Abstract
Chloroplasts contain approximately 80% of total leaf nitrogen and represent a major source of recycled nitrogen during leaf senescence. While bulk degradation of the cytosol and organelles in plants is mediated by autophagy, its role in chloroplast catabolism is largely unknown. We investigated the effects of autophagy disruption on the number and size of chloroplasts during senescence. When leaves were individually darkened, senescence was promoted similarly in both wild-type Arabidopsis (Arabidopsis thaliana) and in an autophagy-defective mutant, atg4a4b-1. The number and size of chloroplasts decreased in darkened leaves of wild type, while the number remained constant and the size decrease was suppressed in atg4a4b-1. When leaves of transgenic plants expressing stroma-targeted DsRed were individually darkened, a large accumulation of fluorescence in the vacuolar lumen was observed. Chloroplasts exhibiting chlorophyll fluorescence, as well as Rubisco-containing bodies, were also observed in the vacuole. No accumulation of stroma-targeted DsRed, chloroplasts, or Rubisco-containing bodies was observed in the vacuoles of the autophagy-defective mutant. We have succeeded in demonstrating chloroplast autophagy in living cells and provide direct evidence of chloroplast transportation into the vacuole.
Chloroplasts contain 75% to 80% of total leaf nitrogen mainly as proteins (Makino and Osmond, 1991). During leaf senescence, chloroplast proteins are gradually degraded as a major source of nitrogen for new growth (Wittenbach, 1978; Friedrich and Huffaker, 1980; Mae et al., 1984), correlating with a decline in photosynthetic activity, while chloroplasts gradually shrink and transform into gerontoplasts, characterized by the disintegration of the thylakoid membranes and accumulation of plastoglobuli (for a recent review, see Krupinska, 2006). Concomitantly, a decline in the cellular population of chloroplasts is also evident in many cases, for example, during natural (Kura-Hotta et al., 1990; Inada et al., 1998), dark-induced (Wittenbach et al., 1982), and nutrient-limited senescence (Mae et al., 1984; Ono et al., 1995), suggesting the existence of a whole chloroplast degradation system. Some electron microscopic studies have shown whole chloroplasts in the central vacuole, which is rich in lytic hydrolases (Wittenbach et al., 1982; Minamikawa et al., 2001). However, there is no direct evidence of chloroplasts moving into the vacuole in living cells and the mechanism of transport is not yet understood (Hörtensteiner and Feller, 2002; Krupinska, 2006).
The most abundant chloroplast protein is Rubisco (EC 4.1.1.39), comprising approximately 50% of the soluble protein (Wittenbach, 1978). The amount of Rubisco decreases rapidly in the early phase of leaf senescence, although more slowly in the later phase (Friedrich and Huffaker, 1980; Mae et al., 1984). In contrast, the chloroplast number remains relatively constant, making it impossible to explain Rubisco loss solely by whole chloroplast degradation. However, the mechanism of intrachloroplastic Rubisco degradation is still unknown (for review, see Feller et al., 2008). Using immunoelectron microscopy, we previously demonstrated in naturally senescing wheat (Triticum aestivum) leaves that Rubisco is released from chloroplasts into the cytoplasm and transported to the vacuole for subsequent degradation in small spherical bodies, named Rubisco-containing bodies (RCBs; Chiba et al., 2003). Similar chloroplast-derived structures were also subsequently confirmed in senescent leaves of soybean (Glycine max) and/or Arabidopsis (Arabidopsis thaliana) by electron microscopy (Otegui et al., 2005), and recently in tobacco (Nicotiana tabacum) leaves by immunoelectron microscopy, although the authors gave them a different name, Rubisco vesicular bodies (Prins et al., 2008). RCBs have double membranes, which seem to be derived from the chloroplast envelope; thus, the RCB-mediated degradation of stromal proteins represents a potential mechanism for chloroplast shrinkage during senescence. We recently demonstrated that Rubisco and stroma-targeted fluorescent proteins can be mobilized to the vacuole by ATG-dependent autophagy via RCBs, using leaves treated with concanamycin A, a vacuolar H+-ATPase inhibitor (Ishida et al., 2008). To investigate further, we wished to observe chloroplast autophagy and degradation directly in living cells to determine whether autophagy is responsible for chloroplast shrinkage and whether it is involved in the vacuolar degradation of whole chloroplasts during leaf senescence.
Autophagy is known to be a major system for the bulk degradation of intracellular proteins and organelles in the vacuole in yeast and plants, or the lysosome in animals (for detailed mechanisms, see reviews by Ohsumi, 2001; Levine and Klionsky, 2004; Thompson and Vierstra, 2005; Bassham et al., 2006). In those systems, a portion of the cytoplasm, including entire organelles, is engulfed in membrane-bound vesicles and delivered to the vacuole/lysosome. A recent genome-wide search confirmed that Arabidopsis has many genes homologous to the yeast autophagy genes (ATGs; Doelling et al., 2002; Hanaoka et al., 2002; for detailed functions of ATGs, see the reviews noted above). Using knockout mutants of ATGs and a monitoring system with an autophagy marker, GFP-ATG8, numerous studies have demonstrated the presence of the autophagy system in plants and its importance in several biological processes (Yoshimoto et al., 2004; Liu et al., 2005; Suzuki et al., 2005; Thompson et al., 2005; Xiong et al., 2005, 2007; Fujiki et al., 2007; Phillips et al., 2008). These articles suggest that autophagy plays an important role in nutrient recycling during senescence, especially in nutrient-starved plants. The atg mutants exhibited an accelerated loss of some chloroplast proteins, but not all, under nutrient-starved conditions and during senescence, suggesting that autophagy is not the sole mechanism for the degradation of chloroplast proteins; other, as yet unidentified systems must be responsible for the degradation of chloroplast contents when the ATG system is compromised (Levine and Klionsky, 2004; Bassham et al., 2006). However, it still remains likely that autophagy is responsible for the vacuolar degradation of chloroplasts in wild-type plants.
Prolonged observation is generally required to follow leaf senescence events in naturally aging leaves and senescence-associated processes tend to become chaotic over time. To observe chloroplast degradation over a short period, and to draw clear conclusions, a suitable experimental model of leaf senescence is required. Weaver and Amasino (2001) reported that senescence is rapidly induced in individually darkened leaves (IDLs) of Arabidopsis, but retarded in plants subjected to full darkness. In addition, Keech et al. (2007) observed a significant decrease of both the number and size of chloroplasts in IDLs within 6 d.
In this study, using IDLs as a senescence model, we aimed to investigate the involvement of autophagy in chloroplast degradation. We show direct evidence for the transport of whole chloroplasts and RCBs to the vacuole by autophagy.
RESULTS
Senescence Induced in IDLs Is Normal in Both Wild-Type and atg Mutant Arabidopsis
To examine the role of autophagy in chloroplast degradation, we chose a T-DNA insertion double mutant of ATG4, atg4a4b-1, which completely lacks autophagy (Yoshimoto et al., 2004). We first observed senescence in IDLs of wild type and atg4a4b-1. Dark treatment was applied to the expanding third and fourth rosette leaves (Fig. 1A, day 0) when both wild-type and atg4a4b-1 plants started bolting under long-day conditions. During the treatment, there were no apparent differences between wild type and atg4a4b-1 in the control condition or in the unshaded leaves of the IDL-treated plants (Fig. 1A, day 3). In both plants, darkened leaves became pale after 5-d treatment (Fig. 1A, day 5). SAG12 and SEN1 are well-known senescence markers (Weaver et al., 1998). In IDLs, expressions of both genes were accelerated. Over the course of leaf senescence, photosynthesis-related nuclear genes were preferentially down-regulated. The expression of RBCS2B, coding for the Rubisco small subunit, and CAB2B, coding for the light-harvesting chlorophyll a/b binding protein, was suppressed in IDLs more rapidly than under control condition in both plants.
Figure 1.
Induction of senescence in IDLs of wild-type and atg4a4b-1 plants. A, Photographs of wild-type and atg4a4b-1 plants before (day 0) and after (day 3) the 3-d treatment and their control leaves and IDLs after the 5-d treatment (day 5). The third and fourth rosette leaves, in order of appearance (indicated on the day 0 plants), were individually covered just after bolting and the plants grown for a further 5 d. B, Expression of SAG12, SEN1, RBCS2B, and CAB2B in control leaves and IDLs of wild-type and atg4a4b-1 plants during the 5-d treatment. Total RNA from third and fourth leaves of each plant was isolated and subjected to semiquantitative RT-PCR using gene-specific primers. 18s rRNA was used as an internal standard.
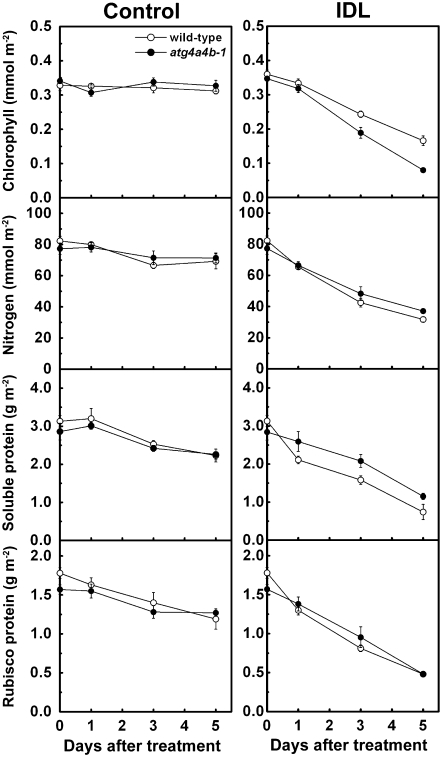
In both wild type and atg4a4b-1, a decrease in the amount of chlorophyll in IDLs was readily observable (Fig. 1A), as compared to control conditions (Fig. 2). Under control conditions, leaf nitrogen, soluble protein, and Rubisco protein concentrations slowly declined in both wild-type and the mutant plants (Fig. 2). By contrast, IDLs exhibited much higher rates of loss of them in both plants (Fig. 2). These data confirmed that senescence is rapidly induced in IDLs within short periods under our experimental conditions, similar to previous studies (Weaver and Amasino, 2001; Keech et al., 2007).
Figure 2.
Changes in the chlorophyll, nitrogen, soluble protein, and Rubisco protein concentrations in control leaves and IDLs of wild type (white circles) and atg4a4b-1 (black circles) over the treatment period. Data are means ± sd (n = 3).
The Number and Size of Chloroplasts Decreased in Wild-Type Arabidopsis, But Not in atg Mutants
In IDLs of wild type, all ATG transcripts analyzed were steadily up-regulated during the treatment (Fig. 3), suggesting that autophagy may be responsible for the noted degradative processes during senescence. In atg4a4b-1, the expression of ATG, ATG5, ATG7, and ATG9 were still up-regulated (Fig. 3), although the processing reaction of ATG8 was compromised and the progression of autophagy was completely prevented (Yoshimoto et al., 2004).
Figure 3.
Expression of ATGs in control leaves and IDLs of wild-type and atg4a4b-1 plants over the treatment period. Total RNA from third and fourth leaves of each plant was isolated and subjected to semiquantitative RT-PCR using gene-specific primers. 18s rRNA was used as an internal standard. DAT, Days after treatment.
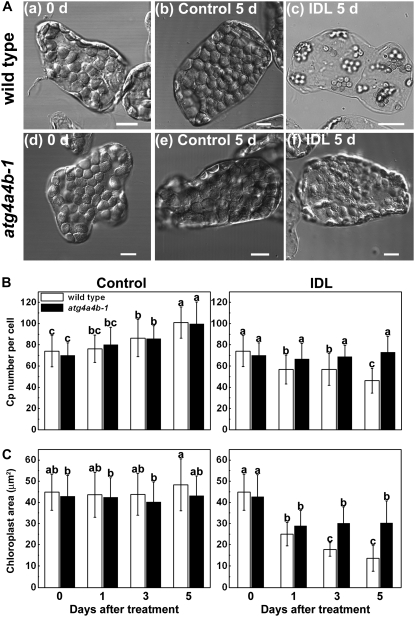
Changes in the number and the area of chloroplasts were measured during the 5-d treatment in both wild type and atg4a4b-1 (Fig. 4). After 5 d under control conditions, chloroplasts still retained their ellipsoid shape and no apparent differences were evident between wild-type and atg4a4b-1 cells (Fig. 4A). By contrast, the occupancy of chloroplasts in the cell apparently decreased in IDLs, with the reduction more severe in wild-type cells than atg4a4b-1 cells (Fig. 4A). After 5-d IDL treatment, chloroplasts had a round shape in wild-type cells (Fig. 4A). The chloroplast number gradually increased in both plants under control conditions (Fig. 4B), suggesting that mesophyll cells were still slightly expanding during the experimental period. Conversely, in the IDL treatment, chloroplast number significantly decreased in wild-type cells, while remaining constant in atg4a4b-1 (Fig. 4B). Chloroplast area remained constant in both plants under control conditions (Fig. 4C), but gradually decreased in wild-type plants in the IDL treatment (Fig. 4C). After 5-d treatment, the wild-type chloroplasts had shrunk by one-third. However, in the IDLs of atg4a4b-1, the area decreased after 1 d and remained constant thereafter (Fig. 4C). These results strongly suggest that autophagy is responsible for the decrease in chloroplast number and partly for the decrease in chloroplast size.
Figure 4.
Visible chloroplast catabolism proceeds in IDLs of wild-type plants, but is suppressed in those of atg4a4b-1. A, Differential interference contrast images of chloroplasts in mesophyll cells separated from leaves of wild-type (a) and atg4a4b-1 (d) plants before the treatment, from control leaves of wild-type (b) and atg4a4b-1 (e) plants after the 5-d treatment, and from IDLs of wild-type (c) and atg4a4b-1 (f) plants after the 5-d treatment. Leaves were cut into small pieces, fixed with 3.5% glutaraldehyde, and mesophyll cells were individually dispersed on the glass plate and observed by microscopy. Bars = 10 μm. B and C, Changes in the number (B) and the area (C) of chloroplasts in control leaves and IDLs of wild-type (white columns) and atg4a4b-1 (black columns) plants during the treatment. Chloroplasts in mesophyll cells were identified by those exhibiting chlorophyll autofluorescence during LSCM. The number of chloroplasts per cell and their length and breadth were counted on differential interference contrast images (shown as A) of mesophyll cells separated from leaves. Chloroplast area was calculated using the assumption that chloroplasts were ovals. Data are means ± sd (n = 50 in the no. per cell; n = 45 in the area). Statistical analysis was performed by Tukey-Kramer's HSD test. Different letters in each graph denote differences at P ≤ 0.01.
Direct Evidence for the Mobilization of Whole Chloroplasts and RCBs to the Vacuole for Autophagy in IDLs
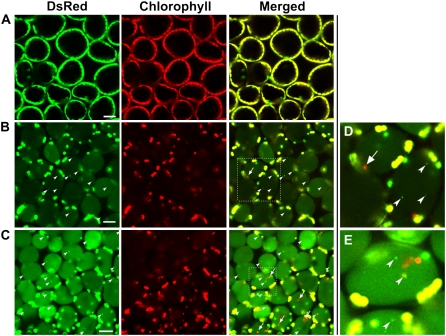
To directly demonstrate the autophagy-dependent transport of whole chloroplasts and RCBs to the vacuole in IDLs, we first observed the fate of chloroplast components in living mesophyll cells by laser-scanning confocal microscopy (LSCM; Fig. 5), using transgenic Arabidopsis expressing chloroplast stroma-targeted DsRed (CT-DsRed; Ishida et al., 2008). As previously reported, DsRed fluorescence was detected within chloroplasts, but not in the vacuole of mesophyll cells when excised leaves of these plants grown under control conditions were immediately observed (Ishida et al., 2008; Fig. 5A). By contrast, a faint signal of DsRed fluorescence was observed in the vacuolar lumen in mesophyll cells of IDLs (Fig. 5B). When the control leaves and IDLs of CT-DsRed-containing plants were analyzed by immunoblotting with anti-DsRed antibodies following SDS-PAGE, a single band of 28.3-kD, which corresponds to the mature form of CT-DsRed after cleavage of the transit sequence, was detected in both leaves (Supplemental Fig. S1). On the other hand, a 34.0-kD band that would correspond to the premature form of DsRed carrying the RECA transit peptide was not found, even upon overexposure of the immunoblot, suggesting that DsRed fluorescence found in the vacuole of IDLs reflects the mobilization of the stroma-localized proteins to the vacuole.
Figure 5.
Visualization of stroma-targeted DsRed and chlorophyll autofluorescence in living mesophyll cells of wild-type plants by LSCM. A and B, Fresh control leaves (A) and IDLs (B) excised from plants after the 5-d treatment and observed immediately. C, IDLs from plants after the 5-d treatment incubated with 1 μm concanamycin A in 10 mm MES-NaOH (pH 5.5) at 23°C for 20 h in darkness. Stroma-targeted DsRed appears green and chlorophyll fluorescence appears red. In merged images, overlap of DsRed and chlorophyll fluorescence appears yellow. Vesicles with DsRed are represented by white arrowheads. Chloroplasts having only chlorophyll fluorescence are represented by arrows. D and E, Magnifications of vesicles and chloroplasts having only chlorophyll fluorescence indicated by the dashed-line areas in B and C, respectively. Bars = 25 μm.
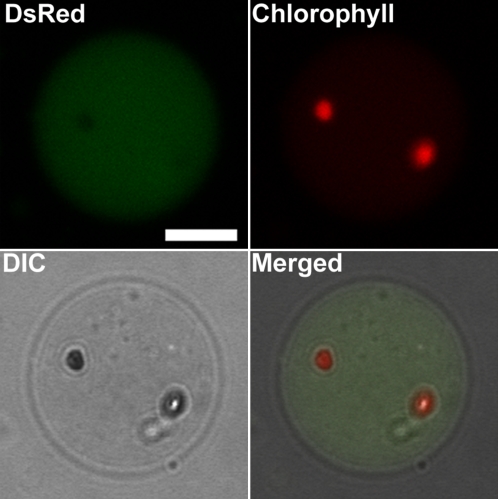
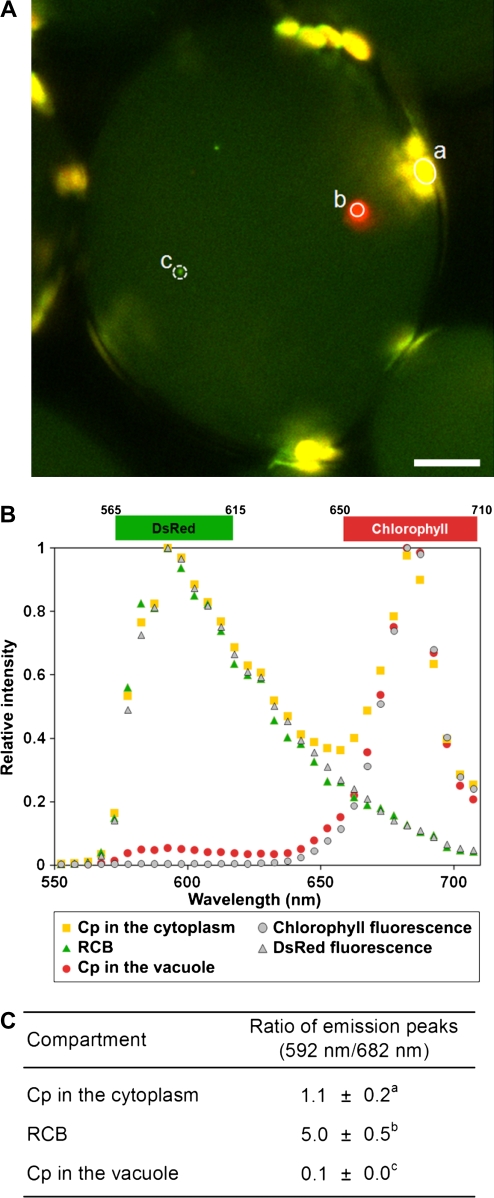
A few spherical DsRed fluorescent bodies, namely, RCBs (Ishida et al., 2008), were also observed in the vacuolar lumen of IDLs (Fig. 5B, arrowhead). Detection of RCBs increased during the IDL treatment (Table I). In addition, some cells in IDLs after 3 and 5 d contained chloroplasts without DsRed fluorescence (Fig. 5B, arrow; Table I). While chloroplasts are normally observed around the cell periphery, the noted chloroplasts exhibited Brownian motion, suggesting a vacuolar location, along with the RCBs. To further confirm the location of chloroplasts without DsRed fluorescence within cells, vacuoles were isolated from IDLs. DsRed-free chloroplasts were found within some of the isolated vacuoles exhibiting slow random motion (Fig. 6; Supplemental Video S1). DsRed-free chloroplasts in the vacuole were 3 to 4 μm in diameter, slightly smaller than chloroplasts in the cytoplasm, but rather bigger than RCBs, which were approximately 1 μm in diameter. When the fluorescence spectra of these chloroplasts in the vacuole and RCBs were investigated in living cells of IDLs (Fig. 7), RCBs exhibited the same fluorescence spectrum as chlorophyll-free amyloplasts in the roots of the transgenic Arabidopsis expressing plastid-targeted DsRed. Meanwhile, chloroplasts in the vacuole had no fluorescent peak corresponding to DsRed, but showed the specific chlorophyll autofluorescence spectrum. These data suggest that RCBs and chloroplasts in the vacuole are clearly distinguishable. Concanamycin A, an inhibitor of vacuolar H+-ATPase, is used to suppress vacuolar lytic activity by increasing the interior pH of the vacuole and to visualize autophagic bodies (Yoshimoto et al., 2004; Thompson et al., 2005). RCBs have also been identified in living cells in the presence of concanamycin A (Ishida et al., 2008). In light of these reports, IDLs were excised and treated with concanamycin A to monitor accumulated substances in the vacuole more clearly. After the treatment, stroma-targeted DsRed fluorescence was clearly observable in the vacuolar lumen (Fig. 5C) and both RCBs and chloroplasts were visible in higher numbers in the presence of concanamycin A after the treatment (Fig. 5, B and C; Table I).
Table I.
Quantitative analysis of vacuolar transfers of RCBs, chloroplasts, and stroma-targeted DsRed
RCB and chloroplast columns indicate cell numbers having RCBs or chloroplasts in the vacuole per 100 cell sections observed by LSCM. Intensity of DsRed in the vacuole of wild-type background plants represents the relative intensity of DsRed accumulated in the vacuole as the IDL 5-d + concanamycin A (conA) 20-h criterion. Fluorescence intensity is measured at cell center spots of images captured using the same laser power and detector gain. Values are means ± sd (n = 50). Different letters represent significant difference statistically analyzed by Tukey-Kramer's HSD test (P ≤ 0.01). The correlation coefficient between treatment time and RCB observability is 0.9867.
| Treatment | Span for Treatment | RCB
|
Whole Cp
|
Intensity of DsRed in Vacuole | ||
|---|---|---|---|---|---|---|
| Wild Type | atg4a4b-1 | Wild Type | atg4a4b-1 | |||
| Control | 5 d | 0 | 0 | 0 | 0 | 4.1 ± 2.6d |
| IDLs | 1 d | 21 | 0 | 0 | 0 | 20.0 ± 8.1c |
| 3 d | 49 | 0 | 4 | 0 | 32.6 ± 13c | |
| 5 d | 66 | 0 | 5 | 0 | 68.3 ± 20b | |
| 5 d + conA 20 h | 97 | 0 | 43 | 0 | 100.0 ± 37a | |
Figure 6.
Detection of chloroplasts exhibiting chlorophyll fluorescence in vacuoles isolated from wild-type IDLs. Vacuoles were released from the thermally lysed protoplasts, which were prepared from 5-d-treated IDLs of plants expressing stroma-targeted DsRed, and were fractionated by a Ficoll density gradient. Stroma-targeted DsRed appears green, chlorophyll fluorescence appears red, and an image by differential interference contrast (DIC) is shown in gray. Some isolated vacuoles accumulated both stroma-targeted DsRed and chloroplasts shown as the overlap of DIC images and chlorophyll fluorescence. Merged images are taken from Supplemental Video S1. Bar = 10 μm.
Figure 7.
Distinction between RCBs and chloroplasts incorporated into the vacuole by fluorescence spectra. A, Visualization of RCBs and chloroplasts in the vacuole of excised IDLs by LSCM with a spectral detector. Emission between 565 and 615 nm reflecting DsRed appears pseudo-colored green, and emission between 650 and 710 nm reflecting chlorophyll fluorescence appears pseudo-colored red. The merged image is shown in which the overlap of DsRed and chlorophyll appears in yellow. Bar = 10 μm. B, Difference in fluorescence spectra of RCBs and chloroplasts in the vacuole. Spectra between 550 and 710 nm of a chloroplast in the cytoplasm (indicated by circle a in A), a chloroplast in the vacuole (circle b in A), and a RCB (circle c in A) are obtained at 5-nm resolution and are shown as yellow squares, green triangles, and red circles, respectively. Chlorophyll fluorescence of a wild-type chloroplast (gray circles) and DsRed fluorescence of a chlorophyll-free plastid in roots of the transgenic plants (gray triangles) are shown as references. C, Difference in the ratio of peak of DsRed fluorescence (592 nm) per peak of chlorophyll fluorescence (682 nm) of RCBs and chloroplasts in the vacuole. Data are means ± sd (n = 10). Statistical analysis was performed by t test. Different letters denote differences at P ≤ 0.01.
In contrast to the results in wild-type plants, DsRed fluorescence was not observed in the vacuoles of IDLs or control condition leaves excised from CT-DsRed transgenic atg4a4b-1 plants (Table I; Supplemental Fig. S2, A and C). Even after concanamycin A treatment, accumulation of RCBs and chloroplasts exhibiting chlorophyll autofluorescence in the vacuole was not observed in atg4a4b-1 in either the leaves of control conditions or IDLs (Table I; Supplemental Fig. S2, B and D).
DISCUSSION
Although pioneer studies suggested the vacuolar uptake of chloroplasts during leaf senescence (Wittenbach et al., 1982; Minamikawa et al., 2001), direct evidence and the underpinning mechanisms have remained elusive. In addition, recent reverse-genetic studies have revealed that autophagy is responsible for the vacuolar degradation of cellular components in plants (Yoshimoto et al., 2004; Suzuki et al., 2005; Thompson et al., 2005; Xiong et al., 2005, 2007; Phillips et al., 2008). In this study, we showed that the atg4s mutation, which compromises the progression of autophagy, prevents the decrease in chloroplast number and, in part, the decrease in chloroplast size during IDL senescence. We also provide direct evidence of chloroplasts as well as their derivative structures, RCBs, being taken up by the vacuole in an autophagy-dependent manner using live cell imaging and cellular fractionation.
In IDLs of atg4a4b-1, after an initial decrease in chloroplast area, the area remained constant over the treatment period, whereas it significantly decreased in IDLs of wild-type plants (Fig. 4C). Rubisco concentration declined similarly in wild-type and atg4a4b-1 plants during entire IDL treatment period (Fig. 2), suggesting that chloroplast shrinkage does not necessarily correlate with the loss of protein. RCBs accumulate in the vacuole of IDLs in wild-type plants, but not at all in atg4a4b-1, suggesting that the derivation of RCBs from chloroplasts is partly responsible for their shrinkage. Chloroplasts in IDLs of atg4a4b-1 were a jagged ellipsoid shape, whereas they were smooth and round in IDLs of wild-type plants (Fig. 4A). A previous electron microscopy study indicated that RCBs have double membranes that are derived from the chloroplast envelope (Chiba et al., 2003). These observations indicate that the RCB formation is accompanied by the loss of chloroplast envelope resulting in chloroplast shrinkage. However, despite being unable to form RCBs, the chloroplast area decreased by approximately 70% in atg4a4b-1 within 1 d after the darkness was imposed. Although the specific cause for this decrease in chloroplast area is unknown, it may be related to the degradation of starch granules that occupy a large volume in the chloroplasts. Starch content rapidly decreases when leaves or plants are placed in darkness, even in isolated chloroplasts (Stitt and Heldt, 1981).
During IDL-induced senescence, in spite of the lack of autophagy in atg4a4b-1, the amounts of Rubisco protein and leaf nitrogen decreased at almost the same rate as control plants (Fig. 2). This suggests the presence of multiple protein degradation pathways, and that such pathways are enhanced when autophagy is defective. This may explain the increased rate of chlorophyll loss in atg mutants compared with wild-type plants (Fig. 2). This suggests that the thylakoid membranes are degraded faster in atg4a4b-1 than in wild type. However, the loss of chlorophyll did not correlate with the chloroplast shrinkage (Figs. 2 and 4), the reasons for which are unknown. Rubisco content generally decreases at a faster rate than chlorophyll content during senescence. Therefore, it is possible that a significant perturbation in senescence progression occurs when autophagy is defective because of the importance of autophagy for chloroplast degradation. Meanwhile, the plants subjected to full darkness ceased growth and leaf senescence progressed more slowly than in the IDL treatment. In the leaves of completely darkened plants, the declines in chloroplast size and number, and in Rubisco, chlorophyll and nitrogen content in both wild type and atg4a4b-1 were reduced (data not shown). Thus, the senescence of IDLs is not caused solely by the effect of darkness, but seems to be dependent upon the nutrient requirements of developing tissues.
Compared to natural and nutrient-limited senescence, which progresses gradually from the older leaves in a plant and the older parts of leaves, senescence induced by IDL treatment takes place regardless of cell age and is regulated at the cellular level. Following Weaver and Amasino (2001), using a leaf that had an IDL treatment applied using a cover that has a hole in the center, we confirmed that both visible leaf yellowing and accumulation of stroma-targeted DsRed, RCBs, and chloroplasts in the vacuole are observed only in the darkened areas of the leaf (data not shown). IDL-induced senescence, including autophagy induction, may have a physiological role in the efficient allocation of nutrients under dark conditions. IDL treatment may induce intracellular degradation more aggressively than natural aging. Therefore, IDL treatment is a useful model for observation of chloroplast degradation both in number and size in a short period; however, it is unclear how closely IDL senescence resembles natural senescence. For example, chloroplast shrinkage precedes the decrease in chloroplast numbers observed during natural and nutrient-limiting senescence (Ono et al., 1995; Inada et al., 1998). When natural aging leaves were subjected to 20-h darkness with concanamycin A, autophagy of only RCBs, not whole chloroplasts, was observed (Ishida et al., 2008). Therefore, the occurrence of autophagy of whole chloroplasts and RCBs may be dependent upon the cellular status and may be a highly regulated phenomenon for chloroplast degradation.
Recently, it has been shown that senescence-associated vacuoles (SAVs) are involved in the degradation of chloroplastic components in senescing leaves of tobacco (Martínez et al., 2008). SAVs preferentially contain stromal proteins, such as Rubisco and Gln synthetase, and chlorophyll a in some cases, but do not contain thylakoid proteins. These features are similar to those for RCBs, except that RCBs do not exhibit chlorophyll autofluorescence (Fig. 7). Most importantly, SAVs are present in leaves of the atg7 mutant of Arabidopsis (Otegui et al., 2005) in which the ATG gene-dependent autophagy, and therefore RCB production, is defective (Doelling et al., 2002; Thompson et al., 2005). Therefore, it should be considered that at least two independent transporting pathways, the ATG gene-dependent autophagy to the central vacuole and ATG-independent unidentified mechanisms to SAVs, are responsible for the degradation of stromal proteins outside chloroplasts. At present, however, we can only rarely visualize the existence of stroma-targeted fluorescent proteins other than those located in chloroplasts, including stromules and the central vacuole in natural senescing leaves or IDLs of both wild-type and autophagy-defective Arabidopsis (Ishida et al., 2008; this study).
MATERIALS AND METHODS
Plant Materials and Treatment
The Arabidopsis (Arabidopsis thaliana L. Heynh.) ecotype Wassilewskija and a T-DNA knockout mutant of atg4a4b-1 with this ecotype (Yoshimoto et al., 2004) were used in this study. Transgenic Arabidopsis Wassilewskija expressing stroma-targeted DsRed was previously described (Ishida et al., 2008). Transgenic Arabidopsis Wassilewskija with atg4a4b-1 background expressing stroma-targeted DsRed was obtained by sexual crosses. Plants were grown hydroponically on horticultural rockwool in a controlled-environment growth chamber with a 14-h photoperiod (220 μmol quanta m−2 s−1) and 22°C day/20°C night temperature.
IDL Treatment
The expanding third and fourth rosette leaves of 19-d-old plants were used for IDL treatments, except for vacuole isolation. IDL treatments were conducted as previously described (Weaver and Amasino, 2001; Keech et al., 2007), except, in the IDL treatment, third and fourth rosette leaves were covered with aluminum foil for 1, 3, or 5 d.
Semiquantitative Reverse Transcription-PCR
RNA was isolated from leaves using the RNeasy plant mini kit (Qiagen). RNA concentrations were measured spectrophotometrically and an RNA gel was run from each batch of RNA samples to check the quality of the RNA and the accuracy of the concentration. Total RNA was treated with DNase (DNA-free; Ambion) prior to the synthesis of first-strand cDNA by Superscript III first-strand synthesis system for reverse transcription (RT)-PCR with random hexamer primers (Invitrogen). Gene-specific primers used for senescence marker gene PCR were 5′-GATGAAGGCAGTGGCACACCAA-3′ and 5′-TCCCACACAAACATACACAATTAAAAGC-3′ for SAG12 (Panchuk et al., 2005); 5′-ATCACGAATTGGAAACTGG-3′ and 5′-CTTTCCTCCATCGGAAG-3′ for SEN1 (Hanaoka et al., 2002); 5′-ACCTTCTCCGCAACAAGTGG-3′ and 5′-GAAGCTTGGTGGCTTGTAGG-3′ for RBCS2B (Acevedo-Hernández et al., 2005); and 5′-TTGAAGGCTACAGAGTCGCAGGAAA-3′ and 5′-CACTCACGAAGCAAAGACTGAAGCA-3′ for CAB2B (Panchuk et al., 2005). Gene-specific primers used for autophagy gene PCR were 5′-ATGAAGGCTTTATGTGATAGATTTGTTC-3′ and 5′-TCAGAGCATTTGCCAGTCATCTTCA-3′ for ATG4a; 5′-ATGAAGGCTTTATGTGATAGATTTGTTC-3′ and 5′-GTCACACAATGAAAAGAATGGCTAGGAG-3′ for ATG4b (Yoshimoto et al., 2004); 5′-ATGGCGAAGGAAGCGGTCA-3′ and 5′-CACAAAGGAGATCGAAAAGAACAC-3′ for ATG5 (Thompson et al., 2005); 5′-AGCTCTTGAAGACCCTTCTGTG-3′ and 5′-AATCTGAGTCGCGCCAATC-3′ for ATG7; 5′-GTCCTCGACCCAGTAGGAACTATG-3′ and 5′-AGAAACTGCTTCCCAAGAGAATCTG-3′ for ATG9; and QuantumRNA 18s internal standards for 18s ribosomal RNA (rRNA; Ambion). PCR was terminated after 28 cycles for SAG12, 23 cycles for SEN1, 20 cycles for RBCS2B, CAB2B, 14 cycles for 18s rRNA, 24 cycles for ATG4a, ATG4b, and 26 cycles for ATG5, ATG7, and ATG9.
Quantification of Chlorophyll, Leaf Nitrogen, Soluble Proteins, and Rubisco Protein
Frozen third and fourth rosette leaves were homogenized in a chilled mortar and pestle in 50 mm sodium phosphate buffer (pH 7.5) containing 2 mm iodoacetic acid, 0.8% (v/v) 2-mercaptoethanol, and 5% (v/v) glycerol (Makino et al., 1988). Chlorophyll was determined by the method of Arnon (1949). Total leaf nitrogen was determined from part of the homogenate with Nessler's reagent after Kjeldahl digestion. Triton X-100 (0.1%, final concentration) was added to the remaining homogenate, which was then centrifuged at 15,000g for 10 min at 4°C, before the determination of soluble proteins and Rubisco in the supernatant. Soluble proteins were measured according to Bradford (1976) using a Bio-Rad protein assay with bovine serum albumin as the standard. The Rubisco content was also determined in the supernatant spectrophotometrically by formamide extraction of the Coomassie Brilliant Blue R-250-stained bands corresponding to the large and small subunits of Rubisco separated by SDS-PAGE using calibration curves made with bovine serum albumin.
Measurement of the Number and Size of Chloroplasts
Leaves were fixed by a procedure described by Pyke and Leech (1991). Fixed leaf tissues were dispersed and pressed on a glass slide. Individually separated cells were observed by LSCM performed with a Nikon C1si system equipped with a CFI Plan Apo VC60× water immersion objective (NA = 1.20; Nikon). Chloroplasts were identified by chlorophyll autofluorescence and the number and the size were measured in differential interference contrast images. Chloroplast number was counted with changing focus to avoid duplicate and uncounted chloroplasts. Measurements of chloroplast length and breadth were taken only on chloroplasts in the center of the cell, while chloroplast areas were calculated with the assumption that chloroplasts were oval.
Image Analysis with LSCM
LSCM was performed with a Nikon C1si system equipped with a CF1 Plan Apo VC60 × water immersion objective (NA = 1.20; Nikon). Leaves were mounted on slides with coverslips. DsRed was excited with the combination of the 488-nm line of a multi-argon ion laser and the 543-nm line of a helium-neon laser. Chlorophyll was excited with the 488-nm line of a multi-Argon ion laser. The emission of DsRed and chlorophyll was detected between 590 and 650 nm, and over 650 nm by a multichannel detector with filters. For obtaining fluorescence spectra of RCBs and chloroplasts in the vacuole, DsRed and chlorophyll were excited using the above method and fluorescence spectra between 550 and 710 nm were obtained with a 5-nm resolution using the spectral detector of the Nikon C1si system. The concanamycin A treatment was applied with the procedure described in Ishida et al. (2008).
Isolation of Vacuoles from IDLs
Vacuoles were isolated by the method of Robert et al. (2007), with a slight modification that applied gradient fractionation with 0%, 6%, and 10% Ficoll solution.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Diagram of the gene construct of stroma-targeted DsRed.
Supplemental Figure S2. Visualization of stroma-targeted DsRed and chlorophyll autofluorescence in living mesophyll cells of atg4a4b-1.
Supplemental Video S1. Movement of chloroplasts lacking CT-DsRed in a vacuole isolated from IDLs of wild-type plants. The movie runs at 3× normal speed.
Supplementary Material
Acknowledgments
We thank Dr. Louis Irving and Dr. Maureen Hanson for critical reading of the manuscript.
This work was supported by Grants-in-Aid for Scientific Research (grant nos. 19039004, 20780044, and 20200061 to H.I.) and the Ministry of Agriculture, Forestry and Fisheries of Japan (grant for the Integrated Research Project for Plant, Insect and Animal using Genome Technology [grant no. GPN 0007] to A.M.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Hiroyuki Ishida (hiroyuki@biochem.tohoku.ac.jp).
The online version of this article contains Web-only data.
Open access articles can be viewed online without a subscription.
References
- Acevedo-Hernández GJ, Leon P, Herrera-Estrella LR (2005) Sugar and ABA responsiveness of a minimal RBCS light-responsive unit is mediated by direct binding of ABI4. Plant J 43 506–519 [DOI] [PubMed] [Google Scholar]
- Arnon DI (1949) Copper enzymes in isolated chloroplasts—polyphenoloxidase in beta-vulgaris. Plant Physiol 24 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham DC, Laporte M, Marty F, Moriyasu Y, Ohsumi Y, Olsen LJ, Yoshimoto K (2006) Autophagy in development and stress responses of plants. Autophagy 2 2–11 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 72 248–254 [DOI] [PubMed] [Google Scholar]
- Chiba A, Ishida H, Nishizawa NK, Makino A, Mae T (2003) Exclusion of ribulose-1,5-bisphosphate carboxylase/oxygenase from chloroplasts by specific bodies in naturally senescing leaves of wheat. Plant Cell Physiol 44 914–921 [DOI] [PubMed] [Google Scholar]
- Doelling JH, Walker JM, Friedman EM, Thompson AR, Vierstra RD (2002) The APG8/12-activating enzyme APG7 is required for proper nutrient recycling and senescence in Arabidopsis thaliana. J Biol Chem 277 33105–33114 [DOI] [PubMed] [Google Scholar]
- Feller U, Anders I, Mae T (2008) Rubiscolytics: fate of Rubisco after its enzymatic function in a cell is terminated. J Exp Bot 59 1615–1624 [DOI] [PubMed] [Google Scholar]
- Friedrich JW, Huffaker RC (1980) Photosynthesis, leaf resistances, and ribulose-1,5-bisphosphate carboxylase degradation in senescing barley leaves. Plant Physiol 65 1103–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y, Yoshimoto K, Ohsumi Y (2007) An Arabidopsis homolog of yeast ATG6/VPS30 is essential for pollen germination. Plant Physiol 143 1132–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaoka H, Noda T, Shirano Y, Kato T, Hayashi H, Shibata D, Tabata S, Ohsumi Y (2002) Leaf senescence and starvation-induced chlorosis are accelerated by the disruption of an Arabidopsis autophagy gene. Plant Physiol 129 1181–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörtensteiner S, Feller U (2002) Nitrogen metabolism and remobilization during senescence. J Exp Bot 53 927–937 [DOI] [PubMed] [Google Scholar]
- Inada N, Sakai A, Kuroiwa H, Kuroiwa T (1998) Three-dimensional analysis of the senescence program in rice (Oryza sativa L.) coleoptiles—investigations of tissues and cells by fluorescence microscopy. Planta 205 153–164 [DOI] [PubMed] [Google Scholar]
- Ishida H, Yoshimoto K, Izumi M, Reisen D, Yano Y, Makino A, Ohsumi Y, Hanson MR, Mae T (2008) Mobilization of Rubisco and stroma-localized fluorescent proteins of chloroplasts to the vacuole by an ATG gene-dependent autophagy process. Plant Physiol 148 142–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keech O, Pesquet E, Ahad A, Askne A, Nordvall D, Vodnala SM, Tuominen H, Hurry V, Dizengremel P, Gardestrom P (2007) The different fates of mitochondria and chloroplasts during dark-induced senescence in Arabidopsis leaves. Plant Cell Environ 30 1523–1534 [DOI] [PubMed] [Google Scholar]
- Krupinska K (2006) Fate and activity of plastids during leaf senescence. In RR Wise, JK Hoober, eds, The Structure and Function of Plastids. Springer, Dordrecht, The Netherlands, pp 433–449
- Kura-Hotta M, Hashimoto H, Satoh K, Katoh S (1990) Quantitative-determination of changes in the number and size of chloroplasts in naturally senescing leaves of rice seedlings. Plant Cell Physiol 31 33–38 [Google Scholar]
- Levine B, Klionsky DJ (2004) Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 6 463–477 [DOI] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Czymmek K, Talloczy Z, Levine B, Dinesh-Kumar SP (2005) Autophagy regulates programmed cell death during the plant innate immune response. Cell 121 567–577 [DOI] [PubMed] [Google Scholar]
- Mae T, Kai N, Makino A, Ohira K (1984) Relation between ribulose bisphosphate carboxylase content and chloroplast number in naturally senescing primary leaves of wheat. Plant Cell Physiol 25 333–336 [Google Scholar]
- Makino A, Mae T, Ohira K (1988) Differences between wheat and rice in the enzymic properties of ribulose-1,5-bisphosphate carboxylase oxygenase and the relationship to photosynthetic gas-exchange. Planta 174 30–38 [DOI] [PubMed] [Google Scholar]
- Makino A, Osmond B (1991) Effects of nitrogen nutrition on nitrogen partitioning between chloroplasts and mitochondria in pea and wheat. Plant Physiol 96 355–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez DE, Costa ML, Gomez FM, Otegui MS, Guiamet JJ (2008) ‘Senescence-associated vacuoles’ are involved in the degradation of chloroplast proteins in tobacco leaves. Plant J 56 196–206 [DOI] [PubMed] [Google Scholar]
- Minamikawa T, Toyooka K, Okamoto T, Hara-Nishimura I, Nishimura M (2001) Degradation of ribulose-bisphosphate carboxylase by vacuolar enzymes of senescing French bean leaves: immunocytochemical and ultrastructural observations. Protoplasma 218 144–153 [DOI] [PubMed] [Google Scholar]
- Ohsumi Y (2001) Molecular dissection of autophagy: two ubiquitin-like systems. Nat Rev Mol Cell Biol 2 211–216 [DOI] [PubMed] [Google Scholar]
- Ono K, Hashimoto H, Katoh S (1995) Changes in the number and size of chloroplasts during senescence of primary leaves of wheat grown under different conditions. Plant Cell Physiol 36 9–17 [Google Scholar]
- Otegui MS, Noh YS, Martínez DE, Vila Petroff MG, Staehelin LA, Amasino RM, Guiamet JJ (2005) Senescence-associated vacuoles with intense proteolytic activity develop in leaves of Arabidopsis and soybean. Plant J 41 831–844 [DOI] [PubMed] [Google Scholar]
- Panchuk II, Zentgraf U, Volkov RA (2005) Expression of the Apx gene family during leaf senescence of Arabidopsis thaliana. Planta 222 926–932 [DOI] [PubMed] [Google Scholar]
- Phillips AR, Suttangkakul A, Vierstra RD (2008) The ATG12-conjugating enzyme ATG10 is essential for autophagic vesicle formation in Arabidopsis thaliana. Genetics 178 1339–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins A, Van Heerden PDR, Olmos E, Kunert KJ, Foyer CH (2008) Cysteine proteinases regulate chloroplast protein content and composition in tobacco leaves: a model for dynamic interactions with ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) vesicular bodies. J Exp Bot 59 1935–1950 [DOI] [PubMed] [Google Scholar]
- Pyke KA, Leech RM (1991) Rapid image-analysis screening-procedure for identifying chloroplast number mutants in mesophyll-cells of Arabidopsis thaliana (L.) Heynh. Plant Physiol 96 1193–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert S, Zouhar J, Carter C, Raikhel N (2007) Isolation of intact vacuoles from Arabidopsis rosette leaf-derived protoplasts. Nat Protocols 2 259–262 [DOI] [PubMed] [Google Scholar]
- Stitt M, Heldt HW (1981) Physiological rates of starch breakdown in isolated intact spinach-chloroplasts. Plant Physiol 68 755–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki NN, Yoshimoto K, Fujioka Y, Ohsumi Y, Inagaki F (2005) The crystal structure of plant ATG12 and its biological implication in autophagy. Autophagy 1 119–126 [DOI] [PubMed] [Google Scholar]
- Thompson AR, Doelling JH, Suttangkakul A, Vierstra RD (2005) Autophagic nutrient recycling in Arabidopsis directed by the ATG8 and ATG12 conjugation pathways. Plant Physiol 138 2097–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AR, Vierstra RD (2005) Autophagic recycling: lessons from yeast help define the process in plants. Curr Opin Plant Biol 8 165–173 [DOI] [PubMed] [Google Scholar]
- Weaver LM, Amasino RM (2001) Senescence is induced in individually darkened Arabidopsis leaves but inhibited in whole darkened plants. Plant Physiol 127 876–886 [PMC free article] [PubMed] [Google Scholar]
- Weaver LM, Gan SS, Quirino B, Amasino RM (1998) A comparison of the expression patterns of several senescence-associated genes in response to stress and hormone treatment. Plant Mol Biol 37 455–469 [DOI] [PubMed] [Google Scholar]
- Wittenbach VA (1978) Breakdown of ribulose bisphosphate carboxylase and change in proteolytic activity during dark-induced senescence of wheat seedlings. Plant Physiol 62 604–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach VA, Lin W, Hebert RR (1982) Vacuolar localization of proteases and degradation of chloroplasts in mesophyll protoplasts from senescing primary wheat leaves. Plant Physiol 69 98–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Contento AL, Bassham DC (2005) AtATG18a is required for the formation of autophagosomes during nutrient stress and senescence in Arabidopsis thaliana. Plant J 42 535–546 [DOI] [PubMed] [Google Scholar]
- Xiong Y, Contento AL, Nguyen PQ, Bassham DC (2007) Degradation of oxidized proteins by autophagy during oxidative stress in Arabidopsis. Plant Physiol 143 291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto K, Hanaoka H, Sato S, Kato T, Tabata S, Noda T, Ohsumi Y (2004) Processing of ATG8s, ubiquitin-like proteins, and their deconjugation by ATG4s are essential for plant autophagy. Plant Cell 16 2967–2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.