivocally that not only are the enzymes of the fermentative pathway induced but that fermentation is also operating in vivo.
Unlike the observations on the hypoxic treatment, the abundance of PDC and ADH enzyme took 2 d of pyruvate feeding to increase (Fig. 6, C and D). This observation is especially interesting because the root internal oxygen concentration (Fig. 6A) was much lower in the pyruvate-fed plants than in the hypoxic treatment. As in the hypoxic treatment, the steady-state levels of pyruvate and acetaldehyde within the root did not change after feeding pyruvate (Fig. 6, F and G). However, the strong increase of respiratory oxygen consumption as well as the increase of the level ethanol within the roots after adding pyruvate to the medium are a clear indication for the uptake of pyruvate by the roots and subsequent metabolization.
The observation that the induction of fermentation is delayed in pyruvate-fed roots even though the internal oxygen concentration was much lower than in roots from the hypoxia treatment indicates that feeding pyruvate attenuates signals that regulate fermentative activity in response to low oxygen. To investigate this further, we tested the correlation between the induction of fermentation and the energy status, expressed as the ratio of ATP to ADP. In the hypoxia-treated plants, the energy status dropped during the first day, whereas in pyruvate-fed roots the energy status did not decrease before the second day of the treatment (Fig. 6H). Obviously, a decrease in oxygen is linked with the induction of fermentative enzymes via changes in the energy status.
adh-null or adh− Mutants Have Increased Pyruvate Levels under Normoxia
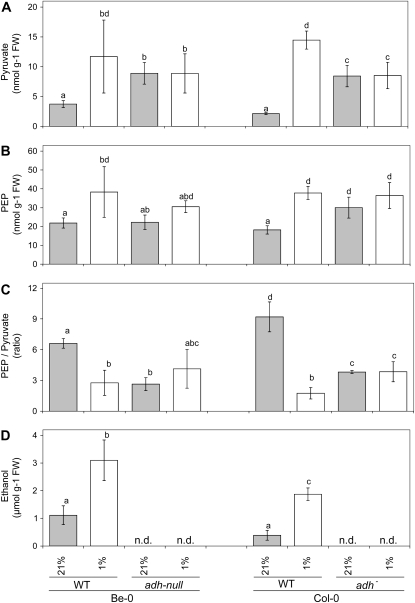
Pyruvate is the primary substrate for fermentation in plants. As the availability of pyruvate was shown to affect respiratory oxygen consumption as well as the plant internal oxygen concentration, it was investigated whether fermentative metabolism is involved in the regulation of the pyruvate content in plants. Therefore, we compared pyruvate accumulation in two adh mutants in different Arabidopsis ecotypes (Bensheim [Be-0] and Columbia [Col-0]) with the pyruvate levels in wild-type plants of the same ecotype. The adh mutant in the Be-0 background is a loss-of-function (adh-null) mutant without ADH activity under anoxia (Jacobs et al., 1988), whereas the mutation in the Col-0 background leads to a severe reduction-of-function (adh−) phenotype, as it is still able to induce the expression of mutated mRNA, but ethanol production under anoxia is reduced to very low levels (Banti et al., 2008). Seedlings were kept either at ambient oxygen or under hypoxic conditions for 24 h. Subsequently, the levels of pyruvate and phosphoenolpyruvate (PEP) were measured and compared to the levels in wild-type plants (Fig. 7). In both wild-type accessions, PEP and pyruvate accumulated significantly during the low oxygen treatment (P < 0.05). The ratio PEP to pyruvate dropped significantly (P < 0.05), pointing to an increased metabolic flux of carbon mediated by the enzyme pyruvate kinase (PK). In a parallel experiment, it was observed that ethanol emission from the roots of wild-type Col-0 plants significantly increased 4.8 times under near anoxia (1% [v/v] oxygen in air) as compared to the normoxic control (P < 0.05; Fig. 7D). From roots of wild-type Be-0, it leaked 2.7 times more ethanol into the medium during the near anoxic treatment as compared to normoxia (P < 0.05; Fig. 7D). Therefore, it is plausible to assume that the Pasteur effect (i.e. the activation of glycolysis when fermentation is induced) attributes to the changes described in the ratio PEP to pyruvate.
Figure 7.
The impact of ADH activity in 3-week-old Arabidopsis seedlings on the levels of pyruvate (A), PEP (B), and the ratio PEP to pyruvate (B) was tested under ambient air conditions (21% [v/v] oxygen in the air; gray bars) or after 24 h of a near-anoxic treatment with air containing 1% (v/v) oxygen (white bars). The seedlings used for these analyses were growing on sterile horizontal agar-plates. The levels of pyruvate and PEP were measured in plants of the wild-type (WT) Arabidopsis accessions Be-0 and Col-0 and compared with the values measured in two independent adh mutants (adh-null in the background of Be-0, and adh− in the Col-0 background). In a parallel experiment, ethanol production by the two wild-type accessions was determined (D) by measuring ethanol leakage into liquid incubation medium surrounding the seedlings, which was either aerated with ambient air, or with air containing 1% (v/v) oxygen. Multiple comparison analysis by the Holm-Sidak test following a two-way ANOVA indicated the oxygen-induced differences between the levels of pyruvate, PEP, and the PEP-to-pyruvate ratio in wild-type plants as significant (P < 0.05). Within knockout lines, all values measured were not statistically different from each other (P < 0.05). Bars represent the mean of at least three measurements ± se. Bars marked with the same letter do not differ significantly from each other. n.d., Not determined.
Both the adh-null and the adh− mutant line had significantly increased levels of pyruvate as compared to wild-type plants under normoxic conditions (P < 0.05; Fig. 7). Concomitantly, the PEP to pyruvate ratio was much lower than in wild-type plants. Upon low-oxygen treatment, no further increase of the level of pyruvate or any significant change in the PEP to pyruvate ratio was detected in either of the adh mutants lines (P < 0.05).
The increased levels of pyruvate in the adh mutant lines compared to wild type can be interpreted that the alcohol fermentation pathway also plays a role under aerobic conditions. This idea is supported by the observation mentioned above that even under aerobic conditions, a small amount of ethanol could be observed. This implies that fermentation not only functions to regenerate NAD from NADH to keep glycolysis running, but it also helps to lower the level of pyruvate, thereby preventing the induction of oxygen consumption by pyruvate as described before.
DISCUSSION
Plants do not have an efficient distribution system for oxygen throughout their tissues. Therefore, oxygen can reach very low internal levels (Geigenberger et al., 2000; Gibon et al., 2002; Rolletschek et al., 2002; Vigeolas et al., 2003; van Dongen et al., 2004). Several studies showed that energy-generating and -consuming metabolic pathways are adjusted to the actual oxygen availability (for review, see Geigenberger, 2003). Until now, little is known about the mechanisms by which these adaptive responses are triggered in plants. In this study, we used hydroponically grown pea roots to investigate the regulation of respiration in relation to the plant internal oxygen concentration.
Regulation of Respiration by Low Oxygen Involves Two Components Acting at Different Oxygen Concentrations in Plants
Under normal conditions, pea roots have internal oxygen concentrations that are about 40% of air saturation (Fig. 1), similar to what has been described for other tissues (Geigenberger et al., 2000; Rolletschek et al., 2002; van Dongen et al., 2003, 2004). When the external oxygen concentration is reduced 4-fold, the internal oxygen concentration goes down only 2-fold (Fig. 1, hypoxia treatment), indicating an adaptive regulation of the oxygen consumption rate helping the tissue to prevent anoxia. Indeed, it can be observed that the rate of oxygen consumption depends on the oxygen availability. As shown in Figure 3, a reduction of the oxygen availability results in a slow but linear decrease of the respiratory rate until a remaining oxygen concentration of 20% of air saturation. Below this concentration, a steep decline of the oxygen consumption rate can be observed. Such biphasic kinetic behavior can only be explained when at least two controlling mechanisms are involved in parallel to control respiratory activity as a function of the oxygen availability. It is not credible to describe the kinetic behavior simply by a passive dependence to the oxygen concentration such as Michaelis-Menten kinetics superimposed on a linear diffusion component. An important argument for this is that the internal oxygen concentration reaches values close to anoxia after feeding pyruvate, whereas the oxygen consumption rate becomes much higher than under control conditions. This indicates that the oxygen affinity of the respiratory system must be high enough not to limit respiration, even if the oxygen concentration decreases below 25% of air saturation.
It is therefore concluded that the rate of respiration is closely regulated by the oxygen availability in a concentration-dependent manner. It is suggested that this mechanism helps to determine the steady-state oxygen concentration within plant tissue. The control mechanism comprises at least two components, each acting at different oxygen concentrations.
Control of the Internal Oxygen Concentration by the Level of Respiratory Substrates
In contrast to the inhibition of respiration under hypoxia, feeding pyruvate or succinate to the roots led to a sharp increase in the oxygen consumption rate (Fig. 2B). This coincides with a severe drop in the plant internal oxygen concentration (Figs. 1 and 2A) even when the nutrient solution was well aerated and the oxygen concentration of this solution never decreased below 80% of air saturation. The first day after the pyruvate treatment started, the tissue in the middle of the root became almost anoxic (Fig. 1). Interestingly, the biphasic characteristic shape of the kinetic curves is independent of the substrate being added to the medium (Fig. 4). Apparently, feeding organic acids does not change the mechanism by which respiration is reduced when the oxygen availability goes down, but the strong increase of the oxygen consumption rate as compared to the control treatment (compare with Fig. 3) does lead to a severe drop in the plant internal oxygen concentration, for which the adaptive response to hypoxia cannot compensate anymore. It is noteworthy that the maximum rate of respiration does not increase when extra Suc of Glc is supplied to the plants within this time frame. Apparently, glycolysis is able to regulate the amount of pyruvate that becomes available for respiration, thereby regulating the internal oxygen concentration of the tissue.
From these data it is concluded that the internal oxygen concentration depends on the rate of respiratory oxygen consumption and that this is linked to the amount of substrate that is available for respiration. Apparently, the glycolytic pathway is capable of buffering the supply of substrate to the TCA cycle, thereby affecting not only the respiratory activity but also the plant internal oxygen concentration.
Several control sites are suggested to be able to regulate the flux of substrate into the TCA cycle. Firstly, glycolytic activity could control the production of pyruvate (for review, see Plaxton and Podestá, 2006). Important regulatory enzymes in the glycolytic pathway are PK (converting PEP directly into pyruvate) and PEP carboxylase (which is the first step of an alternative pathway converting PEP into pyruvate). Interestingly, Hatzfeld and Stitt (1991) showed that only the stimulation of glycolysis by PK resulted in an increase of oxygen consumption. Moreover, analysis of a suite of metabolite levels of glycolysis and the TCA cycle after decreasing the oxygen concentration from 100% to 20% air saturation in tuber tissue revealed PK as one of the several regulatory sites that lead to an inhibition of respiration (Geigenberger et al., 2000). Therefore, one possible mechanism for the regulation of oxygen consumption and thus plant internal oxygen concentration is via the control of PK activity. This hypothesis is in good agreement with our present observation that the availability of pyruvate affects the internal oxygen concentration. Several allosteric regulation mechanisms that affect PK activity are already described (Podesta and Plaxton, 1991, 1992) and it will be of substantial interest to investigate the regulation of PK in response to low oxygen and to investigate the adaptive low oxygen response in plants with altered PK activity.
Interestingly, pyruvate levels were strongly increased in tubers expressing a more effective but less energy-saving route of Suc degradation via invertase (Trethewey et al., 1998) or Suc phosphorylase (Trethewey et al., 2001). The increase in pyruvate levels in these lines was accompanied by a stimulation of respiration and a strong decrease in internal oxygen concentration in growing tubers (Bologa et al., 2003). Interestingly, both COX- and AOX-dependent pathways of respiration were up-regulated in the transgenic tubers compared to wild type (Centeno et al., 2008).
A second control mechanism of interest is the regulation of the import of pyruvate into the TCA cycle by the pyruvate dehydrogenase (PDH) complex that catalyzes the conversion of pyruvate into acetyl-CoA. The activity of the PDH complex is subject to a variety of regulatory mechanisms. First, the PDH complex is feedback regulated by NADH (Randall and Miernyk, 1990). So, when oxygen is completely absent and NADH accumulates, pyruvate will not be shuttled into the TCA cycle anymore. This would result in a reduction of the respiratory rate. Second, the PDH complex is inactivated by phosphorylation via PDH kinase (Tovar-Méndez et al., 2003). Interestingly, this kinase is inhibited by pyruvate. So, the activity of the PDH complex is stimulated when pyruvate is present.
A better understanding of the importance of the possible control sites will need reversed genetic approaches and more studies resolving the subcellular compartmentation of pyruvate and NADH between cytosol, plastid, and mitochondria. Such studies would be most interesting to obtain information about the fine regulation of the pyruvate control mechanism at low oxygen.
The Role of COX and AOX in the Control of the Internal Oxygen Concentration
An alternative regulatory mechanism to control respiratory activity is located within the mitochondrial electron transport chain itself. There are two different enzymes that use oxygen as a substrate, COX and AOX. In the first pathway, COX (also known as complex-IV) is catalyzing the transfer of electrons from cytochrome c to molecular oxygen, thereby transporting protons across the mitochondrial inner membrane. The second respiratory pathway is catalyzed by the enzyme AOX, which transfers electrons directly from ubiquinol to oxygen without carrying protons across the membrane. Therefore, the alternative pathway of respiration via AOX has a lower potential to produce ATP from oxygen than the COX pathway has.
Pyruvate and other α-ketoacids have a significant stimulating effect on the activity of AOX (Millar et al., 1993; Vanlerberghe et al., 1995). Although the significance of this control mechanism in vivo has been discussed (Vanlerberghe and McIntosh, 1997; Pastore et al., 2001), it could be shown in transgenic potato (Solanum tuberosum) that a decrease in the level of pyruvate by silencing the expression of cytosolic PK in tubers leads to a concomitant decrease in the amount of AOX protein (Oliver et al., 2008). In line with these earlier observations, pyruvate feeding to pea roots strongly induced the rate of oxygen consumption (Figs. 2 and 4). However, from our data it cannot be concluded if this is the result of an activation of the oxidases or due to an increase of the oxidase protein abundance.
To find out how the individual respiratory pathways react on changes in the oxygen availability, the activity of either AOX or COX was inhibited using the specific chemical inhibitors SHAM and KCN, respectively. The experimental data show that both pathways are present in roots, though the capacity of the AOX pathway is much less than the capacity of the cytochrome pathway. Most interesting, there was a differential effect of hypoxia on the kinetic behavior of the individual pathways. In the presence of SHAM, the remaining activity of the COX pathway is still showing the biphasic deactivation at low oxygen, ultimately leading to a complete stop of the oxygen consumption. In contrast to this, the cyanide-resistant pathway, which is mainly due to the activity of AOX, does only show linear inhibition by low oxygen but remains active until all oxygen is absolutely being consumed.
Previously, it was shown for bovine heart COX that preincubation of the enzyme complex at low oxygen concentrations reduced Vmax and slightly increased Km for oxygen (Chandel et al., 1996). It is tempting to speculate that plant COX can be regulated in a similar way, whereas AOX does not possess such a regulatory mechanism. Nevertheless, because of the lower oxygen-to-energy efficiency of AOX, it is reasonable to assume that AOX activity in vivo should never exceed COX activity under hypoxic conditions. This could simply be achieved when the affinity for oxygen of COX is much higher than that of AOX. Also, it cannot be excluded that the breakdown of energy parameters after KCN addition and further detrimental effects of this prevented the adaptive response of AOX to oxygen. Direct analyses of kinetic properties on purified COX and AOX will be necessary to clarify this further.
Induction of Fermentation Is Mediated by a Drop in the Energy Status
The best-known function of fermentative metabolism is to recycle NADH to NAD to avoid depletion of the cytosolic NAD pool and inhibition of glycolysis when oxidative phosphorylation is limited. Because all prominent fermentative pathways use pyruvate as initial substrate, the glycolytic flux is maintained or even increased to secure ATP production via glycolysis. The results of our biochemical approaches show that controlling the level of pyruvate is very important for the regulation of oxygen consumption when the oxygen availability becomes low. Moreover, we showed by using Arabidopsis knockout lines that were impaired in ADH activity that the alcohol fermentation pathway is important for balancing pyruvate levels under normoxic conditions. Apparently, fermentation in plants serves not only to regenerate NAD from NADH under anoxic conditions but also to regulate the pyruvate level to control the respiration rate.
Induction of fermentation under aerobic conditions has been described earlier (Bucher et al., 1995; Tadege and Kuhlemeier, 1997). In germinating pollen, it was found that at normal oxygen concentrations, fermentative pyruvate degradation occurs in order to increase the flux of carbon from pyruvate into respiration via PDC and the glyoxylate cycle. Our data indicate that the function of fermentative metabolism under aerobic conditions to regulate respiratory activity is much more general than previously assumed.
As alcohol fermentation is apparently not limited to anoxic conditions only, the question is raised of what factor initiates the induction of PDC and ADH under low oxygen. In Figure 6, the induction of activity of both of these enzymes is compared to the internal oxygen concentration as well as the energy charge of the tissue. Plants that were treated with pyruvate in the nutrient solution had lower internal oxygen concentrations as compared the hypoxic treatment (Fig. 6A), but both PDC and ADH were not induced after 1 d (Fig. 6, C and D). Induction of fermentation correlated well with the energy status of the tissue, expressed here as the ratio of ATP to ADP. In the hypoxia-treated plants, the energy status dropped during the first day, whereas in pyruvate-fed roots, the energy status did not decrease before the second day of treatment (Fig. 6F). In parallel to this, the activity of both PDC and ADH were induced. This indicates that pyruvate attenuates the induction of fermentation at low oxygen by keeping the energy status constant. Obviously, the induction of fermentative enzymes is not exclusively dependent on the oxygen concentration, but it is also linked to changes in the energy status. As ADH expression is one of the most sensitive reactions to hypoxia being affected by a mild decrease in oxygen availability already (van Dongen et al., 2009), it is plausible to assume that direct effects of oxygen on the expression level of the gene are subsequently modified by the energy state of the tissue.
CONCLUSION
From the data presented in this study, it is concluded that the plant internal oxygen concentration is regulated via adjusting the respiratory activity of the tissue to the actual oxygen availability. This adaptive response is suggested to postpone or even prevent anoxia in the tissue when the oxygen availability decreases. The decline in respiratory activity as a function of the oxygen concentration can be described by two different phases, indicating that more than one regulatory mechanism must exist. In contrast to COX, which shows the biphasic response to hypoxia, the decrease in AOX activity when the oxygen availability goes down is just linearly related to the oxygen concentration. This indicates that the various terminal oxidases of the mitochondrial electron transport chain have different regulation mechanisms to adjust their activity to the internal oxygen concentration.
Furthermore, our results show that mechanisms to adjust the oxygen consumption and thus the internal oxygen concentration are linked to the availability of pyruvate. Also, under aerobic conditions, fermentative metabolism plays an important role in balancing the level of pyruvate in the cell. Evidence is shown that the energy status of the tissue affects the induction of fermentative enzymes under low oxygen.
MATERIALS AND METHODS
Plant Material
Pea (Pisum sativum ‘Snap Sugar Boys’) was grown in hydroponic culture as described in Zabalza et al. (2005). The nutrient solution was continuously aerated and was replaced every 4 d to prevent excessive growth of bacteria and algae in the solution. For the experiments, plants that were 12 d old were used. Suc, Glc, pyruvate, and succinate were supplied to the nutrient solution at a final concentration of 8 mm. Hypoxia was applied by aerating the nutrient solution with a gas mixture of oxygen and nitrogen supplied with 0.035% CO2 to achieve a final oxygen concentration in the nutrient solution of 25% of air saturation. Sampling was done in the middle of the light period. The pyruvate concentration in the nutrient solution was evaluated to study its stability in the nutrient solution. The initial concentration (8 mm) hardly changed during the first day of treatment (7.27 ± 0.33 mm pyruvate after 24 h either in the absence or presence of plants). However, during the second day, the pyruvate concentration in the nutrient solution reduced to 5.78 ± 1.01 mm without plants and to 1.23 ± 0.37 mm with plants growing in the nutrient solution.
Respiratory activity of Arabidopsis (Arabidopsis thaliana) ecotype Col-0 roots was investigated on hydroponically grown plant material. For this, seeds were surface sterilized with 70% ethanol and 10% sodium hypochlorite and rinsed several times with distilled water before sowing them on stone wool plugs kept in a polysterene tray (Klem plug standard, Grodan BV) that was soaked in nutrient solution [slightly modified from Loqué et al., 2003: 1 mm NH4NO3, 1 mm KH2PO4, 1 mm MgSO4, 250 μm CaCl2, 0.1 mm Na-Fe-EDTA, 50 μm KCl, 50 μm H3BO3, 5 μm MnSO4, 1 μm ZnSO4, 1 μm CuSO4, and 0.1 μm (NH4)6Mo7O24]. Seeds were incubated for 3 d at 4°C in darkness before the trays were transferred to a phytotron (12-h day/12-h night; light intensity, 150 μmol s−1 m−2 photosynthetically active radiation; temperature, 20°C/18°C; relative humidity of the air, 70%) and placed in a 10l tank filled with nutrient solution that was refreshed twice a week. Roots from 3-week-old plants were used for the respiratory oxygen consumption experiments.
Three-week old Arabidopsis seedlings from a ADH loss-of-function (adh-null) mutant (Arabidopsis ecotype Be-0, ethyl methanesulfonate mutagenized; Jacobs et al., 1988) and a reduction-of-function (adh−) mutant in the Col-0 background (T-DNA knockout, NASC ref. N552699; Banti et al., 2008) were grown on vertical agar plates containing half-strength Hoagland nutrient solution supplement with 2% Suc and treated with normal air containing 21% oxygen (normoxia) or 1% oxygen (hypoxia) and 0.035% CO2 for 24 h as described by van Dongen et al. (2009). For the determination of ethanol production by Arabidopsis, seedlings were transferred from the plates to liquid medium that was in equilibrium with air containing either 21% or 1% of oxygen. The ethanol concentration of the incubation medium was determined after incubation overnight (Banti et al., 2008).
Oxygen Measurements
The oxygen concentration of the nutrient solution and the plant internal oxygen contents were measured using an optical oxygen sensor (Microx TX2, PreSens Precision Sensing).
Additionally, the plant internal oxygen concentrations were verified, using a self-made laser-based apparatus for miniaturized optical oxygen measurements. The setup, which is able to differentiate between sample fluorescence and sensor phosphorescence, allows the use of ultrafine fiber-optic sensors with tip diameters down to 10 μm. The setup and the data evaluation are described by Löhmannsröben et al. (2005) and by Schmälzlin et al. (2005). This kind of measurements allows precise determination of internal oxygen concentrations with minimal disturbance of the tissue.
Respiration Measurements
Respiratory oxygen consumption was measured using Clark-type electrodes (Hansatech Oxygraph, H.Saur Laborbedarf) using small (5–10 mm) root pieces incubating in fresh solution with the same composition as the nutrient solution the roots were taken from. Measurements were done at 25°C in a final volume of 1 mL containing 25 mg of thoroughly rinsed intact roots, and the cuvette was tightly closed to prevent diffusion of oxygen from the air. The alternative pathway was inhibited with 20 mm SHAM (1 m stock solution in methoxyethanol) and to inhibit the cytochrome pathway, 200 μm KCN was added (stock solution in 20 mm HEPES, pH 8.0). The optimal inhibitor concentrations were determined from a titration curve as explained by Møller et al. (1988).
Metabolite and Enzyme Activity Measurements
Metabolites were extracted from frozen root material by TCA extraction. PEP and pyruvate were measured enzymatically as in van Dongen et al. (2004). The amount of ATP and ADP was measured by HPLC (van Dongen et al., 2004).
PDC (EC 4.1.1.1) and ADH (EC 1.1.1.1) activities were assayed in desalted extract as described by Gaston et al. (2002).
To measure ethanol and acetaldehyde in pea roots, 500 mg root material was homogenized in 5 mL of 1 n HClO4 in a Teflon-sealed test tube. After 15-min incubation at 70°C, a 1-mL gas sample was taken from the headspace and injected into a gas chromatograph (6890 HP; Hewlett-Packard) equipped with a HP-Innowax column and a flame ionization detector. Ethanol production by Arabidopsis seedlings was performed enzymatically as described by Banti et al. (2008).
Western Blots
Total protein was isolated from roots that were quickly frozen in liquid nitrogen as described by Zabalza et al. (2005). Western blots were produced according to standard techniques. PDC antibody was kindly provided by Dr. Stephan König (Martin-Luther University, Halle-Wittenberg, Germany). Fumarase was analyzed using antibodies raised against Arabidopsis fumarase (Nunes-Nesi et al., 2007). ADH antibody was produced by a custom peptide facility (Biogenes) and a short conjugated peptide as antigen (C-KGTFYGNYKPRTDL-COOH). Antibody was raised in rabbit using standard protocols from the manufacturer. Primary antibody dilution was 1:2,000 for PDC and 1:500 for ADH. Antirabbit IgG peroxidase (Sigma-Aldrich) was used as secondary antibody at a dilution of dilution 1:200,000.
Acknowledgments
The authors thank Pierdomenico Perata for providing the ADH mutants, Karin Koehl for establishing the Arabidopsis hydroponics, and Mark Stitt and Hans Lambers for helpful discussions. Tine Schmaedicke and Gustavo Garijo are acknowledged for their experimental assistance.
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. Ge 878/1–5), by the Ministry of Education and Science (Spain; grant no. AGL–2004–03784/AGR; predoctoral fellowship to L.O.), and by the Public University of Navarre (predoctoral fellowship to M.I.).
The author responsible for the distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Joost T. van Dongen (dongen@mpimp-golm.mpg.de).
Open access articles can be viewed online without a subscription.
References
- Armstrong W, Strange ME, Cringle S, Beckett PM (1994) Microelectrode and modelling study of oxygen distribution in roots. Ann Bot (Lond) 74 287–299 [Google Scholar]
- Banti V, Loreti E, Novi G, Santaniello A, Alpi A, Perata P (2008) Heat acclimation and cross-tolerance against anoxia in Arabidopsis. Plant Cell Environ 31 1029–1037 [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J, Chang R (2005) Sensing and signalling in response to oxygen deprivation in plants and other organisms. Ann Bot (Lond) 96 507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Serres J, Voesenek LACJ (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59 313–339 [DOI] [PubMed] [Google Scholar]
- Benson BB, Krause D (1980) The concentration and isotopic fractionation of gases dissolved in freshwater in equilibrium with the atmosphere. 1. Oxygen. Limnol Oceanogr 25 662–671 [Google Scholar]
- Bologa KL, Fernie AR, Leisse A, Loureiro ME, Geigenberger P (2003) A bypass of sucrose synthase leads to low internal oxygen and impaired metabolic performance in growing potato tubers. Plant Physiol 132 2058–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher M, Brander KA, Sbicego S, Mandel T, Kuhlemeier C (1995) Aerobic fermentation in tobacco pollen. Plant Mol Biol 28 739–750 [DOI] [PubMed] [Google Scholar]
- Centeno DC, Oliver SN, Nunes-Nesi A, Geigenberger P, Machado DN, Ehlers Loureiro M, Silva MAP, Fernie AR (2008) Metabolic regulation of pathways of carbohydrate oxidation in potato (Solanum tuberosum) tubers. Physiol Plant 133 744–754 [DOI] [PubMed] [Google Scholar]
- Chandel NS, Budinger GRS, Schumacker PT (1996) Molecular oxygen modulates cytochrome c oxidase functions. J Biol Chem 271 18672–18677 [DOI] [PubMed] [Google Scholar]
- Drew MC (1997) Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol 48 223–250 [DOI] [PubMed] [Google Scholar]
- Gaston S, Zabalza A, González EM, Arrese-Igor C, Aparicio-Tejo PM, Royuela M (2002) Imazethapyr, an inhibitor of the branched-chain amino acid biosynthesis, induces aerobic fermentation in pea plants. Physiol Plant 114 524–532 [DOI] [PubMed] [Google Scholar]
- Geigenberger P (2003) Response of plant metabolism to too little oxygen. Curr Opin Plant Biol 6 247–256 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Fernie AR, Gibon Y, Christ M, Stitt M (2000) Metabolic activity decreases as an adaptative response to low internal oxygen in growing potato tubers. Biol Chem 381 723–740 [DOI] [PubMed] [Google Scholar]
- Gibon Y, Vigeolas H, Tiessen A, Geigenberger P, Stitt M (2002) Sensitive and high throughput metabolite assays for inorganic pyrophosphate, ADPGlc, nucleotide phosphates, and glycolytic intermediates based on a novel enzymatic cycling system. Plant J 30 221–235 [DOI] [PubMed] [Google Scholar]
- Hatzfeld WD, Stitt M (1991) Regulation of glycolysis in heterotrophic cell suspension cultures of Chenopodium rubrum in response to proton fluxes at the plasmalemma. Physiol Plant 81 103–110 [Google Scholar]
- Jacobs M, Dolferus R, Van den Bossche D (1988) Isolation and biochemical analysis of ethyl methanesulfonate-induced alcohol dehydrogenase null mutants of arabidopsis thaliana (L.) Heynh. Biochem Genet 26 105–122 [DOI] [PubMed] [Google Scholar]
- Loef I, Stitt M, Geigenberger P (2001) Increased levels of adenine nucleotides modify the interaction between starch synthesis and respiration when adenine is supplied to discs from growing potato tubers. Planta 212 782–791 [DOI] [PubMed] [Google Scholar]
- Loqué D, Tillard P, Gojon A, Lepetit M (2003) Gene expression of the NO3- transporter NRT1.1 and the nitrate reductase NIA1 is repressed in Arabidopsis roots by NO2-, the product of NO3- reduction. Plant Physiol 132 958–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löhmannsröben HG, Beck M, Hildebrandt N, Schmälzlin E, van Dongen JT (2005) New challenges in biophotonics: laser-based fluoroimmuno analysis and in-vivo optical oxygen monitoring. Proc SPIE 6157 61570E [Google Scholar]
- Millar AH, Hoefnagel MHN, Day DA, Wiskich JT (1996) Specificity of the organic acid activation of alternative oxidase in plant mitochondria. Plant Physiol 111 613–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AH, Wiskich JT, Whelan J, Day DA (1993) Organic acid activation of the alternative oxidase of plant mitochondria. FEBS Lett 329 259–262 [DOI] [PubMed] [Google Scholar]
- Miyashita Y, Dolferus R, Ismond KP, Good AG (2007) Alanine aminotransferase catalyses the breakdown of alanine after hypoxia in Arabidopsis thaliana. Plant J 49 1108–1121 [DOI] [PubMed] [Google Scholar]
- Møller IM, Berczi A, van der Plas LHW, Lambers H (1988) Measurement of the activity and capacity of the alternative pathway in intact plant tissues: identification of problems and possible solutions. Physiol Plant 72 642–649 [Google Scholar]
- Nunes-Nesi A, Carrari F, Gibon Y, Sulpice R, Lytovchenko A, Fisahn J, Graham J, Ratcliffe RG, Sweetlove LJ, Fernie AR (2007) Deficiency of mitochondrial fumarase activity in tomato plants impairs photosynthesis via an effect on stomatal function. Plant J 50 1093–1106 [DOI] [PubMed] [Google Scholar]
- Oliver SN, Lunn JE, Urbanczyk-Wochniak E, Lytovchenko A, van Dongen JT, Faix B, Schmälzlin E, Fernie AR, Geigenberger P (2008) Decreased expression of cytosolic pyruvate kinase in potato tubers leads to a decline in pyruvate resulting in an in vivo repression of the alternative oxidase. Plant Physiol 148 1640–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore D, Trono D, Laus MN, Di Fonzo N, Passarella S (2001) Alternative oxidase in durum wheat mitochondria. Activation by pyruvate, hydroxypyruvate and glyoxylate and physiological role. Plant Cell Physiol 42 1373–1382 [DOI] [PubMed] [Google Scholar]
- Plaxton WC, Podestá FE (2006) The functional organization and control of plant respiration. Crit Rev Plant Sci 25 159–198 [Google Scholar]
- Podesta FE, Plaxton WC (1991) Kinetic and regulatory properties of cytosolic pyruvate kinase from germinating castor oil seeds. Biochem J 279 495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podesta FE, Plaxton WC (1992) Plant cytosolic pyruvate kinase: a kinetic study. Biochim Biophys Acta 1160 213–220 [DOI] [PubMed] [Google Scholar]
- Randall DD, Miernyk JA (1990) The mitochondrial pyruvate dehydrogenase complex. In PJ Lee, ed, Methods in Plant Biochemistry, Vol 3. Enzymes of Primary Metabolism. Academic Press, London, pp 175–192
- Rolletschek H, Borisjuk L, Koschorreck M, Wobus U, Weber H (2002) Legume embryos develop in a hypoxic environment. J Exp Bot 53 1099–1107 [DOI] [PubMed] [Google Scholar]
- Schmälzlin E, van Dongen JT, Klimant I, Marmodée B, Steup M, Fisahn J, Geigenberger P, Löhmannsröben HG (2005) An optical multi-frequency phase modulation method using micro-beads for measuring intracellular oxygen concentrations in plants. Biophys J 89 1339–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, ap Rees T (1979) Effects of anaerobiosis on carbohydrate oxidation by roots of Pisum sativum. Phytochemistry 18 1453–1458 [Google Scholar]
- Tadege M, Kuhlemeier C (1997) Aerobic fermentation during tobacco pollen development. Plant Mol Biol 35 343–354 [DOI] [PubMed] [Google Scholar]
- Tovar-Méndez A, Miernyk JA, Randall DD (2003) Regulation of pyruvate dehydrogenase complex activity in plant cells. Eur J Biochem 270 1043–1049 [DOI] [PubMed] [Google Scholar]
- Trethewey RN, Fernie AR, Bachmann A, Fleischer-Notter H, Geigenberger P, Willmitzer L (2001) Expression of a bacterial sucrose phosphorylase in potato tubers results in a glucose-independent induction of glycolysis. Plant Cell Environ 24 357–365 [Google Scholar]
- Trethewey RN, Geigenberger P, Riedel K, Hajirezaei MR, Sonnewald U, Stitt M, Riesmeier JW, Willmitzer L (1998) Combined expression of glucokinase and invertase in potato tubers leads to a dramatic reduction in starch accumulation and a stimulation of glycolysis. Plant J 15 109–118 [DOI] [PubMed] [Google Scholar]
- van Dongen JT, Roeb GW, Dautzenberg M, Froehlich A, Vigeolas H, Minchin PEH, Geigenberger P (2004) Phloem import and storage metabolism are highly coordinated by the low oxygen concentrations within developing wheat seeds. Plant Physiol 135 1809–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen JT, Schurr U, Pfister M, Geigenberger P (2003) Phloem metabolism and function have to cope with low internal oxygen. Plant Physiol 131 1529–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen JT, Fröhlich A, Ramírez-Aguilar SJ, Schauer N, Fernie AR, Erban A, Kopka J, Clark J, Langer A, Geigenberger P (2009) Transcript and metabolite profiling of the adaptive response to mild decreases in oxygen concentration in the roots of Arabidopsis plants. Ann Bot (Lond) 103 269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh LM (1997) Alternative oxidase: from gene to function. Annu Rev Plant Physiol Plant Mol Biol 42 703–734 [DOI] [PubMed] [Google Scholar]
- Vanlerberghe GC, Day DA, Wiskich JT, Vanlerberghe AE, McIntosh L (1995) Alternative oxidase activity in tobacco leaf mitochondria: dependence on tricarboxylic acid cycle-mediated redox regulation and pyruvate activation. Plant Physiol 109 353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigeolas H, van Dongen JT, Waldeck P, Huhn D, Geigenberger P (2003) Lipid storage metabolism is limited by the prevailing low oxygen concentrations oilseed rape. Plant Physiol 33 2048–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabalza A, González EM, Arrese-Igor C, Royuela M (2005) Fermentative metabolism is induced by inhibiting different enzymes of the branched-chain amino acid biosynthesis pathway in pea plants. J Agric Food Chem 53 7486–7493 [DOI] [PubMed] [Google Scholar]