Abstract
Roots respond not only to gravity but also to moisture gradient by displaying gravitropism and hydrotropism, respectively, to control their growth orientation, which helps plants obtain water and become established in the terrestrial environment. As gravitropism often interferes with hydrotropism, however, the mechanisms of how roots display hydrotropism and differentiate it from gravitropism are not understood. We previously reported MIZU-KUSSEI1 (MIZ1) as a gene required for hydrotropism but not for gravitropism, although the function of its protein was not known. Here, we found that a mutation of GNOM encoding guanine-nucleotide exchange factor for ADP-ribosylation factor-type G proteins was responsible for the ahydrotropism of Arabidopsis (Arabidopsis thaliana), miz2. Unlike other gnom alleles, miz2 showed no apparent morphological defects or reduced gravitropism. Instead, brefeldin A (BFA) treatment inhibited both hydrotropism and gravitropism in Arabidopsis roots. In addition, a BFA-resistant GNOM variant, GNM696L, showed normal hydrotropic response in the presence of BFA. Furthermore, a weak gnom allele, gnomB/E, showed defect in hydrotropic response. These results indicate that GNOM-mediated vesicular trafficking plays an essential role in hydrotropism of seedling roots.
Stationary growth is a distinct feature of plants and distinguishes them from other organisms. Plants have evolved a variety of mechanisms for responding to environmental cues, which enables them to survive in the presence of limited resources or environmental stresses. One of the most important growth adaptations plants have acquired is tropism, growth response that involves bending or curving of plant organs toward or away from a stimulus. For example, roots display tropisms in response to environmental cues such as gravity, light, touch, and moisture (Darwin and Darwin, 1880; Takahashi, 1997; Correll and Kiss, 2002; Monshausen et al., 2008). Gravitropism has been the subject of intense study, while other tropic responses of roots have been less well characterized. There is some evidence of hydrotropism in roots, but this response has proven difficult to differentiate from gravitropism, as the latter always interferes with hydrotropism (Jaffe et al., 1985; Takahashi, 1994; Takahashi, 1997). The demonstration of true hydrotropism in roots has facilitated the identification of some of the physiological aspects of hydrotropism and its existence in a wide range of plant species. However, the underlying mechanisms that regulate hydrotropism remain unknown. The limited supply of water and precipitation in many parts of the world greatly affects agriculture and ecosystems. Elucidating the molecular mechanism of hydrotropism in roots is therefore important not only for understanding how terrestrial plants adapt to changes in moisture, but also for improving crop yields and biomass production.
The isolation and analysis of hydrotropism-deficient mutants using the model plant species Arabidopsis (Arabidopsis thaliana) represents a potent tool for dissecting the molecular mechanism of hydrotropism. Previously, we isolated an ahydrotropic mutant of Arabidopsis, mizu-kussei1 (miz1), and showed that MIZ1 encodes a protein of unknown function (Kobayashi et al., 2007). In light of both the physiological features of hydrotropism, as well as what we have learned from genetic studies of other tropisms, it is unlikely that miz1 alone governs the hydrotropic response. In support of this, we have identified a second ahydrotropic mutant, miz2, a unique allele of gnom that confers ahydrotropic but not agravitropic growth, which implies distinct roles of vesicular trafficking between hydrotropism and gravitropism in roots.
RESULTS AND DISCUSSION
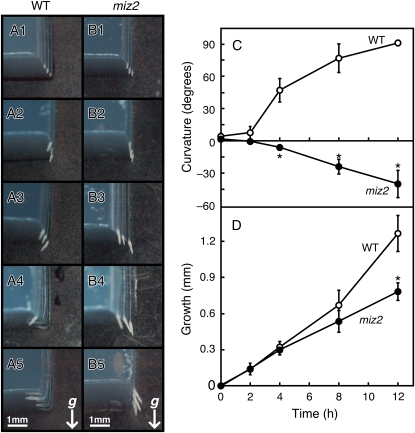
A hydrotropic mutant of Arabidopsis, miz2, was isolated by an experimental procedure previously described (Kobayashi et al., 2007). Initial analysis showed that miz2 appeared to be totally lacking root hydrotropism (Fig. 1, A and B). We next analyzed the kinetics of the hydrotropic response of miz2 roots. In the presence of a moisture gradient, vertically positioned wild-type roots gradually bent toward the source of moisture in 1% (w/v) agar and had grown to contact the agar by 12 h post-hydrostimulation. In contrast, miz2 roots did not display hydrotropic bending. Instead, they tended to bend away from the agar surface (Fig. 1C). This wrong-way curvature has been often observed during ahydrotropic response in several species and is likely due to a loss of turgidity on the dry side of the root (Takahashi, 1994; Kobayashi et al., 2007). There were no significant differences in root elongation rate between wild-type and miz2 roots until 8 h, and the differences became apparent 8 to 12 h after hydrostimulation (Fig. 1D). This was most likely due to the enhanced elongation of wild-type roots as the tips came into contact with the source of moisture, because there were no obvious differences in root elongation when seedlings were grown under conventional conditions (data not shown). The growth rate of miz2 roots did not differ from that of wild-type roots under humidity-saturated conditions.
Figure 1.
Hydrotropism and elongation of miz2 and wild-type (WT) roots. A and B, Hydrotropic curvatures of WT (A1–A5) and miz2 (B1–B5) roots at 0 h (A1 and B1), 2 h (A2 and B2), 4 h (A3 and B3), 8 h (A4 and B4), and 12 h (A5 and B5) after the start of hydrotropic stimulation. The arrow (g) indicates the direction of the gravitational force. Scale bars = 1 mm. C and D, Time course of hydrotropic curvature (C) and elongation (D) of miz2 and WT roots. White circles, WT roots; black circles, miz2 roots. Data represents the means ± sd of three independent experiments (n = 20 for each experiment). Asterisks indicate statistically significant differences, as determined by the Student's two-tailed t test (P < 0.01).
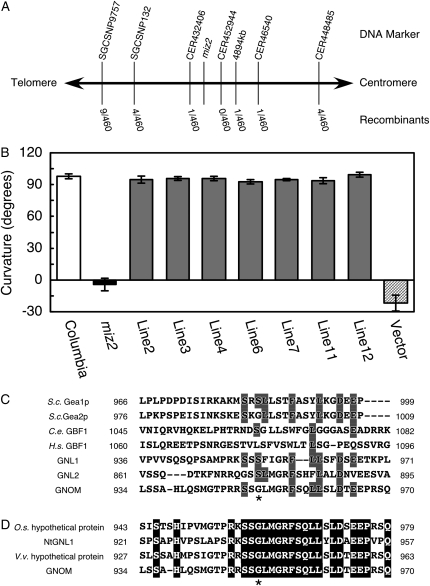
The miz2 mutation was mapped to a site on chromosome 1 (At1g13980), between the simple sequence-length polymorphism markers CER432406 and 4894kb. This genomic locus was different from that of miz1, which suggested that miz2 was not allelic to miz1 (Fig. 2A). Complementation analysis using a genomic fragment of wild-type At1g13980 confirmed that miz2 is a new recessive mutant allele of gnom (Fig. 2B). The mutation in miz2 consisted of a single base change of G to A, which resulted in a single amino acid substitution in GNOM, Gly951Glu.
Figure 2.
Identification of the miz2 gene. A, Map-based cloning of the miz2 locus. DNA markers and the number of recombinants observed for the corresponding marker, as a fraction of the total number of chromosomes, are shown. B, Complementation test for hydrotropism in miz2 plants. Hydrotropic curvature 12 h after hydrostimulation is presented. White bar, wild type; black bar, miz2; gray bars, independently generated transgenic lines; hatched bar, vector control. Data represents the means ± sd of at least 17 individuals. C, Amino acid sequence alignment of the miz2 mutation and the flanking residues for the following Gea/GNOM/GBF family members: budding yeast Gea1p and Gea2p (S.c. Gea1p and S.c. Gea2p, respectively), Caenorhabditis elegans GBF1 (C.e. GBF1), human GBF1 (H.s. GBF1), and Arabidopsis GNL1 (At5g39500) and GNL2 (At5g19610) gene products. Sequences were aligned with GNOM using ClustalW (Thompson et al., 1994). Identical residues are shaded. D, Amino acid sequence alignment of the miz2 mutation and the neighboring amino acids for plant GNOM homologs. Putative proteins of rice (O.s. hypothetical protein; EAY91294), grape (V.v. hypothetical protein; AM461845), and tobacco GNOM-like1 (NtGNL1; EF520731) proteins were aligned with GNOM using ClustalW (Thompson et al., 1994). Identical residues are highlighted. In both C and D, asterisks indicate the mutated residue in miz2.
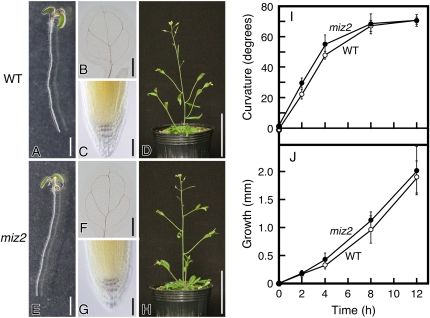
GNOM (also called EMB30 or VAN7) encodes a guanine-nucleotide exchange factor for ADP-ribosylation factor-type G proteins (ARF-GEF). Loss-of-function alleles of Arabidopsis GNOM lead to severe defects in apical-basal pattern formation in the embryo (Mayer et al., 1993; Steinmann et al., 1999). Nearly all of the defects associated with gnom alleles involve altered auxin transport, and proper localization of the auxin efflux carrier has been shown to require GNOM function (Steinmann et al., 1999; Geldner et al., 2003). The missense mutation we identified in miz2 was downstream of the central catalytic Sec7 domain of GNOM. Weak gnom alleles carrying mutations in this C-terminal region have defects in postembryonic development (Geldner et al., 2004). Moreover, the no hydrotropic response1 (nhr1) mutant exhibits altered root elongation and root cap development (Eapen et al., 2003); thus, while the nhr1gene has not been identified, it is reasonable to predict that the ahydrotropic response of miz2 plants is due to defects in root development. We therefore examined whether miz2 mutants had defects in root morphology or not. In contrast to other weak alleles of gnom, there were no obvious differences in the morphological features of either the roots or shoots of wild-type and miz2 plants, including root tip morphology (Fig. 3, A, C–E, G, and H; Supplemental Fig. S1). In addition, no obvious phenotypes in vascular development, which is often observed in weak alleles of gnom, were observed (Fig. 3, B and F). These results demonstrate that the miz2 mutation unlikely affects the functions of GNOM required for proper developmental patterning.
Figure 3.
Morphological features and gravitropism of miz2 and wild-type (WT) plants. WT (A) and miz2 (E) seedlings; vascular patterns in the cotyledons of WT (B) and miz2 (F) seedlings; iodine-stained columella cells of WT (C) and miz2 (G) plants; and inflorescence of 4-week-old WT (D) and miz2 (H) seedlings are shown. Scale bars = 2 mm (A and E), 500 μm (B and F), 50 μm (C and G), and 5 cm (D and H). I and J, Gravitropism of WT and miz2 seedling roots. Curvature (I) and growth (J) were measured 0, 2, 4, 8, and 12 h after gravitropic stimulation. White circles, WT; black circles, miz2. Data represents the means ± sd of three independent experiments (n = 20 for each experiment).
As mentioned above, mutations in GNOM generally disrupt the polarity of auxin transport and thereby cause defects in gravitropism (Geldner et al., 2004). As gravitropism has been shown to interfere with hydrotropism (Jaffe et al., 1985; Takahashi, 1994, 1997; Kobayashi et al., 2007), we were interested in whether the defect in hydrotropism of miz2 mutants was due to altered gravitropism. To test whether the miz2 mutation altered gravitropism, we compared the kinetics of gravitropic growth of wild-type and miz2 plants. Unexpectedly, there were no significant differences in gravitropic curvature or growth between wild-type and miz2 plants (Fig. 3, I and J). This was in a striking contrast to the phenotype of gnomB/E, a weak allele of gnom, which displays a significant defect in root gravitropism (Geldner et al., 2004).
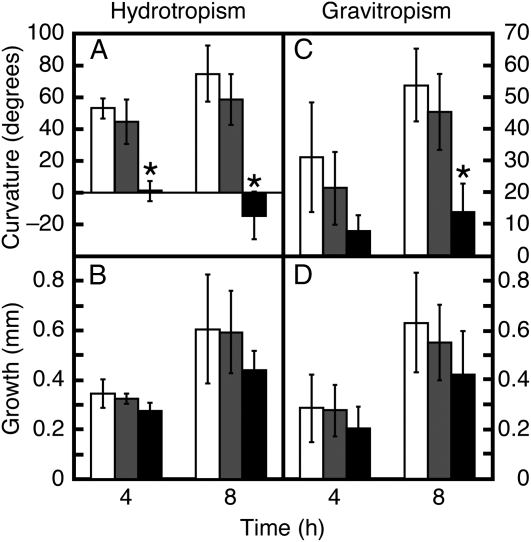
Previously, it was reported that GNOM-mediated vesicular trafficking is inhibited by brefeldin A (BFA) in Arabidopsis root cells (Geldner et al., 2003). To examine the involvement of vesicular trafficking in root hydrotropism, we treated wild-type seedlings with BFA and monitored the effect on hydrotropism. As shown in Figure 4A, treatment with BFA inhibited the development of root hydrotropic curvature in a dose-dependent manner. Thus, the hydrotropic response of miz2 was phenocopied by treatment of wild-type plants with BFA at a concentration of 10−5 m, which demonstrated that vesicle trafficking was involved in root hydrotropism. In contrast to miz2 mutants, BFA treatment to wild-type seedlings also decreased root gravitropic response (Fig. 4, C and D), which probably is attributable to the inhibition of proper auxin transport. On the other hand, our recent demonstration showed that polar auxin transport is not essential for root hydrotropism in Arabidopsis. Namely, Arabidopsis roots treated with inhibitors of auxin efflux, TIBA or NPA, showed normal hydrotropic response, while their gravitropic response was substantially reduced (Kaneyasu et al., 2007). Also, hydrotropic response of pin2/wav6-52 mutant roots in which polar auxin transport and gravitropism were altered, did not differ from that of wild-type roots (Takahashi et al., 2002). We further confirmed the apparent normality of auxin transport in miz2 by monitoring DR5∷GUS expression, which is widely used to visualize auxin gradients in roots (Geldner et al., 2004). We found no obvious alteration in auxin response gradients between wild type and miz2 (Supplemental Fig. S2). In addition, localization of PIN1 protein in the roots of miz2 did not differ from that of the wild-type roots (data not shown). Thus, it is likely that miz2 affects hydrotropism by a mechanism apart from the role of GNOM that regulates polar auxin transport.
Figure 4.
BFA inhibits both hydrotropism and gravitropism. Wild-type seedlings were treated with BFA at concentrations of 10−6 m (gray bars) and 10−5 m (black bars), and hydrotropism (A and B) and gravitropism (C and D) were compared to mock-treated seedlings (white bars). Curvature (A and C) and growth (B and D) were collected 4 and 8 h after hydrotropic and gravitropic stimulation. Data represents the means ± sd of three independent experiments (at least 15 individuals for each hydrotropism assay and 10 individuals for each gravitropism assay). Asterisks indicate statistically significant differences, as determined by the Student's two-tailed t test (P < 0.01).
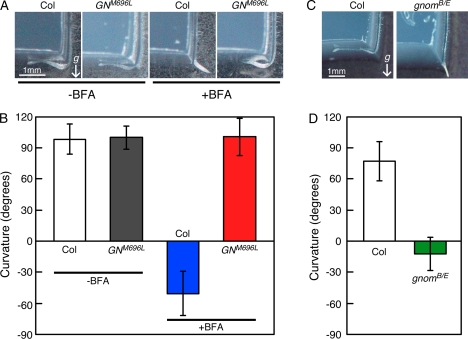
Because phenotypes of miz2 differed from those of other known gnom alleles, we confirmed the involvement of GNOM in root hydrotropism using Arabidopsis seedlings that express the BFA-resistant GNOM variant, GNM696L (Geldner et al., 2003). Root hydrotropic response was completely inhibited by BFA treatment in wild-type plants, whereas Arabidopsis seedlings expressing GNM696L displayed normal hydrotropic response irrespective of the BFA treatment (Fig. 5, A and B). This result virtually confirmed that miz2 is a novel allele of gnom and that GNOM function is essential for root hydrotropic response. In addition, we investigated the hydrotropic response of gnomB/E, a weak allele of gnom, to reveal the specificity of miz2 mutation on root hydrotropism. Seedlings of gnomB/E showed no hydrotropic response as miz2 did (Fig. 5, C and D), suggesting a stronger requirement of GNOM for hydrotropism relative to gravitropism.
Figure 5.
Hydrotropic response of gnom variants. A and B, Effect of BFA on root hydrotropic response of a GNOM BFA-resistant line. Seedlings expressing BFA-resistant GNOM (GNM696L) were subjected to hydrotropic assay together with those of wild type (Col). Seedlings were treated with BFA at a concentration of 10−5 m, and hydrotropism was compared to mock-treated seedlings. Photomicrographs (A) and curvature (B) were collected 12 h after hydrotropic stimulation. Data in B represent the means ± sd of 16 individuals. White bar, Control roots of wild type; gray bar, control roots of GNM696L; blue bar, BFA-treated roots of wild type; red bar, BFA-treated roots of GNM696L. C and D, Hydrotropic response of a weak gnom allele, gnomB/E. Seedlings of gnomB/E were subjected to hydrotropic assay together with those of wild type (Col). Photomicrographs (C) and curvature (D) were collected as described above. Data in D represent the means ± sd of 28 and 23 for Col and gnomB/E individuals, respectively. White bar, wild type; green bar, gnomB/E. In A and C, the arrow (g) indicates the direction of the gravitational force.
GNOM is a member of the Gea/GBF/GNOM subfamily of ARF-GEF proteins (Jackson and Casanova, 2000). Members of this subfamily contain a significant level of similarity in the regions of the Sec7 domain as well as upstream and downstream of the Sec7 domain. While the Sec7 domain of ARF-GEFs is well characterized, relatively little is known about these conserved flanking regions. The only information that has been shown is that the N-terminal DCB-domain of GNOM is required for the direct interaction between DCB, HUS, and SEC7 domains of GNOM molecules (Anders et al., 2008). The amino acid substitution of miz2 was located in the C-terminal region of GNOM, which includes a 300-amino acid region located downstream of the Sec7 domain that is shared specifically by members of the Gea/GBF/GNOM family (Jackson and Casanova, 2000). The overall C-terminal region of GNOM is believed to be required for full auxin canalization, but no functions other than regulating auxin transport polarity have been shown. While the amino acid residue affected by the miz2 mutation was not conserved among Gea/GBF/GNOM family members, it was conserved among GNOM homologs of other plant species such as grape (Vitis vinifera), tobacco (Nicotiana tabacum), and rice (Oryza sativa; Fig. 2, C and D). In budding yeast (Saccharomyces cerevisiae), the corresponding C-terminal region of Gea1p and Gea2p, which are homologues of GNOM, is required for their ability to interact with the Golgi protein Gmh1p (Chantalat et al., 2003). While the precise role of Gmh1p is unclear, it is probable that such C-terminal binding protein of GNOM confers its full activity necessary for specific plant phenomena.
In conclusion, we have shown that GNOM is involved in the regulation of an important step in hydrotropism in addition to its role in different aspects of plant development, including the gravitropic response. Our results clearly suggest that vesicular trafficking plays crucial roles in both hydrotropism and gravitropism, but their regulatory mechanisms differ from one another in the two tropisms. Further analysis of miz2 mutants will shed a new light on the importance of membrane trafficking in some of the unsolved issues of plant biology such as hydrotropism in roots.
MATERIALS AND METHODS
Plant growth conditions, the procedure for the hydrotropism and gravitropism assays, and the screening of miz mutants have been described previously (Kobayashi et al., 2007). A miz2 homozygous mutant (ecotype Columbia) was crossed with Landsberg erecta (Ler) wild-type plants to generate a mapping population. In the F2 population, miz2 mutant seedlings were selected based on an impaired hydrotropic response 12 h after exposure to a moisture gradient, and their genomic DNA was isolated from the leaf tissues. Polymorphisms between Columbia and Ler ecotypes were analyzed using a combination of cleaved amplified polymorphic sequence analysis and simple sequence length polymorphism markers, and data obtained from The Arabidopsis Information Resource and the Monsanto Arabidopsis Polymorphism and Ler Sequence collection (Jander et al., 2002). For complementation analysis, a 11.4-kb genomic fragment of the GNOM gene, containing approximately 6.2 kb upstream of the ATG start codon and approximately 0.4 kb downstream of the stop codon, was subcloned into pPCR-Script Amp SK+ (Stratagene) according to the manufacturer's instructions. After sequencing, the 11.4-kb GNOM fragment was excised by restriction digestion with KpnI and SacI and inserted into the KpnI-SacI sites of the binary plant transformation vector pPZP211 (Hajdukiewicz et al., 1994). Further complementation analyses were performed as described previously (Kobayashi et al., 2007). Treatment with BFA was performed as previously described (Kaneyasu et al., 2007), with the exception that the stock solution of BFA (Sigma Chemicals) was prepared at a concentration of 1,000× in dimethyl sulfoxide. As a control, an equivalent volume of dimethyl sulfoxide was added to the culture medium. We observed root tip morphology of propidium iodide stained wild-type and miz2 roots under FV-1000 confocal laser scanning microscopy (Olympus). GUS staining and microscopic observations were done as described previously (Kobayashi et al., 2007).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers NC_001142 (S.c. Gea1), NC_001137 (S.c. Gea2), NM_067121 (C.e. GBF1), NM_004193 (H.s. GBF1), NM_123312 (AtGNL1), NM_121966 (AtGNL2), U36432 (AtGNOM), AP005778 (O.s. hypothetical protein), EF520731 (NtGNL1), and AM461845 (V.v. hypothetical protein).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Primary root meristem of miz2 mutant.
Supplemental Figure S2. Auxin response gradients in miz2 root tips upon exogenous auxin treatment.
Supplementary Material
Acknowledgments
We thank Prof. Gerd Jürgens of the University of Tübingen for kindly providing us with seeds of the gnomB/E and GNOMM696L (BFA-resistant) lines and for critical reading of the manuscript. We thank Prof. Tom J. Guilfoyle of University of Missouri (Columbia, MO) for allowing us to use DR5∷GUS Arabidopsis plants. We also thank Prof. Peter W. Barlow of University of Bristol (Bristol, UK), Prof. Kiyotaka Okada of National Institute of Basic Biology (Okazaki, Japan), and Prof. Kotaro Yamamoto of Hokkaido University (Sapporo, Japan) for their helpful discussions and critical reading of the manuscript. We are grateful to our laboratory members, Yoko Kakimoto, Atsuhi Ooba, Mayumi Nakayama, and Yoshie Ito for their assistance, and Prof. Atsushi Higashitani (Tohoku University) for his helpful discussions.
This work was supported by the Program for Promotion of Basic Research Activities for Innovative Biosciences (to Y.M.), by the Japan Society for the Promotion of Science (Grants-in-Aid for Scientific Research B, no. 20370017, to H.T.; and a Research Fellowship for Young Scientists to A.K.), by the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grants-in-Aid for Scientific Research on Priority Areas, no. 19039005, to H.T.), and by the “Ground-based Research Announcement for Space Utilization,” promoted by the Japan Space Forum (to H.T.).
The author responsible for the distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Hideyuki Takahashi (hideyuki@ige.tohoku.ac.jp).
The online version of this article contains Web-only data.
Open access articles can be viewed online without a subscription.
References
- Anders N, Nielsen M, Keicher J, Stierof YD, Furutani M, Tasaka M, Skiriver K, Jürgens G (2008) Membrane association of the Arabidopsis ARF exchange factor GNOM involves interaction of conserved domains. Plant Cell 20 142–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantalat S, Courbeyrette R, Senic-Matuglia F, Jackson CL, Goud B, Peyroche A (2003) A novel Golgi membrane protein is a partner of the ARF exchange factors Gea1p and Gea2p. Mol Biol Cell 14 2357–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll MJ, Kiss JZ (2002) Interactions between gravitropism and phototropism in plants. J Plant Growth Regul 21 89–101 [DOI] [PubMed] [Google Scholar]
- Darwin C, Darwin F (1880) The Power of Movement in Plants. John Murray, London
- Eapen D, Barroso ML, Campos ME, Ponce G, Corkidi G, Dubrovsky JG, Cassab GI (2003) A no hydrotropic response root mutant that responds positively to gravitropism in Arabidopsis. Plant Physiol 131 536–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jürgens G (2003) The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112 219–230 [DOI] [PubMed] [Google Scholar]
- Geldner N, Richter S, Vieten A, Marquardt S, Torres-Ruiz RA, Mayer U, Jürgens G (2004) Partial loss-of-function alleles reveal a role for GNOM in auxin transport-related, post-embryonic development of Arabidopsis. Development 131 389–400 [DOI] [PubMed] [Google Scholar]
- Hajdukiewicz P, Svab Z, Maliga P (1994) The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol 25 989–994 [DOI] [PubMed] [Google Scholar]
- Jackson CL, Casanova JE (2000) Turning on ARF: the Sec7 family of guanine-nucleotide-exchange factors. Trends Cell Biol 10 60–67 [DOI] [PubMed] [Google Scholar]
- Jaffe MJ, Takahashi H, Biro RL (1985) A pea mutant for the study of hydrotropism in roots. Science 230 445–447 [DOI] [PubMed] [Google Scholar]
- Jander G, Norris SR, Rounsley SD, Bush DF, Levin IM, Last RL (2002) Arabidopsis map-based cloning in the post-genome era. Plant Physiol 129 440–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneyasu T, Kobayashi A, Nakayama M, Fujii N, Takahashi H, Miyazawa Y (2007) Auxin response, but not its polar transport, plays a role in hydrotropism of Arabidopsis roots. J Exp Bot 58 1143–1150 [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Takahashi A, Kakimoto Y, Miyazawa Y, Fujii N, Higashitani A, Takahashi H (2007) A gene essential for hydrotropism in roots. Proc Natl Acad Sci USA 104 4724–4729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U, Büttner G, Jürgens G (1993) Apical-basal pattern formation in the Arabidopsis embryo: studies on the role of the gnom gene. Development 117 149–162 [Google Scholar]
- Monshausen GB, Sarah JS, Gilroy S (2008) Touch sensing and thigmotropism. In S Gilroy, PH Masson, eds, Plant Tropisms. Blackwell Publishing, Ames, IA, pp 91–122
- Steinmann T, Geldner N, Grebe M, Mangold S, Jackson CL, Paris S, Gälweller L, Palme K, Jürgens G (1999) Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science 286 316–318 [DOI] [PubMed] [Google Scholar]
- Takahashi H (1994) Hydrotropism and its interaction with gravitropism in roots. Plant Soil 165 301–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H (1997) Hydrotropism: the current state of our knowledge. J Plant Res 110 163–169 [DOI] [PubMed] [Google Scholar]
- Takahashi N, Goto N, Okada K, Takahashi H (2002) Hydrotropism in abscisic acid, wavy, and gravitropic mutants of Arabidopsis thaliana. Planta 216 203–211 [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.