Abstract
Pathogen-induced stomatal closure is part of the plant innate immune response. Phytopathogens using stomata as a way of entry into the leaf must avoid the stomatal response of the host. In this article, we describe a factor secreted by the bacterial phytopathogen Xanthomonas campestris pv campestris (Xcc) capable of interfering with stomatal closure induced by bacteria or abscisic acid (ABA). We found that living Xcc, as well as ethyl acetate extracts from Xcc culture supernatants, are capable of reverting stomatal closure induced by bacteria, lipopolysaccharide, or ABA. Xcc ethyl acetate extracts also complemented the infectivity of Pseudomonas syringae pv tomato (Pst) mutants deficient in the production of the coronatine toxin, which is required to overcome stomatal defense. By contrast, the rpfF and rpfC mutant strains of Xcc, which are unable to respectively synthesize or perceive a diffusible molecule involved in bacterial cell-to-cell signaling, were incapable of reverting stomatal closure, indicating that suppression of stomatal response by Xcc requires an intact rpf/diffusible signal factor system. In addition, we found that guard cell-specific Arabidopsis (Arabidopsis thaliana) Mitogen-Activated Protein Kinase3 (MPK3) antisense mutants were unresponsive to bacteria or lipopolysaccharide in promotion of stomatal closure, and also more sensitive to Pst coronatine-deficient mutants, showing that MPK3 is required for stomatal immune response. Additionally, we found that, unlike in wild-type Arabidopsis, ABA-induced stomatal closure in MPK3 antisense mutants is not affected by Xcc or by extracts from Xcc culture supernatants, suggesting that the Xcc factor might target some signaling component in the same pathway as MPK3.
Foliar bacterial phytopathogens initially colonize the leaf surface as epiphytes, but subsequently become endophytes as the infection progresses. Because bacteria cannot directly penetrate the leaf epidermis, endophytic colonization occurs through natural openings, such as hydathodes and stomata, or through accidental wounds. Stomata are small pores located in the leaf surface that allow plants to exchange gases with the environment. Stomatal apertures are finely regulated in response to hormones and environmental factors such as light intensity, air humidity, and CO2 concentration, which allow the plant to maximize CO2 intake required for photosynthesis, while minimizing water loss. Several internal and external stimuli, such as the hormone abscisic acid (ABA), low humidity, or a high concentration of CO2, can bring about stomatal closure through a reduction in turgor of the two guard cells that constitute the stomatal pore. This is achieved at least in part through the efflux of osmotically active ions from these cells (Schroeder et al., 2001; Pandey et al., 2007).
Because stomata are the most abundant pores in the leaf surface, they are potential candidates to serve as a way of entry of pathogens into the leaf. During evolution, stomata have acquired the capacity of responding not only to changing concentration of gases and to internal stimuli, but also to the presence of microorganisms on the leaf surface. The fungal elicitors oligogalacturonic acid and chitosan are known to promote stomatal closure (Lee et al., 1999; Klusener et al., 2002), and, more recently, it has been shown that both phytopathogenic and nonphytopathogenic living bacteria can also promote stomatal closure through the pathogen-associated molecular patterns (PAMPs), flagellin, and lipopolysaccharide (LPS; Melotto et al., 2006). Therefore, stomata effectively function as part of the plant innate immune response.
Pathogens have in turn evolved strategies to overcome stomatal defense. For example, the toxin fusicoccin, produced by the fungal phytopathogen Fusicoccum amygdali, promotes stomatal opening through the activation of a plasma membrane H+-ATPase (Emi et al., 2001). The phytopathogenic fungi Rhynchosporium secalis and Plasmopara viticola have also been reported to modulate stomatal behavior (Allegre et al., 2007). One phytopathogenic bacterium, Pseudomonas syringae pv tomato (Pst) strain DC3000, has also been found to modulate stomatal movements through coronatine (Melotto et al., 2006), a secreted polyketide toxin. The enzymes required for the biosynthesis of this toxin are encoded in a plasmid or chromosome of some pathovars of P. syringae (Young et al., 1992; Bender et al., 1999; Melotto et al., 2006).
Little is known about the signaling events downstream of PAMPs in guard cells leading eventually to promotion of stomatal closure. The fungal elicitors oligogalacturonic acid and chitosan promote the synthesis of reactive oxygen species (ROS) in guard cells (Lee et al., 1999). More recently, it was found that bacteria-induced stomatal closure in Arabidopsis (Arabidopsis thaliana) requires the synthesis of nitric oxide and that is compromised in mutant plants with reduced levels of salicylic acid or ABA, as well as in the guard cell-specific OST1 kinase mutants (Melotto et al., 2006). Responses to pathogens, as well as to many environmental stimuli and hormones, are mediated by mitogen-activated protein kinase (MAPK) cascades in plants as well as in other eukaryotes (Morris, 2001; Colcombet and Hirt, 2008). Because MAPKs are also known to play a role in the control of stomatal movements (Wang and Song, 2008), they are good candidates to participate in the stomatal response to pathogens. In particular, Mitogen-Activated Protein Kinase3 (MPK3) is activated in mesophyll protoplasts (Asai et al., 2002; Merkouropoulos and Shirsat, 2003) and in seedlings (Heese et al., 2007) following recognition of the bacterial elicitor flagellin, and is also involved in the control of stomatal movements in response to ABA and H2O2 (Gudesblat et al., 2007). MPK3 is also activated by ROS (Kovtun et al., 2000), which are believed to have both signaling and effector roles in the response against pathogens (Apel and Hirt, 2004). In addition, MPK3 gene transcription and/or kinase activity occurs upon interaction with fungi or with the fungal elicitor chitin (Schenk et al., 2000; Miya et al., 2007), and this kinase participates in a cascade required for the synthesis of a phytoalexin induced by the fungal pathogen Botrytis cinerea (Ren et al., 2008).
Xanthomonas campestris pv campestris (Xcc) is a bacterial intravascular phytopathogen that is the causal agent of the black rot of crucifers. It has a broad host range that includes a majority of members of the Brassicaceae family. Whereas Xcc is widely considered to use hydathodes and wounds as preferential ways of entry into the leaf, it can also penetrate this organ through stomata (Buell, 2002). In Arabidopsis, Xcc 8004 can enter the leaf through both hydathodes and stomata, and the preferred route of entry depends both on the particular Arabidopsis ecotype and on environmental conditions (Hugouvieux et al., 1998). Because at least under some conditions and in certain ecotypes Xcc can enter Arabidopsis leaves through stomata, it is likely that Xcc possesses some mechanism to overcome stomatal defense.
The rpf/diffusible signal factor (DSF) gene cluster of Xcc controls the synthesis of factors required for pathogenicity and for epiphytic survival. It regulates genes involved in motility, toxin, oxidative-stress resistance, aerobic respiration, biofilm formation, and the synthesis of extracellular hydrolytic enzymes and extracellular polysaccharides such as xanthan (Tang et al., 1991; Dow and Daniels, 1994; Barber et al., 1997; Slater et al., 1997; Vojnov et al., 2001; He et al., 2006; Torres et al., 2007). Transcriptional control of these genes is mediated by a DSF (cis-11-methyl-2-dodecenoic acid) that is responsible for cell-to-cell signaling (Barber et al., 1997; Wang et al., 2004). The DSF is synthesized by the products of the genes rpfB and rpfF belonging to the rpf virulence-regulation cluster, and is sensed by a two-component signal transduction system consisting of RpfC and RpfG. This event leads to degradation of cyclic di-GMP and subsequent activation of genes in the DSF/rpf regulon (Mole et al., 2007). Disruption of the rpfF or rpfC genes reduces the pathogenicity, the resistance to oxidative stress, and the ability of Xcc to form a biofilm (He et al., 2006; Torres et al., 2007). Many of the genes under control of the rpf/DSF system are required for the initial stages of endophytic colonization.
In this study, we found that Xcc is capable of reverting both pathogen and ABA-induced stomatal closure in Arabidopsis through a virulence factor that is secreted to the extracellular medium and whose synthesis is regulated by the rpf gene cluster. In addition, we found that expression of MPK3 in guard cells is required for both promotion of stomatal closure by bacteria and for inhibition of ABA-induced stomatal closure by the Xcc-secreted virulence factor.
RESULTS
Xcc Disables Stomatal Defense by a Mechanism Regulated by Cell-to-Cell Signaling
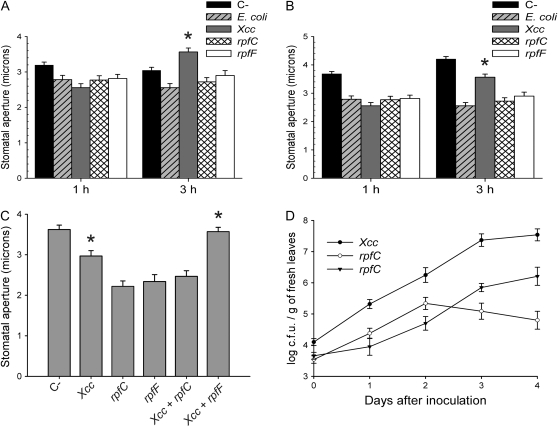
Previous evidence showing that, under some conditions, Xcc is capable of entering Arabidopsis leaves through stomata (Hugouvieux et al., 1998) prompted us to investigate whether this phytopathogen has the ability to manipulate the stomatal defense of the host. These authors reported that Xcc can enter Arabidopsis leaves through stomata in Landsberg erecta (Ler), but not in Columbia (Col-0) ecotype, at 25°C. Therefore, we investigated whether this bacterium has the ability to revert stomatal closure induced by bacteria. For this purpose, we measured promotion of stomatal closure by Xcc and by the nonphytopathogenic bacterium Escherichia coli during 1 and 3 h in both ecotypes. All bacteria promoted stomatal closure after 1 h; however, after 3 h, Xcc, but not E. coli, was capable of reopening stomata in both ecotypes (Fig. 1, A and B), showing that Xcc is capable of manipulating Arabidopsis stomatal movements to gain access into the leaf, similar to what has been described previously for coronatine (Melotto et al., 2006). Subsequent experiments were performed in the Col-0 ecotype.
Figure 1.
Reversal of bacteria-induced stomatal closure by Xcc depends on an intact rpf/DSF signaling system. A and B, Promotion of stomatal closure in epidermal peels from Arabidopsis Ler (A) or Col-0 (B) after incubation with bacterial strains during 1 or 3 h. C, Promotion of stomatal closure after 3 h by wild-type Xcc and rpf mutants, and by coincubation with two different strains. Datasets marked with an asterisk are significantly different from controls (E. coli 3 h in A and B; rpfC and rpfF in C) as assessed by Student's t test: *, P < 0.001. D, Bacterial growth after infection of Col-0 plants by dipping with Xcc strains (107 cfu/mL). The mean and se of three independent experiments are given.
The rpf gene cluster of Xcc regulates many genes required for virulence, and Xcc mutants affected in the synthesis (rpfF) and perception (rpfC) of the Xcc DSF cell-to-cell signaling molecule are less infective in Brassica campestris (Newman et al., 1994) and Nicotiana benthamiana (Torres et al., 2007). In Arabidopsis, both rpfF and rpfC mutants have severely reduced growth in plant tissue (Fig. 1D) and produce less symptoms (data not shown). Because several genes encoded in the rpf gene cluster play important roles during the initial stages of plant colonization and infection, particularly in cell-to-cell signaling, we speculated that this cluster might contain genes required for the modulation of stomatal movements. Therefore, we investigated whether the reduced virulence of the rpfF and rpfC mutants is due, at least in part, to the fact that they are affected in their capacity to revert bacteria-induced stomatal closure. We found that both mutants are unable to revert stomatal closure after 3 h (Fig. 1, A and B), providing strong evidence that genes involved in suppression of stomatal defense are under control of the rpf/DSF system.
Further evidence that the ability of Xcc to manipulate stomatal defense is controlled by the rpf/DSF system was obtained from experiments in which different Xcc strains were coinoculated. Previous reports showed that the synthesis of DSF is tightly regulated by a negative feedback mechanism (Barber et al., 1997; Slater et al., 2000), and that both reduced and increased levels of DSF interfere with biofilm formation and pathogenicity (Torres et al., 2007). Therefore, DSF can only activate transcription of genes in the rpf/DSF cluster when it is present in a certain range of concentrations. Whereas the rpfF mutant does not produce DSF, the rpfC mutant overproduces it due to lack of feedback regulation. If the synthesis of the factor responsible for reversal of stomatal closure is under control of the DSF, we predicted that the excessive amounts of DSF synthesized by the rpfC mutant, but not by rpfF, would interfere with the ability of wild-type Xcc to revert stomatal closure. We effectively found that while coincubation of Xcc with the rpfC mutant completely abolished its capacity to revert stomatal closure after 3 h, the presence of the rpfF mutant did not affect it (Fig. 1C). This result provides further proof that the synthesis of the factor that reverts stomatal closure is under control of the rpf/DSF system.
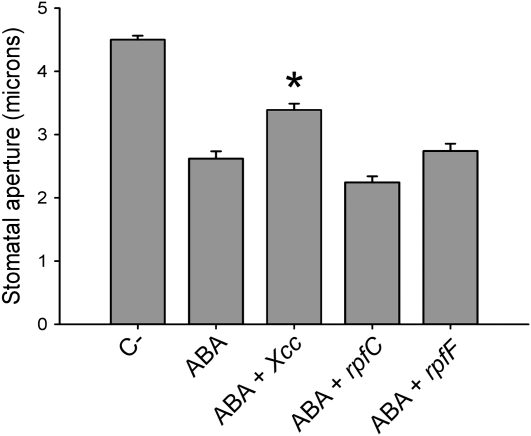
ABA is a main hormone controlling stomatal movements; for this reason, we subsequently investigated whether Xcc is capable of reverting stomatal closure induced by this hormone. We found that coincubation of epidermal peels with ABA and Xcc wild type, but not with rpfF or rpfC mutants, significantly diminished stomatal closure compared to a control treated with ABA alone (Fig. 2).
Figure 2.
Xcc, but not rpfC or rpfF, interferes with promotion of stomatal closure by ABA. Promotion of stomatal closure by ABA for 2 h in the presence of wild type of Xcc or rpf mutants. The dataset marked with an asterisk is significantly different from control (ABA) as assessed by Student's t test: *, P < 0.001. The mean and se of three independent experiments are given.
An Extract from an Xcc Culture Supernatant Affects Stomatal Movements
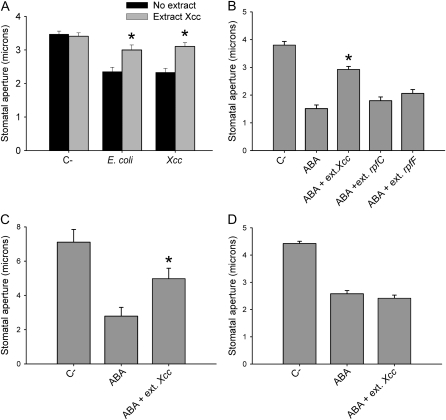
To find out the molecular basis of reversal of stomatal closure by Xcc, we investigated whether the activity responsible for the observed suppression of stomatal defense is secreted out of the bacteria. For this purpose, we extracted supernatants of bacterial cultures of Xcc and of rpfC and rpfF mutants with ethyl acetate. Subsequently, we investigated whether the extracts thus obtained can interfere with stomatal movements. We found that extracts from Xcc partially abolish stomatal closure induced by E. coli, Xcc (Fig. 3A), or ABA (Fig. 3B). Extracts from rpfC or rpfF mutants, however, did not have any inhibitory effect (Fig. 3, A and B). Also, Xcc extracts partially abolished ABA-induced stomatal closure in Vicia fava (Fig. 3C), a species evolutionarily distant from Arabidopsis, indicating that the factor capable of inhibiting stomatal closure probably targets some evolutionarily conserved process. In addition, we observed that the Xcc extract failed to prevent ABA-induced inhibition of light-induced stomatal opening (Fig. 3D), suggesting that the factor present in the Xcc extract acts specifically on a signaling element involved in promotion of closure by ABA. The extract did not affect the capacity of ABA to cause postgermination arrest (data not shown), which provides further evidence that it acts on some signaling component or effector required specifically for stomatal closure.
Figure 3.
Ethyl acetate extracts of Xcc strain culture supernatants affect stomata in a similar way as living bacteria. Promotion of stomatal closure by bacteria for 1 h (A) or ABA for 2 h (B) in the presence of extracts from Xcc strains. C, Promotion of stomatal closure in V. fava by ABA for 2 h in the presence of an extract from Xcc. D, Inhibition of stomatal opening by ABA in Arabidopsis for 2 h in the presence of an extract from Xcc. Datasets marked with an asterisk are significantly different from controls (no extract [A]; ABA [B and C]) as assessed by Student's t test: *, P < 0.001. The mean and se of three (A and B) or two (C and D) independent experiments are given.
The rpfC and rpfF Mutants Are Affected in Their Ability to Migrate through Arabidopsis Epidermal Peels
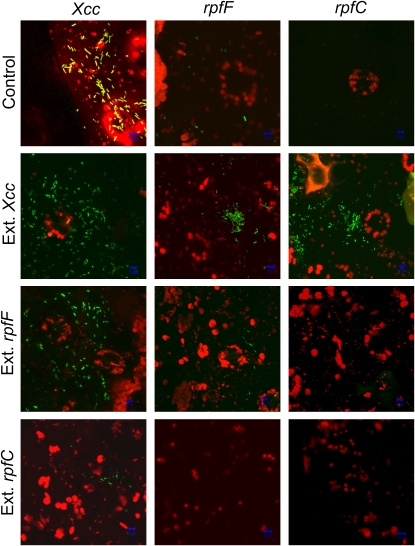
Xcc possess the ability to enter Arabidopsis leaves both through hydathodes and stomata; therefore, to study the migration of bacteria specifically through stomata, we performed an assay of bacterial migration through isolated epidermal peels as described previously by Melotto et al. (2006). In this assay, isolated epidermis of Arabidopsis are floated on a suspension of GFP-labeled bacteria, which are allowed to migrate through stomata for 3 h, and then bacteria having reached the upper side of the epidermis are observed. This assay revealed that both rpfC and rpfF mutants have greatly reduced capacity to migrate through stomata of Arabidopsis (Fig. 4), consistent with their inability to manipulate stomatal movements. The inability of rpfC and rpfF mutants to migrate through stomata in epidermal peels is possibly due to the absence of the factor that affects stomatal movements in these strains. However, due to the pleiotropic effect of mutations in rpfC and rpfF genes, it is a priori not possible to exclude that the expression of other genes involved in the migration through stomatal pores is also affected in these mutants. However, the fact that an Xcc extract restored the capacity of these mutants to migrate through epidermal peels shows that the factor present in the extract suffices to allow migration of rpf mutants through stomatal pores (Fig. 4). Therefore, the inability of rpfC and rpfF mutant strains to move through stomatal pores is likely due to the lack of the factor present in Xcc extracts, rather than to an intrinsic inability to migrate. By contrast, the extracts from rpfC and rpfF mutant culture supernatants failed to restore the capacity of these strains to migrate through epidermis. Consistent with the inhibiting effect of rpfC strain on the reversal of stomatal opening by Xcc (Fig. 1C), an extract from this mutant strain, which contains a high amount of DSF (Torres et al., 2007), inhibited the capacity of the wild-type Xcc to move through stomata (Fig. 4), suggesting again that the synthesis of the Xcc virulence factor is down-regulated by high concentrations of DSF.
Figure 4.
The ability of Xcc to migrate through stomata in isolated epidermis is dependent on a functional rpfF/DSF system. Confocal images of the inner side of detached Arabidopsis epidermis were recorded after floating them for 3 h, with the cuticle side in contact with bacterial suspensions of Xcc, rpfF, or rpfC in the presence or not of extracts of supernatants from Xcc, rpfF, or rpfC cultures. An extract from Xcc, but not from rpfF or rpfC, restored the ability of rpfF and rpfC to migrate through the epidermis. An extract from rpfC, which contains a high concentration of DSF, reduced the ability of Xcc to migrate through stomata. The experiment was repeated with similar results.
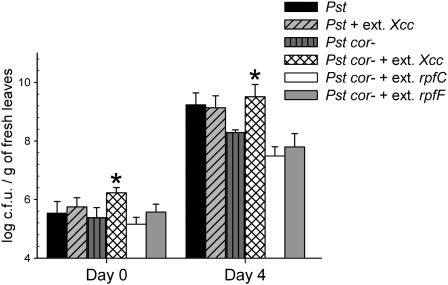
The Xcc Extract Restores the Infectivity of Pst Mutants Impaired in the Synthesis of Coronatine
Next we investigated whether the stomata-modulating activity present in the Xcc extract is relevant for bacterial pathogenicity. Because an Xcc mutant specifically affected in the ability to penetrate through stomata is not available, we analyzed whether an Xcc extract is capable of restoring the infectivity of the Pst DC3118 cor− mutant. This strain is incapable of synthesizing the toxin coronatine and, as a result, is unable to reverse bacteria-induced stomatal closure (Melotto et al., 2006). Arabidopsis plants were infected with wild-type Pst DC3000 and with DC3118 Pst cor− strain in the presence of extracts of Xcc or of rpfC and rpfF mutants. We found that the presence of an extract from Xcc, but not of extracts from rpfC or rpfF mutants, enhanced the capacity of the Pst cor− strain to grow in Arabidopsis leaves both 2 h and 4 d postinfection (Fig. 5). This finding shows that the factor synthesized by Xcc can act as a true virulence factor, enhancing the ability of infection of the Pst cor− mutant strain.
Figure 5.
Ethyl acetate extracts of Xcc strains culture supernatants can complement coronatine deficiency in Pst. Arabidopsis plants were infected by dipping with wild-type Pst DC3000 or coronatine-deficient Pst DC3118 coronatine-deficient mutant strains in the presence of wild-type Xcc or rpf mutant extracts. Bacterial growth was measured after 4 d. The datasets marked with an asterisk are significantly different from controls (Pst cor−) as assessed by Student's t test: *, P < 0.001. The mean and se of two independent experiments are given.
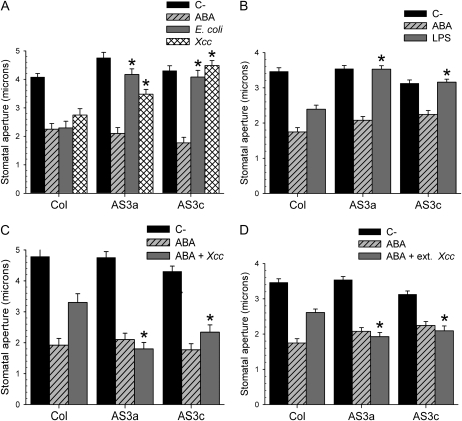
MPK3 Antisense Mutants Display Reduced Sensitivity to Promotion of Closure by Bacteria and LPS
As part of our effort to understand the mechanism of stomatal innate immunity, we investigated the possible signaling role of Arabidopsis MPK3 in bacterial and PAMP signaling in guard cells. We found that previously described Arabidopsis plants expressing an antisense RNA targeted against MPK3 mRNA driven by a guard-cell-specific promoter (Gudesblat et al., 2007) are much less sensitive to promotion of stomatal closure induced by E. coli or Xcc, but not by ABA (Fig. 6A). This result indicates that MPK3 participates in signaling downstream of pathogens in guard cells. Because promotion of stomatal closure by bacterial pathogens is mediated by PAMPs (Melotto et al., 2006), we tested the ability of one of them, LPS, to promote stomatal closure. Again, we observed a diminished response to LPS in MPK3 antisense plants compared with the wild-type plants (Fig. 6B), showing that the MPK3 signaling role in stomatal closure in response to bacteria is linked to the perception of PAMPs.
Figure 6.
Guard-cell-specific MPK3 antisense mutants are impaired in their response to bacteria, LPS, and stomatal inhibiting factor from Xcc. Promotion of stomatal closure in epidermal peels from Col-0 and two MPK3 antisense lines (AS3a and AS3c) for 1 h by bacteria (A) or by LPS (B). MPK3 antisense mutants are insensitive to the inhibitory effect of Xcc (C) or of an Xcc extract (D) on ABA-induced promotion of closure. Datasets marked with an asterisk are significantly different from controls (Col-0 controls in A, B, C, and D) as assessed by Student's t test: *, P < 0.001. The mean and se of two independent experiments are given.
MPK3 Antisense Mutants Are Insensitive to the Inhibition of ABA-Induced Promotion of Closure by Xcc and More Sensitive to Pst Mutants Lacking Coronatine
Because we determined that MPK3 mutants are impaired in bacteria-induced stomatal closure, but respond normally to ABA, we next studied the stomatal response to ABA of these plants in the presence of Xcc or of an Xcc extract. Unlike what happens in the case of wild-type Arabidopsis, neither living Xcc (Fig. 6C) nor the extract from an Xcc culture supernatant (Fig. 6D) prevented ABA-induced stomatal closure in MPK3 antisense plants. This result shows that the inhibitory effect of Xcc on ABA-induced stomatal closure requires the presence of MPK3.
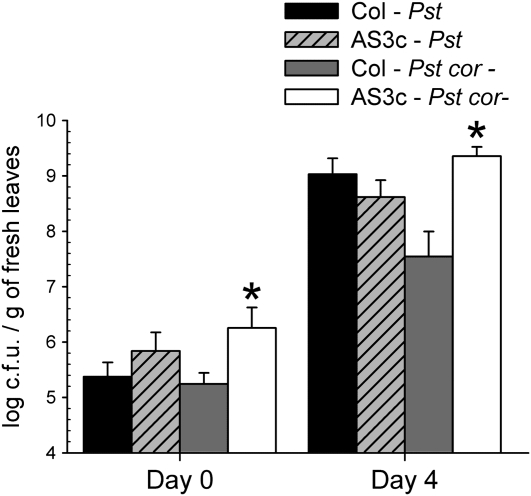
Given that MPK3 antisense plants are unable to close their stomata in response to bacteria, we predicted that in these plants coronatine would not be required for bacterial movement through stomata. When MPK3 mutant plants were infected with the Pst DC3118 strain, incapable of producing coronatine, we effectively observed that they were more sensitive to this strain than wild-type plants (Fig. 7).
Figure 7.
Inhibition of MPK3 expression in guard cells eliminates the requirement of coronatine for Pst penetration through stomata. Bacterial growth in wild-type Col-0 and MPK3 antisense mutant (AS3c) plants 4 d after infection by dipping with wild-type Pst DC3000 or the Pst DC3118 cor− mutant. The datasets marked with asterisks are significantly different from controls (Pst cor− infiltrated in wild-type Col-0) as assessed by Student's t test: *, P < 0.001.The mean and se of two independent experiments are given.
DISCUSSION
In this article, we have attempted to clarify the mechanism of endophytic colonization through stomata during the interaction between the phytopathogenic bacterium Xcc and Arabidopsis. We have found that Xcc initially promotes stomatal closure in Arabidopsis, but later it is capable of reversing it both in Ler and Col-0 ecotypes, allowing penetration into the leaf through stomata. We provide evidence that the activity responsible for reversal of stomatal closure is under control of the rpf/DSF system because we found that mutant bacteria lacking the genes rpfF or rpfC are incapable of reversing bacteria-induced stomatal closure, and that the excess of DSF produced by the rpfC mutant modulates the wild-type Xcc factor production. Xcc can also prevent ABA-induced stomatal closure, which indicates that it inhibits a signaling component or effector that is not involved exclusively in microorganism-induced stomatal responses.
As an initial step in the characterization of the Xcc activity that modulates stomatal movements, Xcc supernatants were extracted with ethyl acetate. A factor present in extracts thus obtained from wild-type Xcc is capable of preventing stomatal closure induced by E. coli, Xcc, and ABA, showing that modulation of stomatal closure by Xcc is achieved through the secretion of a molecule to the extracellular medium. The Xcc extract also prevented ABA-induced stomatal closure in Vicia faba, a distant relative of Arabidopsis; thus, its action is not limited to species within the host range of Xcc and probably targets some evolutionarily conserved signaling component or effector. The Xcc extract did not affect Arabidopsis inhibition of light-induced stomatal opening by ABA, nor did it prevent ABA-induced arrest of germination, indicating that the Xcc activity probably targets some signaling component specifically involved in promotion of stomatal closure.
To evaluate the physiological relevance for infection of the stomata-modulating activity of Xcc, we used both in vitro and in vivo assays. In vitro assays revealed that, unlike wild-type Xcc, rpfF and rpfC mutants are reduced in migration through epidermal peels. However, migration of these mutants through peels was restored by an extract from Xcc culture supernatant. These results provide further evidence that the secreted factor is produced by wild type, but not by mutants, and that is required for the movement of bacteria through stomata. The in vivo assay, performed in the DC3118 cor− Pst mutant, affected in the ability to penetrate through stomata, showed that the factor produced by Xcc was capable of restoring the infectivity of this mutant strain on Arabidopsis plants, strongly suggesting that the ability of Xcc to modulate stomatal activity is relevant for bacterial infectivity.
In this article, we also found that plants expressing an antisense construct targeted against MPK3 under the control of the guard-cell-specific promoter KST1 show virtually no stomatal closure in response to E. coli, Xcc, or LPS, showing that MPK3 is required for bacterial and LPS-induced stomatal closure, and that it therefore likely acts downstream of PAMP receptors in guard cells, as has been described previously for mesophyll protoplasts (Asai et al., 2002; Merkouropoulos and Shirsat, 2003). As expected from these results, the Pst cor− strain can achieve similar levels of infection as wild-type Pst on MPK3 antisense plants because coronatine becomes unnecessary for overcoming stomatal defense in these plants.
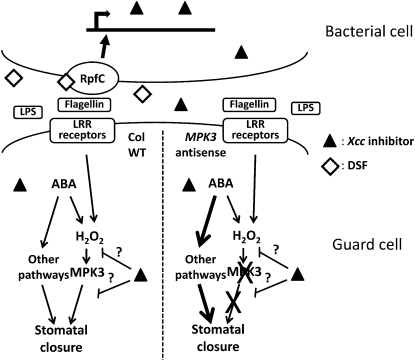
The finding that MPK3 antisense plants, which display wild-type promotion of closure in response to ABA (Gudesblat et al., 2007), are insensitive to the Xcc extract in ABA-induced promotion of closure may provide a clue on the mechanism of inhibition of the Xcc factor. Promotion of closure by ABA relative to wild-type controls in MPK3 antisense lines is normal, except when ABA-induced pH increase is blocked by the weak acid sodium butyrate (Gudesblat et al., 2007), similar to what has been described for the Arabidopsis gpa1 mutants lacking the G protein α-subunit (Wang et al., 2001). MPK3 and GPA1 may therefore act in the same signaling branch, whose absence could be compensated by ABA-induced pH increase, and probably also other signaling components (Wang et al., 2001). This compensation does not work for all closure-promoting stimuli because MPK3 antisense lines are less responsive to exogenous H2O2 (Gudesblat et al., 2007) and to bacteria or purified LPS (this article). Therefore, a possible explanation for the lack of sensitivity of MPK3 antisense lines to the Xcc factor in ABA-induced promotion of closure would be that the Xcc inhibitory factor targets some signaling component acting in the same signaling branch as MPK3 (Fig. 8), something that makes sense given that this branch would be absolutely required of bacteria-induced promotion of closure. In MPK3 antisense lines, there would be functional compensation by other redundant ABA signaling components (indicated by thicker arrows in Fig. 8), which would make ABA-induced promotion of closure no longer reliant on the MPK3 signaling branch, nor on the component targeted by the Xcc factor, effectively making MPK3 antisense plants insensitive to inhibition. This model also predicts high resilience of the guard cell ABA signaling network, which is consistent with the results obtained by modeling signaling events leading to ABA-induced stomatal closure using a dynamic Boolean network (Li et al., 2006).
Figure 8.
Proposed model of the mode of inhibition of stomatal closure by Xcc. The DSF (diamonds), which mediates cell-to-cell communication, interacts with the cell surface receptor RpfC, which in turn triggers signaling that leads to transcription of genes required for the synthesis of the stomatal inhibitory compound (triangles). This molecule diffuses to the extracellular space and gets contact with stomatal guard cells, where it blocks stomatal closure induced by ABA or PAMPs by interfering with some signaling component within a putative signaling pathway branch including H2O2 and MPK3. MPK3 is absolutely required for bacterial or PAMP-induced promotion of closure pathway, as shown by the failure of MPK3 antisense plants to close stomata in response to these stimuli. For ABA, instead, other pathways, including ABA-induced cytosolic pH increase, would compensate for the absence of MPK3. The failure of the stomatal inhibitor to interfere with promotion of closure by ABA in MPK3 antisense plants could be explained by compensatory adjustments within the guard-cell-signaling network, indicated by thick arrows, that would make up for the absence of MPK3. WT, Wild type; LRR, Leu-rich repeat receptor.
The preceding observations suggest that MPK3 participates in a signaling branch downstream of bacterial PAMPs and H2O2 (Fig. 8). This is consistent with previous observations linking PAMPs and fungal elicitors with generation of ROS. Fungal elicitors can induce H2O2 synthesis in guard cells (Lee et al., 1999; Klusener et al., 2002). Furthermore, a recent report shows that the Arabidopsis His kinase AHK5 mutants are affected both in flagellin-induced stomatal closure and in flagellin-induced H2O2 synthesis (Desikan et al., 2008). Since MPK3 is activated in response to H2O2 (Kovtun et al., 2000), it is conceivable that both ABA and PAMPs, which induce H2O2 synthesis, activate MPK3 to promote closure (Fig. 8). Interestingly enough, GPA1 has been proposed to act upstream of ROS production in guard cells (Li et al., 2006) and has been shown to be necessary for flg22-induced inhibition of stomatal opening in Arabidopsis (Zhang et al., 2008).
The structure of the virulence factor present in Xcc extracts is currently under investigation. Preliminary purification using a molecular sieve revealed that the activity resides in a small molecule of <2,000 D. This molecule has high thermal stability because activity disappeared only after 1 h of incubation at 100°C. The only other bacterial virulence factor with capacity to modulate stomatal closure described so far is coronatine from Pst. It is very unlikely that this toxin is also produced by Xcc because the enzymes required for its biosynthesis are encoded in a plasmid or chromosome of only some pathovars of P. syringae (Young et al., 1992; Bender et al., 1999; Melotto et al., 2006). In addition, the Xcc factor failed to inhibit root growth (data not shown) as has been described for coronatine (Feys et al., 1994).
It is well established that to colonize a host successfully, phytopathogens have evolved mechanisms to evade or subvert the plant defenses (Ritter and Dangl, 1996; Jamir et al., 2004; Metz et al., 2005; Nomura et al., 2005; Abramovitch et al., 2006; Nomura et al., 2006; Yun et al., 2006; Rigano et al., 2007). Many phytopathogens have been reported to enter leaves through stomata. Stomata have often been considered as passive gates for pathogen entrance into the leaves. Nevertheless, the fact that plants can actively close stomata in response to microorganisms clearly indicates that stomata can play an active role in the defense against pathogens. On the other hand, the description of the toxins fusicoccin, coronatine, and the factor reported in this article capable of disabling stomatal defense, suggest that active modulation of stomatal apertures could be a widespread strategy evolved by phytopathogens that gain entry into the leaf via stomata to disable stomatal defense. Identification of the molecular nature of the Xcc factor and its the target of action could not only shed light on the mechanism of regulation of stomatal movements, but also provide a novel target to design crops resistant to Xcc.
MATERIALS AND METHODS
Plant Material
Arabidopsis (Arabidopsis thaliana L. Heynh.) ecotype Col-0, Ler, and MPK3 guard cell-specific antisense line (Gudesblat et al., 2007) seeds were surface sterilized in 10% (v/v) commercial bleach with 0.01% (v/v) Tween 20 for 10 min and rinsed four times in sterile water. Seeds were plated in petri dishes containing one-half-strength Murashige and Skoog medium with 1% (w/v) Suc and 0.6% (w/v) agar. Seeds on plates were maintained in the dark for 2 to 3 d at 4°C to break dormancy before being transferred to a growth room at 22°C to 23°C, with a 12-h light photoperiod under light intensity of 90 μE m−2 s−1. After 5 or 6 d, seedlings were transferred to pots containing a mixture of vermiculite, peat, and perlite (1:1:1) and fertilized every 2 d. Vicia faba plants were grown in soil inside a growth room at 22°C to 23°C, with a 16-h light photoperiod.
Chemicals
ABA (mixed isomers; Sigma) was used at a final concentration of 20 μm from a 50-mm stock solution in ethanol. Pseudomonas aeruginosa LPS (Sigma) was diluted in 10:10 MES-KOH buffer, pH 6.15, and 10 mm KCl, which also contained 0.25 mm MgCl2 and 0.1 mm CaCl2 as a 1 mg/mL stock, and used at a final concentration of 100 μg/mL.
Stomatal Aperture Bioassays
For all experiments, epidermal peels from the two or three youngest fully expanded leaves from 3- to 4-week-old, unbolted Arabidopsis plants were used. Unless otherwise stated, bioassays were performed in Col-0 cultivar. To measure promotion of stomatal closure, epidermal peels were floated in 10:10 buffer under light (under the same conditions as used previously for plant growth) for at least 2 h. Then ABA, LPS, and bacterial suspensions were added to the incubation medium, and peels were further incubated as indicated. For the inhibition of opening experiments, peels were floated in the dark in 10:0 buffer (10 mm MES-KOH, pH 6.15) for 2 h to promote stomatal closure. Peels were then transferred to 10:10 buffer containing ABA for a further 2 h and were subsequently placed on a microscope slide, where apertures of 40 stomata from each experiment were measured in a Carl Zeiss microscope (400×) with the aid of an eyepiece micrometer. Data are presented as the average from 80 to 120 aperture measurements per treatment, collected from two or three independent experiments. For V. faba stomatal bioassays, peels were obtained from mature leaves of 2- to 3-week-old plants. Assays were performed as described for Arabidopsis, except that CO2-free 10:10 buffer was used.
Bacterial Strains
Xcc strains 8004 (wild type; Daniels et al., 1984), 8523 (rpfF∷Tn5lac; Tang et al., 1991), and 8557 (rpfC∷pUIRM504; Slater et al., 2000) were grown in peptone, yeast, and malt extract medium (Cadmus et al., 1976). Escherichia coli DH5α were grown at 37°C in Luria-Bertani medium (Sambrook et al., 1989). Pst DC3000 and mutant derivatives were cultured at 28°C in Luria-Bertani medium supplemented with appropriate antibiotics. All strains were grown overnight with the appropriate antibiotics. Bacteria were collected by centrifugation and resuspended in 10:10 buffer. The final bacterial concentration for stomatal bioassays was 108 cfu/mL for all strains. In coincubation experiments, 108 cfu/mL of each strain were added to the incubation buffer.
Preparation of Extracts of Bacterial Culture Supernatants
One hundred-milliliter cultures of Xanthomonas campestris strains were grown overnight in peptone, yeast, and malt extract medium. Bacteria were centrifuged for 30 min at 6,000g. The supernatant was transferred to a new centrifuge bottle and centrifuged for 90 min at 20,000g. The supernatant was transferred to 50-mL Falcon tubes and was extracted with one-third volume of ethyl acetate. Phases were separated by centrifugation for 15 min at 13,000g. The organic phase was evaporated using a Speedvac concentrator and was resuspended in 500 μL of water. For stomatal bioassays, 6 μL of extracts were used for every milliliter of incubation buffer.
Assays of Bacterial Migration
For the assays of bacterial migration across epidermal peels, X. campestris strains were transformed with the plasmid pRU1319, which expresses the green fluorescent protein (GFPuv; Allaway et al., 2001). Bacteria were cultured and processed as described above. Peels were floated on 10:10 buffer containing bacteria under the light. When extracts were used, resuspended bacteria were preincubated for 30 min with extracts. After 3 h, peels were briefly washed in 10:10 to avoid carryover of bacteria from the incubation medium and observed in a confocal laser-scanning microscope (Carl Zeiss LSM510-Axiovert 100 m, 488-nm excitation with argon laser line, and 505-nm long-pass emission).
Bacterial Growth Assay
Plant inoculations and bacterial growth assays were performed as previously described (Tornero and Dangl, 2001; Yun et al., 2006). Briefly, 3-week-old plants were infected with bacterial suspensions (108 cfu/mL except where indicated) resuspended in 10 mm MgCl2, and Silwet L-77 (200 μL/L). The aerial part of plants growing in pots were submerged upside down in the bacterial solution for 30 s and then covered with a transparent lid. Two hours after inoculation, the lid was removed and the collected sample was considered as zero time point; samples were taken every 24 h over 4 d.
Acknowledgments
We thank Max Dow, Marcelo Yanovsky, and Sheng Yang He for bacterial strains and Philip Poole for providing pRU1319. We are grateful to Tomás Santa Coloma for making the confocal microscope available. A.A.V. is Career Investigator of the Consejo Nacional de Investigaciones Científicas y Técnicas.
This work was supported by the Agencia de Promoción Científica y Tecnológica of Argentina.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Adrián Vojnov (avojnov@fundacioncassara.org.ar).
Open access articles can be viewed online without a subscription.
References
- Abramovitch RB, Anderson JC, Martin GB (2006) Bacterial elicitation and evasion of plant innate immunity. Nat Rev Mol Cell Biol 7 601–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaway D, Schofield NA, Leonard ME, Gilardoni L, Finan TM, Poole PS (2001) Use of differential fluorescence induction and optical trapping to isolate environmentally induced genes. Environ Microbiol 3 397–406 [DOI] [PubMed] [Google Scholar]
- Allegre M, Daire X, Heloir MC, Trouvelot S, Mercier L, Adrian M, Pugin A (2007) Stomatal deregulation in Plasmopara viticola-infected grapevine leaves. New Phytol 173 832–840 [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55 373–399 [DOI] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415 977–983 [DOI] [PubMed] [Google Scholar]
- Barber CE, Tang JL, Feng JX, Pan MQ, Wilson TJ, Slater H, Dow JM, Williams P, Daniels MJ (1997) A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol Microbiol 24 555–566 [DOI] [PubMed] [Google Scholar]
- Bender CL, Alarcon-Chaidez F, Gross DC (1999) Pseudomonas syringae phytotoxins: mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol Mol Biol Rev 63 266–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell CR (2002) Interactions between Xanthomonas species and Arabidopsis thaliana. In CR Somerville, EM Meyerowitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, doi/10.1199/tab.0031 [DOI] [PMC free article] [PubMed]
- Cadmus MC, Rogovin SP, Burton KA, Pittsley JE, Knutson CA, Jeanes A (1976) Colonial variation in Xanthomonas campestris NRRL B-1459 and characterization of the polysaccharide from a variant strain. Can J Microbiol 22 942–948 [DOI] [PubMed] [Google Scholar]
- Colcombet J, Hirt H (2008) Arabidopsis MAPKs: a complex signalling network involved in multiple biological processes. Biochem J 413 217–226 [DOI] [PubMed] [Google Scholar]
- Daniels MJ, Barber CE, Turner PC, Sawczyc MK, Byrde RJ, Fielding AH (1984) Cloning of genes involved in pathogenicity of Xanthomonas campestris pv. campestris using the broad host range cosmid pLAFR1. EMBO J 3 3323–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Horak J, Chaban C, Mira-Rodado V, Witthoft J, Elgass K, Grefen C, Cheung MK, Meixner AJ, Hooley R, et al (2008) The histidine kinase AHK5 integrates endogenous and environmental signals in Arabidopsis guard cells. PLoS One 3 e2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow JM, Daniels MJ (1994) Pathogenicity determinants and global regulation of pathogenicity of Xanthomonas campestris pv. campestris. Curr Top Microbiol Immunol 192 29–41 [DOI] [PubMed] [Google Scholar]
- Emi T, Kinoshita T, Shimazaki K (2001) Specific binding of vf14-3-3a isoform to the plasma membrane H+-ATPase in response to blue light and fusicoccin in guard cells of broad bean. Plant Physiol 125 1115–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys B, Benedetti CE, Penfold CN, Turner JG (1994) Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6 751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudesblat GE, Iusem ND, Morris PC (2007) Guard cell-specific inhibition of Arabidopsis MPK3 expression causes abnormal stomatal responses to abscisic acid and hydrogen peroxide. New Phytol 173 713–721 [DOI] [PubMed] [Google Scholar]
- He YW, Xu M, Lin K, Ng YJ, Wen CM, Wang LH, Liu ZD, Zhang HB, Dong YH, Dow JM, et al (2006) Genome scale analysis of diffusible signal factor regulon in Xanthomonas campestris pv. campestris: identification of novel cell-cell communication-dependent genes and functions. Mol Microbiol 59 610–622 [DOI] [PubMed] [Google Scholar]
- Heese A, Hann DR, Gimenez-Ibanez S, Jones AM, He K, Li J, Schroeder JI, Peck SC, Rathjen JP (2007) The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA 104 12217–12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugouvieux V, Barber CE, Daniels MJ (1998) Entry of Xanthomonas campestris pv. campestris into hydathodes of Arabidopsis thaliana leaves: a system for studying early infection events in bacterial pathogenesis. Mol Plant Microbe Interact 11 537–543 [DOI] [PubMed] [Google Scholar]
- Jamir Y, Guo M, Oh HS, Petnicki-Ocwieja T, Chen S, Tang X, Dickman MB, Collmer A, Alfano JR (2004) Identification of Pseudomonas syringae type III effectors that can suppress programmed cell death in plants and yeast. Plant J 37 554–565 [DOI] [PubMed] [Google Scholar]
- Klusener B, Young JJ, Murata Y, Allen GJ, Mori IC, Hugouvieux V, Schroeder JI (2002) Convergence of Calcium signaling pathways of pathogenic elicitors and abscisic acid in arabidopsis guard cells. Plant Physiol 130 2152–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtun Y, Chiu WL, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA 97 2940–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Choi H, Suh S, Doo IS, Oh KY, Jeong Choi E, Schroeder Taylor AT, Low PS, Lee Y (1999) Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis. Plant Physiol 121 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Assmann SM, Albert R (2006) Predicting essential components of signal transduction networks: a dynamic model of guard cell abscisic acid signaling. PLoS Biol 4 e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto M, Underwood W, Koczan J, Nomura K, He SY (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126 969–980 [DOI] [PubMed] [Google Scholar]
- Merkouropoulos G, Shirsat AH (2003) The unusual Arabidopsis extensin gene atExt1 is expressed throughout plant development and is induced by a variety of biotic and abiotic stresses. Planta 217 356–366 [DOI] [PubMed] [Google Scholar]
- Metz M, Dahlbeck D, Morales CQ, Al Sady B, Clark ET, Staskawicz BJ (2005) The conserved Xanthomonas campestris pv. vesicatoria effector protein XopX is a virulence factor and suppresses host defense in Nicotiana benthamiana. Plant J 41 801–814 [DOI] [PubMed] [Google Scholar]
- Miya A, Albert P, Shinya T, Desaki Y, Ichimura K, Shirasu K, Narusaka Y, Kawakami N, Kaku H, Shibuya N (2007) CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc Natl Acad Sci USA 104 19613–19618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mole BM, Baltrus DA, Dangl JL, Grant SR (2007) Global virulence regulation networks in phytopathogenic bacteria. Trends Microbiol 15 363–371 [DOI] [PubMed] [Google Scholar]
- Morris PC (2001) MAP kinase signal transduction pathways in plants. New Phytol 151 67–89 [DOI] [PubMed] [Google Scholar]
- Newman MA, Conrads-Strauch J, Scofield G, Daniels MJ, Dow JM (1994) Defense-related gene induction in Brassica campestris in response to defined mutants of Xanthomonas campestris with altered pathogenicity. Mol Plant Microbe Interact 7 553–563 [DOI] [PubMed] [Google Scholar]
- Nomura K, Debroy S, Lee YH, Pumplin N, Jones J, He SY (2006) A bacterial virulence protein suppresses host innate immunity to cause plant disease. Science 313 220–223 [DOI] [PubMed] [Google Scholar]
- Nomura K, Melotto M, He SY (2005) Suppression of host defense in compatible plant-Pseudomonas syringae interactions. Curr Opin Plant Biol 8 361–368 [DOI] [PubMed] [Google Scholar]
- Pandey S, Zhang W, Assmann SM (2007) Roles of ion channels and transporters in guard cell signal transduction. FEBS Lett 581 2325–2336 [DOI] [PubMed] [Google Scholar]
- Ren D, Liu Y, Yang KY, Han L, Mao G, Glazebrook J, Zhang S (2008) A fungal-responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis. Proc Natl Acad Sci USA 105 5638–5643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigano LA, Payette C, Brouillard G, Marano MR, Abramowicz L, Torres PS, Yun M, Castagnaro AP, Oirdi ME, Dufour V, et al (2007) Bacterial cyclic beta-(1,2)-glucan acts in systemic suppression of plant immune responses. Plant Cell 19 2077–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter C, Dangl JL (1996) Interference between two specific pathogen recognition events mediated by distinct plant disease resistance genes. Plant Cell 8 251–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch E, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, Somerville SC, Manners JM (2000) Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci USA 97 11655–11660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D (2001) Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52 627–658 [DOI] [PubMed] [Google Scholar]
- Slater H, Alvarez-Morales A, Barber CE, Daniels MJ, Dow JM (2000) A two-component system involving an HD-GYP domain protein links cell-cell signalling to pathogenicity gene expression in Xanthomonas campestris. Mol Microbiol 38 986–1003 [DOI] [PubMed] [Google Scholar]
- Slater JH, Bull AT, Hardman DJ (1997) Microbial dehalogenation of halogenated alkanoic acids, alcohols and alkanes. Adv Microb Physiol 38 133–176 [DOI] [PubMed] [Google Scholar]
- Tang JL, Liu YN, Barber CE, Dow JM, Wootton JC, Daniels MJ (1991) Genetic and molecular analysis of a cluster of rpf genes involved in positive regulation of synthesis of extracellular enzymes and polysaccharide in Xanthomonas campestris pathovar campestris. Mol Gen Genet 226 409–417 [DOI] [PubMed] [Google Scholar]
- Tornero P, Dangl JL (2001) A high-throughput method for quantifying growth of phytopathogenic bacteria in Arabidopsis thaliana. Plant J 28 475–481 [DOI] [PubMed] [Google Scholar]
- Torres PS, Malamud F, Rigano LA, Russo DM, Marano MR, Castagnaro AP, Zorreguieta A, Bouarab K, Dow JM, Vojnov AA (2007) Controlled synthesis of the DSF cell-cell signal is required for biofilm formation and virulence in Xanthomonas campestris. Environ Microbiol 9 2101–2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojnov AA, Slater H, Daniels MJ, Dow JM (2001) Expression of the gum operon directing xanthan biosynthesis in Xanthomonas campestris and its regulation in planta. Mol Plant Microbe Interact 14 768–774 [DOI] [PubMed] [Google Scholar]
- Wang LH, He Y, Gao Y, Wu JE, Dong YH, He C, Wang SX, Weng LX, Xu JL, Tay L, et al (2004) A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol Microbiol 51 903–912 [DOI] [PubMed] [Google Scholar]
- Wang P, Song CP (2008) Guard-cell signalling for hydrogen peroxide and abscisic acid. New Phytol 178 703–718 [DOI] [PubMed] [Google Scholar]
- Wang XQ, Ullah H, Jones AM, Assmann SM (2001) G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science 292 2070–2072 [DOI] [PubMed] [Google Scholar]
- Young SA, Park SK, Rodgers C, Mitchell RE, Bender CL (1992) Physical and functional characterization of the gene cluster encoding the polyketide phytotoxin coronatine in Pseudomonas syringae pv. glycinea. J Bacteriol 174 1837–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun MH, Torres PS, El Oirdi M, Rigano LA, Gonzalez-Lamothe R, Marano MR, Castagnaro AP, Dankert MA, Bouarab K, Vojnov AA (2006) Xanthan induces plant susceptibility by suppressing callose deposition. Plant Physiol 141 178–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, He SY, Assmann SM (2008) The plant innate immunity response in stomatal guard cells invokes G-protein-dependent ion channel regulation. Plant J 56 984–996 [DOI] [PMC free article] [PubMed] [Google Scholar]