Abstract
Biogenesis of thylakoid membranes in both chloroplasts and cyanobacteria is largely not understood today. The vesicle-inducing protein in plastids 1 (Vipp1) has been suggested to be essential for thylakoid membrane formation in Arabidopsis (Arabidopsis thaliana), as well as in the cyanobacterium Synechocystis sp. PCC 6803, although its exact physiological function remains elusive so far. Here, we report that, upon depletion of Vipp1 in Synechocystis cells, the number of thylakoid layers in individual Synechocystis cells decreased, and that, in particular, the content of photosystem I (PSI) complexes was highly diminished in thylakoids. Furthermore, separation of native photosynthetic complexes indicated that PSI trimers are destabilized and the monomeric species is enriched. Therefore, depletion of thylakoid membranes specifically affects biogenesis and/or stabilization of PSI in cyanobacteria.
In chloroplasts and cyanobacteria the energy transfer between PSI and PSII is regulated in a light-dependent manner (for a recent review, see Kramer et al., 2004). The two photosystems are connected by the cytochrome b6f complex, and electron transfer from PSII via the cytochrome b6f complex to PSI is believed to be regulated by the redox state of the plastoquinol pool potentially also involving the cytochrome b6f complex (Fujita et al., 1987; Murakami and Fujita, 1993; Schneider et al., 2001, 2004; Pfannschmidt, 2003; Volkmer et al., 2007). Transfer of light energy to the two photosystems is mediated by light-harvesting complexes, and in cyanobacteria light is harvested by the soluble extramembranous phycobilisomes. The efficient energy transfer to PSI and PSII has to be balanced to synchronize the function of the two photosystems. In response to changing light intensities and qualities, energy coupling between the phycobilisomes and the photosystems changes, which allows a rapid adjustment of light absorbance by the individual photosystems. Furthermore, besides this short-term adaptation mechanism, it has been shown in many studies that on a longer term in cyanobacteria the ratio of the two photosystems changes depending on the light conditions (Manodori and Melis, 1986; Murakami and Fujita, 1993; Murakami et al., 1997). Upon shifting cyanobacterial cells from low-light to high-light growth conditions, the PSI-to-PSII ratio decreases due to selective suppression of the amount of functional PSI. In recent years, some genes have already been identified that are involved in this regulation of the photosystem stoichiometry (Hihara et al., 1998; Sonoike et al., 2001; Fujimori et al., 2005; Ozaki et al., 2007).
Whereas in chloroplasts of higher plants and green algae the amounts of the two photosystems change in response to changing light conditions (Melis, 1984; Chow et al., 1990; Smith et al., 1990; Kim et al., 1993), it has already been noted a long time ago that the chloroplast ultrastructure also adapts to high-light and low-light conditions (Melis, 1984). Chloroplasts of plants grown under low light or far-red light have more thylakoid membranes than chloroplasts of plants grown under high light or blue light (Anderson et al., 1973; Lichtenthaler et al., 1981; Melis and Harvey, 1981). There appears to be a direct correlation between the chlorophyll content and the amount of thylakoids per chloroplast because light harvesting is increased by enhanced chlorophyll and thylakoid membrane content per chloroplast. Thus, chloroplasts adapt to high light both by a reduction of thylakoid membranes and by a decrease in the PSI-to-PSII ratio.
Thylakoid membranes are exclusive features of both cyanobacteria and chloroplasts, and it still remains mysterious how formation of thylakoid membranes is organized. Many cellular processes, like lipid biosynthesis, membrane formation, protein synthesis in the cytoplasm and/or at a membrane, protein transport, protein translocation, and protein folding have to be organized and aligned for formation of internal thylakoid membranes. The recent observation that deletion of the vipp1 gene in Arabidopsis (Arabidopsis thaliana) results in complete loss of thylakoid membranes has indicated that Vipp1 is involved in biogenesis of thylakoid membranes. Further analysis has suggested that Vipp1 could be involved in vesicle trafficking between the inner envelope and the thylakoid membrane of chloroplasts (Kroll et al., 2001). Because of this, the protein was named Vipp1, for vesicle-inducing protein in plastids 1. Depletion of Vipp1 strongly affected the ability of cyanobacterial cells to form proper thylakoid membranes (Westphal et al., 2001) and, consequently, also in cyanobacteria Vipp1 appears to be involved in formation of thylakoid membranes. A Vipp1 depletion strain of Arabidopsis is deficient in photosynthesis, although the defect could not be assigned to a deficiency of a single photosynthetic complex, but appeared to be caused by dysfunction of the entire photosynthetic electron transfer chain (Kroll et al., 2001). Therefore, depletion of Vipp1 in Arabidopsis seems to affect thylakoid membrane formation rather than the assembly of thylakoid membrane protein complexes (Aseeva et al., 2007). However, for cyanobacteria, it is not clear yet how diminishing the amount of thylakoid membrane layers would affect the amount and stoichiometry of the two photosystems.
Here, we present the generation and characterization of a Vipp1 depletion strain of the cyanobacterium Synechocystis sp. PCC 6803. Upon depletion of Vipp1, a decrease in thylakoid membrane pairs in the generated mutant strain and, furthermore, a significant decrease in active PSI centers was observed. Moreover, trimerization of PSI also appeared to be impaired in the mutant strain. These results suggest that thylakoid membrane perturbations caused by the Vipp1 depletion directly affects PSI assembly and stability in cyanobacterial thylakoid membranes.
RESULTS AND DISCUSSION
Whereas it has been suggested that depletion of Vipp1 in Arabidopsis affects thylakoid membrane formation per se, but not the assembly of individual thylakoid membrane protein complexes, it is unclear how depletion of thylakoid membranes would affect the photosystem content and/or stoichiometry in cyanobacteria. Therefore, we have generated and characterized a Vipp1 depletion strain to analyze a potential connection between the amount of thylakoid membranes and the photosystem stoichiometry in Synechocystis PCC 6803 more thoroughly.
Disruption of the vipp1 Gene in Synechocystis
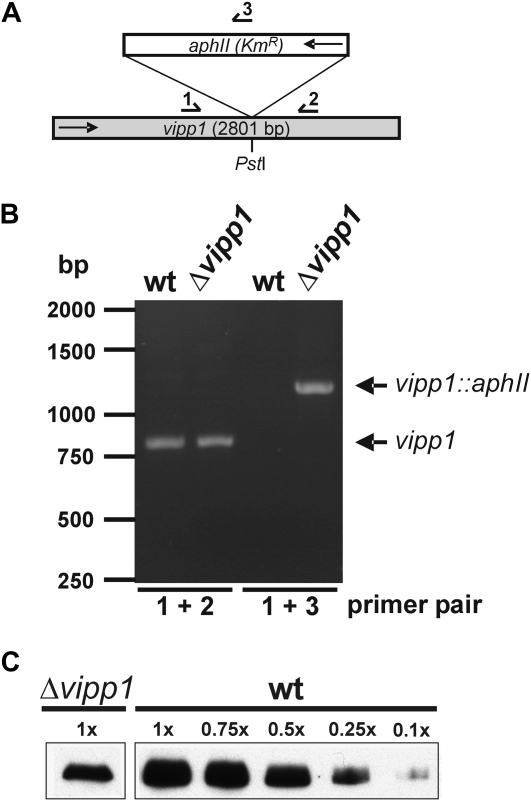
Because the physiological function of Vipp1 is still mysterious, we aimed to characterize the physiological effects of a Vipp1 depletion in Synechocystis in more detail. To this end, the vipp1 (sll0617) gene (Kaneko et al., 1996) was disrupted in Synechocystis PCC 6803 by insertion of a kanamycin resistance cassette into a single PstI site of the gene (Fig. 1A). After transformation of Synechocystis wild-type cells with the plasmid containing the interrupted gene, complete segregation of the mutant should be obtained by growing the cells for various generations on medium containing increasing amounts of kanamycin. Synechocystis contains several identical genome copies and, in each of these, the gene has to be inactivated to obtain a completely segregated disruption strain. To check the segregation state of the Synechocystis Δvipp1 strain, we amplified the vipp1 gene from genomic Synechocystis DNA by PCR. The wild-type vipp1 gene is approximately 800 bp and the size of the PCR fragment should increase to about 2,000 bp when the aphII gene (kanamycin resistance cassette) is introduced into the gene. As can be seen in Figure 1B, besides the wild-type fragment, no fragment corresponding in size to the interrupted vipp1 gene was amplified by PCR when genomic DNA from the vipp1 merodiploid strain was used as a template. However, when primers were used, which anneal at the 5′-end of the vipp1 gene and within the aphII gene (see Fig. 1A), a fragment with the expected size was amplified. These observations verify that some of the vipp1 gene copies are interrupted by the aphII gene in the vipp1 merodiploid strain. However, most of the genomic vipp1 copies remained noninterrupted and, even after growing the cells for 4 years on selective medium, no completely segregated strain has been obtained. This highly supports the assumption that the vipp1 gene is essential for cell viability.
Figure 1.
Disruption of vipp1 in Synechocystis sp. PCC 6803. A, Strategy for disruption of vipp1 in the genome of Synechocystis sp. PCC 6803. The aphII gene confers resistance to kanamycin (KmR). Arrows indicate the direction of the aphII and the vipp1 gene. Numbered arrows indicate primers, which were used for segregation control (compare B). B, The vipp1 gene cannot be deleted completely in Synechocystis. Amplification of the vipp1 gene from wild-type (wt) and Δvipp1 Synechocystis genomic DNA resulted only in an amplification of the wild-type fragment (800 bp) and no fragment, which corresponds in size to the interrupted vipp1 gene (approximately 2,000 bp) was observed. When a primer pair was used, one primer of which anneals to the 5′-end of the vipp1 gene and the other anneals to the aphII gene (compare A), a fragment of about 1,200 bp was amplified, which demonstrates that the aphII gene is interrupted in some genomic vipp1 copies. C, Immunoblot analysis of total cellular extracts from Synechocystis wild type and the Vipp1 depletion strain grown at 120 μmol s−1 m−2. Five micrograms protein have been separated by SDS-PAGE and subsequently analyzed by immunoblot analysis using an α-Vipp1 antibody. For comparison, for the wild-type cell extract, 3.75, 2.5, 1.25, and 0.5 μg protein have also been analyzed.
Because at least in some genomic copies the vipp1 gene was interrupted, we tested whether the Vipp1 content in the depletion strain was decreased when compared to wild-type cells. After growing the cells in liquid cultures enriched with 2% CO2, a significant depletion of Vipp1 in total cellular extracts was observed by immunoblot analysis (Fig. 1C). Based on this analysis, we conclude that the total Vipp1 content in the mutant strain was significantly decreased when compared to wild-type cells.
Because it has been shown that Vipp1 is involved in formation of thylakoid membranes in chloroplasts, we subsequently characterized the ultrastructure of Synechocystis wild-type and Vipp1 depleted cells by electron microscopy (EM), and we determined and compared the amount of thylakoid membrane pairs in the two strains. As can be seen in Figure 2A, after depletion of Vipp1, Synechocystis cells contain less thylakoid membrane pairs than the wild-type strain. To subsequently quantify the observed difference in more detail, we determined the number of thylakoid membrane pairs in wild-type and Vipp1 depleted cells. As can be seen in Figure 2B, under the presented growth conditions, the mutant cells contain on average stacks of two to three pairs of thylakoid layers, whereas the wild-type cells contain on average four to five layers of thylakoid membrane pairs. Therefore, depletion of Vipp1 results in a reduction of thylakoid membrane pairs. Nevertheless, it has to be noted that, although we have tested numerous growth conditions, we were not able to generate a Synechocystis strain with no organized thylakoids and no photosynthetic activity, in contrast to the results reported in Westphal et al. (2001). We always observed a significant amount of internal thylakoid membranes coupled with photosynthetic activity (compare below). Besides the observed reduction in thylakoid membrane pairs in the vipp1 merodiploid strain, the EM data further suggest that the overall thylakoid membrane morphology is disturbed in the mutant strain and the thylakoid membranes are less well arranged than in the wild-type strain. This observation further suggests that Vipp1 is involved in thylakoid membrane biogenesis in cyanobacteria and depletion of Vipp1 might result in formation of less well-organized thylakoid membrane pairs (as further discussed below).
Figure 2.
Depletion of Vipp1 results in a Synechocystis strain with less thylakoid membranes. A, Representative electron micrographs of Synechocystis wild-type (wt) and Vipp1 depleted cells are shown. B, Under the presented growth conditions, the wild-type cells (black bars) had on average four to six layers of thylakoid membranes, whereas the mutant strain (gray bars) had only two to three thylakoid layers. Synechocystis cells were grown at 20 μmol/(m2 s) as described in “Materials and Methods.”
Chlorophyll and Photosystem Content in Vipp1 Depleted Cells
For cyanobacterial wild-type cells, it is well established that shifting cyanobacterial cells from low light to higher light intensities results in a general decrease in the amount of the two photosystems in thylakoid membranes, as well as in a specific decrease of the PSI-to-PSII ratio (see introduction). Therefore, we have determined and compared the chlorophyll content of wild-type and Vipp1 depleted cells at different growth light intensities. As can be seen in Figure 3, the chlorophyll content per cyanobacterial cell significantly decreased in both wild-type and vipp1 merodiploid cells when the cyanobacterial cells were grown under increasing light intensities. Furthermore, the chlorophyll content per cell was at any light intensity lower in vipp1 merodiploid cells when compared to the wild-type strain. For a meaningful comparison of the cellular chlorophyll content, we have quantified the amount of Synechocystis cells per OD750, and Synechocystis cells were counted in a Thoma counting chamber (see “Materials and Methods”). Whereas the overall cell shape and size of the mutant strain appeared not to have changed due to the vipp1 gene interruption, the amount of cells corresponding to an OD750 = 1 was significantly altered. An OD750 = 1 corresponded to 1.98 × 105 cells mL−1 for the wild type and to 2.88 × 105 cells mL−1 for the mutant strain at identical growth conditions. Thus, at the same OD750, the two cultures contain significantly different amounts of cells. Therefore, a simple normalization of cells based on OD750 measurements would have resulted in rather inaccurate data comparison. This observed difference in light scattering was taken into account, and for all further measurements the wild-type and mutant strains were compared on a per-cell basis. The results presented in Figure 3 clearly suggest that the amount of chlorophyll-containing membrane protein complexes (photosystems) decreases with increased light intensities and that in the vipp1 merodiploid strain the total amount of chlorophyll-containing membrane protein complexes is lower at any tested light intensity when compared to wild-type cells.
Figure 3.
Chlorophyll content in Synechocystis wild-type and Vipp1 depleted cells. Chlorophyll content of Synechocystis wild-type (light bars) and Vipp1 depleted cells (gray bars) was determined as described in “Materials and Methods” after growing the cells at different light intensities [20, 40, 80, 120 μmol/(m2 s)]. Chl is shown for 105 cells.
To further characterize the effect of the thylakoid membrane perturbation and reduction on pigment-binding membrane protein complexes, absorbance spectra of whole Synechocystis wild-type and Vipp1 depleted cells were recorded using identical amounts of cells (Fig. 4). These spectra further support the observation that in vipp1 merodiploid cells the cellular concentration of chlorophyll-binding proteins is significantly lower when compared to wild-type cells. Based on the absorbance spectra, the ratio of phycobilisomes to chlorophyll-binding proteins can be estimated (Bennett and Bogorad, 1973). Whereas the pigment content per cell has changed in the mutant strain, the ratio of phycocyanin/chlorophyll, which corresponds to the phycobilisome-to-photosystems ratio, remains largely unchanged (0.51 ± 0.0015 in wild-type cells and 0.53 ± 0.002 in the Vipp1 depletion strain).
Figure 4.
Absorbance spectra of Synechocystis wild-type and vipp1 merodiploid cells. Cell suspensions were diluted to 3 × 105 cells/mL (for details see the text), and spectra were recorded in the range between 350 to 750 nm. Solid line, Wild-type cells; dashed line, vipp1 merodiploid cells.
The observed decrease in the photosystem content per cell could be caused by a general reduction of the content of both PSI and PSII. However, it is also possible that the cellular concentration of one photosystem is more affected. Because in Synechocystis about 90% of all chlorophylls are bound to PSI (Shen et al., 1993), a reduction of PSI, which is often observed when, for example, the photosynthetic electron transfer chain is affected due to mutations (Schneider et al., 2001, 2004; Berry et al., 2002; Volkmer et al., 2007), would result in a more dramatic reduction of the cellular chlorophyll content than reduction of solely PSII. To address the question of whether the cellular content of PSI and PSII is equally affected by the Vipp1 depletion, we have determined the relative amounts and activities of the two photosystems.
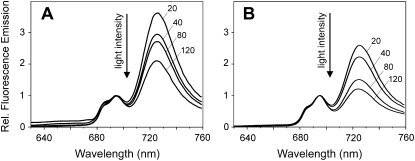
The relative amounts of the two photosystems can be estimated by fluorescence emission spectroscopy at 77K. After excitation of chlorophylls at 435 nm, PSI has a maximal fluorescence emission at 725 nm and PSII at 685 and 695 nm. In Figure 5, fluorescence emission spectra of Synechocystis wild-type and vipp1 merodiploid cells measured after growth at different light intensities are shown. The spectra clearly support the results obtained by UV/VIS spectroscopy and strengthen the assumption that the ratio of PSI to PSII has changed in the mutant when compared to the wild-type strain. The PSI-to-PSII ratio is regulated in a highly dynamic fashion in cyanobacteria and it has been observed that in cyanobacteria the ratio of PSI to PSII depends on the light conditions used for cell growth (Murakami and Fujita, 1993). With increasing light intensities, the amount of PSI is down-regulated, whereas the PSII concentration within membranes remains largely constant. Because of this observation, it appeared possible that reduction of thylakoids resulted in disturbed regulation of the PSI-to-PSII ratio and that the Vipp1 depletion has somehow frozen the cyanobacterial cells in a high light state with less PSI centers in the thylakoid membranes. In wild-type Synechocystis cells, the PSI-to-PSII ratio changed as expected, and with increasing light intensities the amount of PSI centers decreased. However, as for the wild type, we also observed similar changes in the PSI-to-PSII ratio in vipp1 merodiploid cells and also in these cells the ratio of the two photosystems was regulated in a light-dependent manner. When compared to wild-type cells, the relative amount of PSII was higher under all tested growth conditions. Compared to the wild type, either dramatically more PSII centers are present per mutant cell or less PSI. Because the chlorophyll content per cell was significantly decreased in the mutant strain, it appeared unlikely that the amount of PSII was increased, which would have resulted in an increase of chlorophylls per cell. Therefore, we conclude that depletion of thylakoid membranes directly affects accumulation of PSI in Synechocystis cells. Nevertheless, the different cellular concentrations of internal thylakoid membranes and chlorophylls could in general result in different reabsorption of fluorescence emission and, therefore, the 77K fluorescence measurements strongly indicated, but not firmly established, a changed PSI-to-PSII ratio. Because of this, we have subsequently determined the activities of PSI and PSII in Synechocystis wild-type and Vipp1 depleted cells to further support the spectroscopic observations.
Figure 5.
Light-dependent regulation of the PSI-to-PSII ratio in wild-type and vipp1 merodiploid cells. A and B, 77K fluorescence emission spectra of Synechocystis wild-type (A) and vipp1 merodiploid (B) cells after excitation of chlorophylls at 435 nm. Increase of the growth light intensity [20, 40, 80, 120 μmol/(m2 s)] results in a decreased PSI-to-PSII ratio in both the wild-type and Vipp1 depletion strain. At each line, the light intensity, at which the cells were grown, is indicated. Under all tested light intensities, the PSI-to-PSII ratio was decreased when compared to wild-type cells grown under identical growth conditions. For better comparability, the spectra were normalized at 695 nm and the fluorescence emission at 695 nm was set at 1.
Amount and Activity of PSI and PSII
To determine the activity of PSII, the photosynthetic activity of whole Synechocystis cells was measured as described in “Materials and Methods.” Wild-type and the vipp1 merodiploid Synechocystis strain were grown under various light intensities and the rate of oxygen evolution was determined using phenyl-p-benzoquinone (PPBQ) as an electron acceptor at PSII. When normalized to the amount of cells (see above), these measurements suggest that the PSII activity in the vipp1 merodiploid strain has not significantly changed at any light intensity when compared to wild-type cells (Fig. 6). Nonetheless, it appears possible that the PSII activity is slightly increased per cell in the vipp1 merodiploid strain at light intensities up to 80 μmol/(m2 s).
Figure 6.
Oxygen evolution of wild-type (light bars) and vipp1 merodiploid (gray bars) Synechocystis cells. Cells were grown at different light intensities and oxygen evolution was measured using either PPBQ (top) or HCO3− (bottom) as electron acceptor. The statistical significance of the observed difference in oxygen evolution was calculated by a one-tailed t test. For all the observed oxygen evolution rates, the differences between the means were due to chance (P > 0.05) and thus statistically not relevant.
When oxygen evolution at PSII is measured with HCO3− as an electron acceptor, the activity of the entire photosynthetic electron transfer chain can be determined. Interestingly, when normalized to the amount of cells, with this acceptor, the oxygen evolution was about the same for the wild type and the mutant strain within the range of the error (Fig. 6). These data further support the assumption that, upon thylakoid membrane reduction, the amount of active PSII per cell is not significantly altered.
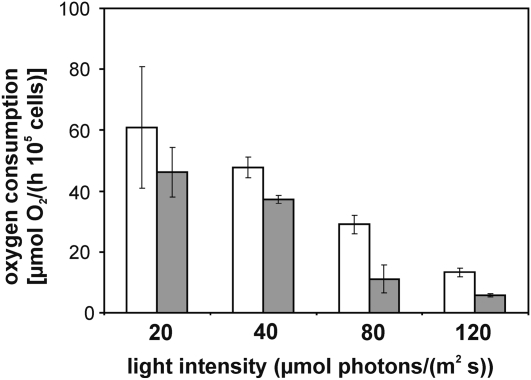
To subsequently determine the PSI activity in Synechocystis wild-type and mutant cells, oxygen consumption at PSI due to the Mehler reaction was measured. As can be seen in Figure 7, the activity of PSI was determined to be decreased relative to the wild-type strain at any growth condition in line with the observations described above. Taken together, these measurements indicate reduced PSI content per cell in the vipp1 merodiploid strain relative to wild-type cells and an unchanged or just marginally increased PSII content. However, even with a reduced amount of PSI, the mutant cells still appear to be able to operate the electron transfer chain at wild-type rates, as shown in Figure 6. Thus, the PSI reduction does not significantly affect the electron transfer chain from water (PSII) via PSI to HCO3−.
Figure 7.
Determination of the PSI activity. PSI activity of Synechocystis cells grown at different light intensities was measured as oxygen consumption due to the Mehler reaction (for details, see “Materials and Methods”). Oxygen consumption of wild-type (white bars) and vipp1 merodiploid (gray bars) cells was normalized to 105 cells. The statistical significance of the observed difference in oxygen consumption was calculated by a one-tailed t test. For the observed oxygen consumption rates at 40, 80, and 120 μmol photons/(m2 s), the differences were statistically relevant at a 1% level (P < 0.01). Due to the relatively high scattering of the measured rates at 20 μmol photons/(m2 s), the observed difference between the means were statistically not relevant (P > 0.05).
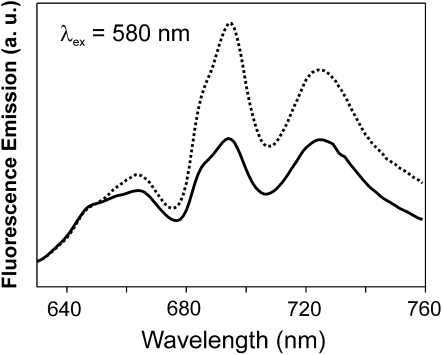
As discussed above, whereas the overall content of the photosystems has been changed in the mutant strain and this strain contains less PSI, the ratio of phycobilisomes to chlorophyll-containing protein complexes has not changed in the vipp1 merodiploid strain. To subsequently analyze the energy coupling between phycobilisomes and the two photosystems, 77K fluorescence emission spectra were recorded after excitation of phycobilisomes at 580 nm. After excitation of the phycobilisomes, the fluorescence emission at 685 and 695 nm was increased in the mutant strain relative to the fluorescence emission of phycobilisomes at 650 and 665 nm (Fig. 8), which suggested an increased energy transfer from phycobilisomes to PSII. Because the ratio of phycobilisomes/chlorophyll is largely unchanged in the mutant strain and because only the content of PSI is reduced in the vipp1 merodiploid cells, the ratio of phycobilisomes to PSII has in fact increased, which is in line with the observed increase in phycobilisomes coupled to the PSII reaction centers (emission at 685 and 695 nm). However, whereas the cellular content of PSI is decreased in the mutant strain, energy transfer from phycobilisomes to PSI was also increased when compared to wild-type cells (emission at 720 nm). These observations suggest that, although the ratio of the two photosystems has changed, the vipp1 merodiploid cells maintain efficient energy coupling between phycobilisomes and PSI. Thus, by an increased energy transfer from phycobilisomes to PSI, the cells may adjust to the decrease in the PSI content and, thereby, retain efficient electron transfer rates, as observed in the whole-cell electron transfer measurements (Fig. 6).
Figure 8.
77K fluorescence emission spectra of Synechocystis wild-type (solid line) and Δvipp1 cells (dotted line) after excitation of phycobilisomes at 580 nm. Cells were grown at 120 μmol/(m2 s). The spectra were normalized at 650 nm. a. u., Arbitrary units.
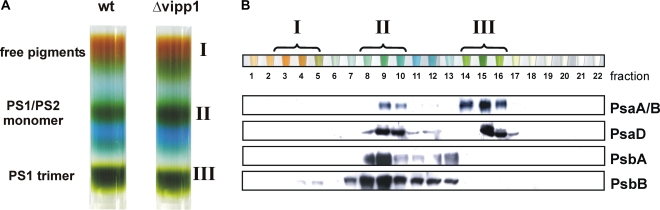
To further characterize the relative amounts of PSI and PSII in the thylakoid membranes of Synechocystis wild-type and mutant cells, we have used Suc gradient centrifugation and blue native (BN)-PAGE analyses. These techniques can be used to separate the native photosystems from each other, as well as to analyze the oligomeric state of the photosystems. Whereas PSI forms trimers in cyanobacteria (Kruip et al., 1994), PSII forms dimers (Boekema et al., 1995). However, whereas both photosystems form distinct oligomers in membranes, the monomeric species are also active and so far the physiological role of the oligomerization is largely enigmatic.
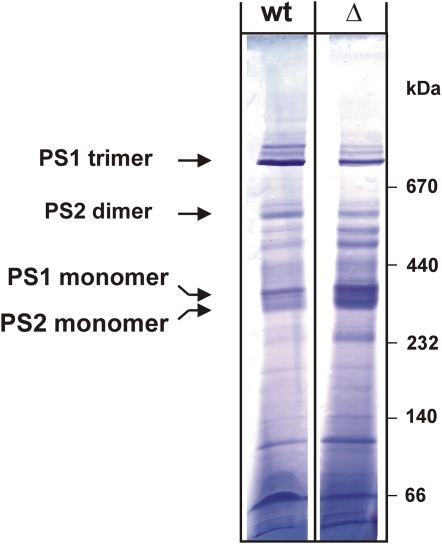
As can be seen in Figure 9, when thylakoid membranes of Synechocystis wild type and the vipp1 merodiploid strain were extracted and separated on a 10% to 30% Suc gradient, differences in the photosystem composition can be observed: To compare the relative amounts of monomeric and trimeric PSI, we have determined the chlorophyll concentration of bands II and III of the Suc gradient. The ratio of chlorophylls enclosed in bands II and III of the gradient was about 3.5 for the wild type and lowered to approximately 1.8 for the mutant strain. Thus, the amount of PSI trimers is significantly reduced in membranes isolated from mutant cells when compared to the wild-type situation. In line with a general PSI reduction, the relative amount of monomeric PSII is increased. In contrast to the trimeric PSI species, in the Suc gradient dimeric PSII is almost not resolved. However, the significantly increased intensity of the PSI/PSII monomer band could not only be caused by the apparent increase of PSII relative to PSI, but also by an increase of the monomeric PSI species. Furthermore, in the mutant cells, we observed more free pigments after centrifugation, which might indicate a general destabilization of PSI and/or PSII in this strain. These observations might indicate that not only the amount of PSI is reduced in thylakoid membranes of the Vipp1 depletion strain, but also the ratio of monomeric PSI to trimeric PSI has changed. To further strengthen the assumption that Vipp1 has an impact on PSI trimerization in membranes, we have additionally analyzed the composition of thylakoid membrane proteins by BN-PAGE. As can be seen in Figure 10, when followed by BN-PAGE, the amount of PSI trimers is also reduced in the mutant cells accompanied by an increase in monomeric PSI. For the wild type, the PSI trimer band contained about 2.5 times more PSI than the monomer band, whereas in the depletion strain the ratio was shifted to about 0.5. Thus, in the mutant strain, the trimeric PSI species is highly destabilized when compared to wild-type membranes. Because the Suc gradients and BN-PAGE gels were loaded on a chlorophyll basis and because in the mutant strain less PSI is present, the relative amount of PSII appears to be increased in the mutant strain when compared to the wild type, in line with the presented spectroscopic observations. Nevertheless, it has to be emphasized that, because, due to technical problems, the gels cannot be loaded on a per-cell basis, this observed relative increase does not reflect the concentrations within a living cell. BN-PAGE, as well as Suc density analysis, only allows comparison of the relative amounts of PSI and PSII in thylakoid membranes. However, besides the observed reduction of PSI trimers, which was also observed after the Suc density gradient centrifugation, the BN-PAGE analysis (Fig. 10) also indicates that the stability of PSII dimers is slightly decreased in the mutant strain because the relative amount of PSII monomers to PSII dimers has changed in the mutant membranes. Thus, the Vipp1 depletion and the resulting thylakoid membrane perturbation appear to affect not only the PSI-to-PSII ratio, but also oligomerization of both photosystems, although PSI seems to be more affected than PSII.
Figure 9.
Separation of membrane protein complexes from wild-type and Vipp1 depleted Synechocystis cells. A, Membrane proteins from Synechocystis wild-type (wt) and Vipp1 depleted cells were solubilized by β-DM and membrane protein complexes were separated on a 10% to 30% Suc density gradient. Three colored bands (I–III) were clearly resolved. In wild-type membranes, band II of the gradient contained about 3.5 times more chlorophyll than band III, whereas in the depletion strain, this ratio was lowered to about 1.8. B, Individual fractions of the gradient from wild-type membranes were analyzed by immunoblot analysis. The individual membrane protein complexes were identified using antibodies directed against PSI (PsaA, B, D) and PSII (PsbA, B) subunits. Fraction 1 corresponds to the top of the Suc gradient.
Figure 10.
Coomassie-stained BN-PAGE gel of membrane protein complexes isolated from wild-type (wt) and Vipp1 depleted (Δ) Synechocystis cells. For identification of the photosystems, the individual membrane protein complexes were identified with antibodies directed against PSI and PSII subunits (see Fig. 9 legend) after separation in a second dimension by SDS-PAGE (data not shown). Densities of the PSI monomer and trimer bands in BN-PAGE gels were determined using the program Scion Image (Scion Corp.). Based on these measurements, the wild type contained at least 2.5 times more trimeric over monomeric PSI, whereas in the depletion strain, this ratio was shifted to approximately 0.5. [See online article for color version of this figure.]
On the Function of Vipp1
Biogenesis of thylakoid membranes is neither understood in chloroplasts nor in cyanobacteria. The discovery that Vipp1 could be involved in thylakoid membrane formation in chloroplasts was a major breakthrough and indicated a direct link between a single protein and thylakoid membrane organization via a vesicular trafficking system (Kroll et al., 2001). It has subsequently been shown that Vipp1 can form large oligomeric ring structures (Aseeva et al., 2004), although the exact physiological function of Vipp1 still remains mysterious.
The results presented in this study suggest an in vivo function of Vipp1 not only in thylakoid membrane organization, but also in biogenesis of cyanobacterial membrane-bound protein complexes. A Vipp1 depletion strain of Arabidopsis is deficient in photosynthesis, although the defect could not be assigned to a deficiency of a single photosynthetic complex, but appeared to be caused by dysfunction of the entire photosynthetic electron transfer chain (Kroll et al., 2001). Therefore, it has been suggested that depletion of Vipp1 in Arabidopsis affects thylakoid membrane formation rather than the assembly of thylakoid membrane protein complexes (Aseeva et al., 2007). In contrast, depletion of the vipp1 gene product resulted in a rather specific decrease of functional (trimeric) PSI in Synechocystis, whereas the amount of active PSII was not significantly altered. Therefore, the observed effect seems to be more specific for PSI and Vipp1 appears to be involved in biogenesis and/or stabilization of PSI in thylakoid membranes. However, based on the presented results, we cannot clearly distinguish between a direct effect of Vipp1 on PSI biogenesis and/or stability and a more indirect effect of Vipp1 on, for example, membrane formation, which could subsequently affect PSI stability or biogenesis in the membrane. In several recent studies, it has been observed that defects in thylakoid membrane formation in chloroplasts and cyanobacteria are caused by depletion of important lipids or of pigments (for a recent overview, see Vothknecht and Westhoff, 2001). After deletion of genes involved in myxoxanthophyll biosynthesis, thylakoid membranes in the resulting Synechocystis cells were highly disorganized and myxoxanthophyll biogenesis is therefore critical for thylakoid membrane organization (Mohamed et al., 2005). In chloroplasts, defects in thylakoid membrane formation have been linked to deficiencies in chlorophyll biosynthesis (Falbel and Staehelin, 1994; Runge et al., 1995). Furthermore, synthesis of galactolipids is essential for thylakoid membrane formation (Kobayashi et al., 2007) and also negatively charged phospholipids appear to be indispensable for thylakoid membrane formation and function (as summarized in Frentzen, 2004). Taken together, many factors are involved in formation, stabilization, and organization of thylakoid membranes in chloroplasts and cyanobacteria, and Vipp1 could therefore be involved in processes other than a vesicular transfer, as originally suggested.
CONCLUSION
Taken together, we show here that in the cyanobacterium Synechocystis sp. PCC 6803 the synthesis of active PSI depends on the amount of thylakoid membranes present per cell. Down-regulation of the amount of active PSI appears to be a general adaptation mechanism in cyanobacteria. Vipp1 could be directly involved in such mechanisms, resulting in changes of the PSI-to-PSII ratio in response to, for example, increasing light intensities. In contrast, depletion of the vipp1 gene product resulted in a specific decrease of functional (trimeric) PSI in Synechocystis, whereas the amount of functional PSII was not significantly altered and, thus, the observed effect seems to be more specific for PSI.
MATERIALS AND METHODS
Growth Conditions
Synechocystis wild type and the vipp1 merodiploid strain were grown at 30°C in BG11 medium supplemented with 10 mm Glc (Rippka et al., 1979). Kanamycin (30 μg/mL) was added to the growth medium for growth of the vipp1 merodiploid strain. The growth medium was aerated with air enriched with 2% CO2 and cells were grown under constant illumination of 20 to 120 μmol/(m2 s).
Population sizes for the different Synechocystis strains in a cell suspension were determined by direct microscopic counts using a Thoma counting chamber.
Escherichia coli DH5α, which was used for plasmid propagation, was grown in Luria-Bertani medium at 37°C according to standard procedures (Sambrook and Russel, 2001). Luria-Bertani broth and agar were supplemented with 100 μg/mL ampicillin when needed.
Heterologous Expression of Vipp1 from Synechocystis
For raising antibodies against the cyanobacterial Vipp1 protein in rabbits, the vipp1 gene from Synechocystis was amplified by PCR using the two primers vipp5′ 5′-tatgccatatgggattatttgaccgtttagg-3′ and vipp3′ 5′-atacgggatccttacagattatttaaccgacgacg-3′, and genomic Synechocystis DNA as a template. The PCR product was restriction digested with NdeI and BamHI and ligated to the equally restriction-digested plasmid pET19b (Merck). The resulting plasmid pET-SynVipp1 was used to express the Vipp1 protein with an N-terminal His-tag in E. coli BL21 (DE3) cells. For protein expression, cells were grown in 1-L Luria-Bertani medium to an OD600 of approximately 0.6 and protein expression was induced by addition of 0.5 mm isopropyl-β-d-thiogalactopyranoside. After 3 h, cells were harvested and resuspended in 50 mL 20 mm HEPES, pH 7.5, 5 mm EDTA buffer. Cells were subsequently broken by ultrasonic treatment and afterward inclusion bodies were sedimented by centrifugation at 10,000g for 10 min. Proteins were solubilized in buffer A (50 mm sodium phosphate, pH 7.6, 50 mm NaCl) supplemented with 150 mm SDS. After 10-min incubation at room temperature, the samples were centrifuged at 10,000g, and the supernatant was loaded onto a nickel-nitrilotriacetic acid agarose column (Qiagen). The column was washed two times with buffer A + 150 mm SDS, two times with the same buffer at pH 7.2, and proteins were finally eluted in buffer with 10 mm SDS at pH 6.5.
Preparation of Cyanobacterial Membranes
Synechocystis cells were harvested in the mid-log growth phase and pelleted by centrifugation (5,000g, 10 min, 4°C). Cells were resuspended in buffer (50 mm HEPES, pH 7.0, 5 mm MgCl2, 25 mm CaCl2, 10% [v/v] glycerol) and disrupted in a bead beater homogenizer (BioSpec) using 0.5-mm glass beads. Glass beads, unbroken cells, and cell debris were removed by centrifugation for 5 min at 4°C and 5,000g. After centrifugation at 100,000g and 4°C for 40 min, the membrane pellet was resuspended in a buffer at a chlorophyll concentration of about 1 mg chl/mL.
SDS-PAGE and Immunoblot Analysis
For immunoblot analysis, membranes were extracted with SDS sample buffer and proteins were loaded on a 12% polyacrylamide gel. Subsequently, SDS-PAGE proteins were transferred to a polyvinylidene difluoride membrane using a semidry blotter from Bio-Rad. The rabbit primary antibodies and the goat anti-rabbit secondary antibody (Sigma) were used at dilutions of 1:2,000 and 1:10,000, respectively. To visualize the cross-reacting protein bands, membranes were incubated with the ECL kit from Pierce.
Suc Density Gradient Centrifugation and BN-PAGE Analyses
For analyses of membrane protein complexes in Synechocystis cells, the membranes were always prepared freshly as described in Dühring et al. (2006).
Thylakoid membranes were extracted with 1% n-dodecyl-β-d-maltoside and separation of protein complexes in a 10% to 30% Suc density gradient was done as described in Rögner et al. (1990). Individual fractions of the gradient were analyzed by immunoblot analyses to determine the fractions containing PSI or PSII. Antibodies were directed against the PSI subunits PsaA, PsaB, and PsaD and against the PSII subunits PsbA and PsbB. PsaA/B, PsbA, and PsbB antibodies were a kind gift of M. Rögner (Ruhr-University Bochum, Germany). The antibody directed against PsaD was from Agrisera.
For BN-PAGE analyses, Synechocystis membranes were extracted with 1% n-dodecyl-β-d-maltoside, and membrane protein complexes were separated exactly as described in detail in Dühring et al. (2006, 2007). Individual lanes were separated in a second dimension on a 12% SDS gel (Laemmli, 1970) and PSI and PSII were identified by immunoblot analyses using the antibodies described above.
Inactivation of the vipp1 Gene
Inactivation of the Synechocystis vipp1 gene (sll0617) was essentially carried out as described (Westphal et al., 2001). A kanamycin resistance cassette, derived from pBSL14 (Alexeyev, 1995), was introduced into a single PstI site within the vipp1 gene of pET-SynVipp1. Five micrograms of the plasmid were used to transform Synechocystis wild-type cells following the procedure described in Williams (1988). For selection of kanamycin-resistant mutants, solid BG11 medium was supplemented with increasing kanamycin concentrations (5–50 μg/mL).
Absorbance and 77K Fluorescence Emission Spectroscopy
Absorbance spectra of whole cells in the visible range were recorded with a Perkin-Elmer Lambda 25 spectrophotometer. Low-temperature fluorescence emission spectra were recorded at 77K with an Aminco Bowman Series 2 fluorimeter. Monochromators were set to a slit width of 4 nm. Cells were adjusted to a chlorophyll concentration of about 3 μm in BG11 and frozen in liquid nitrogen. Chlorophylls were excited at 435 nm and phycobilisomes at 580 nm.
Chlorophyll concentration was determined according to Porra et al. (1989) after extraction of whole cells with 100% methanol. Phycocyanin/chlorophyll content was determined from whole-cell absorbance spectra as described (Bennett and Bogorad, 1973).
Oxygen Evolution and Consumption Measurements
Oxygen evolution was measured in 1-mL cell suspension (OD750 of approximately 1, about 5 μg/mL chlorophyll) using a fiber-optic oxygen meter (PreSens) under actinic light (600 μmol photons m−2 s−1). Synechocystis wild-type and Vipp1 depleted cells were harvested in the mid-log phase and diluted to an OD750 = 1 in BG11 medium. Three independent cultures of each analyzed strain were measured three times each. Photosynthetic rates were determined in the presence of 10 mm NaHCO3 and PSII activity in the presence of 0.5 mm PPBQ.
PSI activity was measured as oxygen consumption in the presence of 20 μm 3-(3,4-dichlorophenyl)-1,1-dimethylurea, 2 mm NaN3, 200 μm 2,3,5,6-tetramethyl-1,4-phenylenediamine, 5 mm sodium ascorbate, and 200 μm methyl viologen. A baseline was recorded in dark and light. Oxygen consumption due to the Mehler reaction was determined as corrected oxygen consumption in the light by oxygen consumption in the dark.
EM
The morphology of Synechocystis cells was studied by examining ultrathin sections with a PHILIPS CM10 electron microscope (Fei Company) equipped with a Bioscan camera (model 792; Gatan). The electron micrographs taken at an acceleration voltage of 80 kV were processed by a digital image system (digital micrograph; Gatan). The specimens were prepared routinely (Golecki, 1988) for thin sectioning by collecting the cells by centrifugation and fixing in 2% glutardialdehyde in cacodylate buffer (pH 7.0) and 1% osmium tetroxide in veronal acetate buffer (pH 7.0). After embedding in epoxy resin ultrathin sections were produced. The sections were stained with uranyl acetate followed by lead citrate.
To determine the number of thylakoid layers per cell, more than 100 cells of each Synechocystis wild type and Vipp1 depletion strain were evaluated on ultrathin sections.
Acknowledgments
We thank C. Escher for excellent technical assistance and M. Schroda and C. Dreher for stimulating discussions. We also thank M. Rögner (Ruhr-University Bochum) for the kind gift of antibodies and A. Wilde (Liebig-University Giessen) for help with the BN-PAGE analyses.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (SCHN 690/3–1).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Dirk Schneider (dirk.schneider@biochemie.uni-freiburg.de).
Some figures in this article are displayed in color online but in black and white in the print edition.
References
- Alexeyev MF (1995) Three kanamycin resistance gene cassettes with different polylinkers. Biotechniques 18 52, 54, 56 [PubMed] [Google Scholar]
- Anderson JM, Goodchild DJ, Boardman NK (1973) Composition of the photosystems and chloroplast structure in extreme shade plants. Biochim Biophys Acta 325 573–585 [DOI] [PubMed] [Google Scholar]
- Aseeva E, Ossenbühl F, Eichacker LA, Wanner G, Soll J, Vothknecht UC (2004) Complex formation of Vipp1 depends on its alpha-helical PspA-like domain. J Biol Chem 279 35535–35541 [DOI] [PubMed] [Google Scholar]
- Aseeva E, Ossenbuhl F, Sippel C, Cho WK, Stein B, Eichacker LA, Meurer J, Wanner G, Westhoff P, Soll J, et al (2007) Vipp1 is required for basic thylakoid membrane formation but not for the assembly of thylakoid protein complexes. Plant Physiol Biochem 45 119–128 [DOI] [PubMed] [Google Scholar]
- Bennett A, Bogorad L (1973) Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol 58 419–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry S, Schneider D, Vermaas WF, Rögner M (2002) Electron transport routes in whole cells of Synechocystis sp. strain PCC 6803: the role of the cytochrome bd-type oxidase. Biochemistry 41 3422–3429 [DOI] [PubMed] [Google Scholar]
- Boekema EJ, Hankamer B, Bald D, Kruip J, Nield J, Boonstra AF, Barber J, Rögner M (1995) Supramolecular structure of the photosystem II complex from green plants and cyanobacteria. Proc Natl Acad Sci USA 92 175–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow WS, Melis A, Anderson JM (1990) Adjustments of photosystem stoichiometry in chloroplasts improve the quantum efficiency of photosynthesis. Proc Natl Acad Sci USA 87 7502–7506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dühring U, Irrgang KD, Lunser K, Kehr J, Wilde A (2006) Analysis of photosynthetic complexes from a cyanobacterial ycf37 mutant. Biochim Biophys Acta 1757 3–11 [DOI] [PubMed] [Google Scholar]
- Dühring U, Ossenbuhl F, Wilde A (2007) Late assembly steps and dynamics of the cyanobacterial photosystem I. J Biol Chem 282 10915–10921 [DOI] [PubMed] [Google Scholar]
- Falbel TG, Staehelin LA (1994) Characterization of a family of chlorophyll-deficient wheat (Triticum) and barley (Hordeum vulgare) mutants with defects in the magnesium-insertion step of chlorophyll biosynthesis. Plant Physiol 104 639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frentzen M (2004) Phosphatidylglycerol and sulfoquinovosyldiacylglycerol: anionic membrane lipids and phosphate regulation. Curr Opin Plant Biol 7 270–276 [DOI] [PubMed] [Google Scholar]
- Fujimori T, Higuchi M, Sato H, Aiba H, Muramatsu M, Hihara Y, Sonoike K (2005) The mutant of sll1961, which encodes a putative transcriptional regulator, has a defect in regulation of photosystem stoichiometry in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol 139 408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Murakami A, Ohki K (1987) Regulation of photosystem composition in the cyanobacterial photosynthetic system: the regulation occurs in response to the redox state of the electron pool located between the two photosystems. Plant Cell Physiol 28 283–292 [Google Scholar]
- Golecki JR (1988) Analysis of structure and development of bacterial membranes/outer, cytoplasmic, and intracytoplasmic membranes. Method Microbiol 20 61–77 [Google Scholar]
- Hihara Y, Sonoike K, Ikeuchi M (1998) A novel gene, pmgA, specifically regulates photosystem stoichiometry in the cyanobacterium Synechocystis species PCC 6803 in response to high light. Plant Physiol 117 1205–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, et al (1996) Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res 3 109–136 [DOI] [PubMed] [Google Scholar]
- Kim JH, Glick RE, Melis A (1993) Dynamics of photosystem stoichiometry adjustment by light quality in chloroplasts. Plant Physiol 102 181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Kondo M, Fukuda H, Nishimura M, Ohta H (2007) Galactolipid synthesis in chloroplast inner envelope is essential for proper thylakoid biogenesis, photosynthesis, and embryogenesis. Proc Natl Acad Sci USA 104 17216–17221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer DM, Avenson TJ, Edwards GE (2004) Dynamic flexibility in the light reactions of photosynthesis governed by both electron and proton transfer reactions. Trends Plant Sci 9 349–357 [DOI] [PubMed] [Google Scholar]
- Kroll D, Meierhoff K, Bechtold N, Kinoshita M, Westphal S, Vothknecht UC, Soll J, Westhoff P (2001) VIPP1, a nuclear gene of Arabidopsis thaliana essential for thylakoid membrane formation. Proc Natl Acad Sci USA 98 4238–4242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruip J, Bald D, Boekema E, Rögner M (1994) Evidence for the existence of trimeric and monomeric photosystem I complexes in thylakoid membranes from cyanobacteria. Photosynth Res 40 279–286 [DOI] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 680–685 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler H, Buschmann C, Döll M, Fietz HJ, Bach T, Kozel U, Meier D, Rahmsdorf U (1981) Photosynthetic activity, chloroplast ultrastructure, and leaf characteristics of high-light and low-light plants and of sun and shade leaves. Photosynth Res 2 115–141 [DOI] [PubMed] [Google Scholar]
- Manodori A, Melis A (1986) Cyanobacterial acclimation to photosystem I or photosystem II light. Plant Physiol 82 185–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A (1984) Light regulation of photosynthetic membrane structure, organization, and function. J Cell Biochem 24 271–285 [DOI] [PubMed] [Google Scholar]
- Melis A, Harvey GW (1981) Regulation of photosystem stoichiometry, chlorophyll a and chlorophyll b content and relation to chloroplast ultrastructure. Biochim Biophys Acta 637 138–145 [Google Scholar]
- Mohamed HE, van de Meene AM, Roberson RW, Vermaas WF (2005) Myxoxanthophyll is required for normal cell wall structure and thylakoid organization in the cyanobacterium Synechocystis sp. strain PCC 6803. J Bacteriol 187 6883–6892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami A, Fujita Y (1993) Regulation of stoichiometry between PSI and PSII in response to light regime for photosynthesis observed with synechocystis PCC 6714: relationship between redox state of Cyt b6f complex and regulation of PSI formation. Plant Cell Physiol 34 1175–1180 [Google Scholar]
- Murakami A, Kim SJ, Fujita Y (1997) Changes in photosystem stoichiometry in response to environmental conditions for cell growth observed with the cyanophyte Synechocystis PCC 6714. Plant Cell Physiol 38 392–397 [DOI] [PubMed] [Google Scholar]
- Ozaki H, Ikeuchi M, Ogawa T, Fukuzawa H, Sonoike K (2007) Large-scale analysis of chlorophyll fluorescence kinetics in Synechocystis sp. PCC 6803: identification of the factors involved in the modulation of photosystem stoichiometry. Plant Cell Physiol 48 451–458 [DOI] [PubMed] [Google Scholar]
- Pfannschmidt T (2003) Chloroplast redox signals: how photosynthesis controls its own genes. Trends Plant Sci 8 33–41 [DOI] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975 384–394 [Google Scholar]
- Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111 1–61 [Google Scholar]
- Rögner M, Nixon PJ, Diner BA (1990) Purification and characterization of photosystem I and photosystem II core complexes from wild-type and phycocyanin-deficient strains of the cyanobacterium Synechocystis PCC 6803. J Biol Chem 265 6189–6196 [PubMed] [Google Scholar]
- Runge S, van Cleve B, Lebedev N, Armstrong G, Apel K (1995) Isolation and classification of chlorophyll-deficient xantha mutants of Arabidopsis thaliana. Planta 197 490–500 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russel DW (2001) Molecular Cloning: A Laboratory Manual, Ed 3. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Schneider D, Berry S, Rich P, Seidler A, Rögner M (2001) A regulatory role of the PetM subunit in a cyanobacterial cytochrome b6f complex. J Biol Chem 276 16780–16785 [DOI] [PubMed] [Google Scholar]
- Schneider D, Berry S, Volkmer T, Seidler A, Rögner M (2004) PetC1 is the major Rieske iron-sulfur protein in the cytochrome b6f complex of Synechocystis sp. PCC 6803. J Biol Chem 279 39383–39388 [DOI] [PubMed] [Google Scholar]
- Shen G, Boussiba S, Vermaas WF (1993) Synechocystis sp PCC 6803 strains lacking photosystem I and phycobilisome function. Plant Cell 5 1853–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BM, Morrissey PJ, Guenther JE, Nemson JA, Harrison MA, Allen JF, Melis A (1990) Response of the photosynthetic apparatus in Dunaliella salina (green algae) to irradiance stress. Plant Physiol 93 1433–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoike K, Hihara Y, Ikeuchi M (2001) Physiological significance of the regulation of photosystem stoichiometry upon high light acclimation of Synechocystis sp. PCC 6803. Plant Cell Physiol 42 379–384 [DOI] [PubMed] [Google Scholar]
- Volkmer T, Schneider D, Bernat G, Kirchhoff H, Wenk SO, Rögner M (2007) Ssr2998 of Synechocystis sp. PCC 6803 is involved in regulation of cyanobacterial electron transport and associated with the cytochrome b6f complex. J Biol Chem 282 3730–3737 [DOI] [PubMed] [Google Scholar]
- Vothknecht UC, Westhoff P (2001) Biogenesis and origin of thylakoid membranes. Biochim Biophys Acta 1541 91–101 [DOI] [PubMed] [Google Scholar]
- Westphal S, Heins L, Soll J, Vothknecht UC (2001) Vipp1 deletion mutant of Synechocystis: a connection between bacterial phage shock and thylakoid biogenesis? Proc Natl Acad Sci USA 98 4243–4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JGK (1988) Mutations in PSII reaction center. Methods Enzymol 167 766–778 [Google Scholar]