Abstract
Ascorbic acid (AA) protects plants against abiotic stress. Previous studies suggested that this antioxidant is also involved in the control of flowering. To decipher how AA influences flowering time, we studied the four AA-deficient Arabidopsis (Arabidopsis thaliana) mutants vtc1-1, vtc2-1, vtc3-1, and vtc4-1 when grown under short and long days. These mutants flowered and senesced before the wild type irrespective of the photoperiod, a response that cannot simply be attributed to slightly elevated oxidative stress in the mutants. Transcript profiling of various flowering pathway genes revealed a correlation of altered mRNA levels and flowering time. For example, circadian clock and photoperiodic pathway genes were significantly higher in the vtc mutants than in the wild type under both short and long days, a result that is consistent with the early-flowering phenotype of the mutants. In contrast, when the AA content was artificially increased, flowering was delayed, which correlated with lower mRNA levels of circadian clock and photoperiodic pathway genes compared with plants treated with water. Similar observations were made for the autonomous pathway. Genetic analyses demonstrated that various photoperiodic and autonomous pathway mutants are epistatic to the vtc1-1 mutant. In conclusion, our transcript and genetic analyses suggest that AA acts upstream of the photoperiodic and autonomous pathways.
In higher plants, the timing of the transition from the vegetative to the reproductive phase is essential to ensure reproductive success. Flowering time is controlled by external and internal factors that are integrated in a complex gene regulatory network that ensures the expression of flowering genes, resulting in flower formation (Jack, 2004; Corbesier and Coupland, 2005). Environmental factors that regulate flowering include daylength, light, and temperature. The plant hormone GA is an important internal factor that controls flowering. Therefore, we differentiate four flowering pathways in the facultative long-day plant Arabidopsis (Arabidopsis thaliana): the photoperiodic, vernalization, autonomous, and GA pathways.
One of the most important environmental factors that affect floral transition is the change in daylength (photoperiod). A role of photoperiod was originally proposed by Tournois and Klebs (Tournois, 1912; Klebs, 1913). In the 1920s, Garner and Allard (1920, 1923) were the first who discovered that flowering and other developmental responses could be controlled by exposure to short day (SD) or long day (LD) depending on the plant species. They introduced the terms “photoperiod,” which defines the recurring duration of daily light and dark periods, and “photoperiodism,” which defines the responses to photoperiod. Numerous studies have been devoted to elucidating the molecular mechanisms of the photoperiodic flowering pathway (for comprehensive reviews, see Imaizumi and Kay, 2006; Kobayashi and Weigel, 2007). This pathway, which consists of a circadian clock and a circadian-regulated daylength measurement mechanism, promotes flowering specifically under LD. The Arabidopsis circadian clock is set by light signals that are perceived by red and far-red light receptors (phytochromes; primarily PHYA and PHYB) and by blue light receptors (cryptochromes; CRY1 and CRY2). The core oscillator is composed of proteins, including CCA1 (CIRCADIAN CLOCK ASSOCIATED1), LHY (LATE ELONGATED HYPOCOTYL), and TOC1 (TIMING OF CAB EXPRESSION1), that are regulated in a negative-feedback loop (Schaffer et al., 1998; Wang and Tobin, 1998; Strayer et al., 2000; Alabadi et al., 2001). The circadian clock takes part in the daylength measurement mechanism that is determined by the regulation of CONSTANS (CO) gene expression and the light regulation of CO protein stability and activity (Hayama and Coupland, 2004). Induction of flowering in LD is regulated by the gene sequence GIGANTEA (GI)-CO-FLOWERING LOCUS T (FT). In the leaves, GI activates CO transcription regardless of photoperiod (Fowler et al., 1999). CO transcription is regulated by a number of factors, including FKF1, GI, and CDF1 (Fowler et al., 1999; Suárez-López et al., 2001; Imaizumi et al., 2003, 2005; Chen and Ni, 2006; Sawa et al., 2007). CO protein is stabilized by PHYA, CRY1, and CRY2, whereas PHYB promotes the degradation of CO (Valverde et al., 2004). Recently, a role of CRY signaling in suppressing COP1-mediated degradation of CO in the dark was also reported (Jang et al., 2008; Liu et al., 2008). CO induces the expression of the floral integrator FT (Putterill et al., 2004). Finally, FT protein moves through the phloem to the shoot apex, where FT interacts with the FD transcription factor to induce the expression of the floral identity gene APETALA1, which requires the transcription factor LFY (Abe et al., 2005; Wigge et al., 2005; Corbesier et al., 2007). Expression of LFY is up-regulated by FT (Blazquez, 2005).
Vernalization (i.e. the promotion of flowering by low-temperature treatment) acts, in Arabidopsis, by repression of the floral repressor FLOWERING LOCUS C (FLC; Martinez-Zapater et al., 1994). Through the action of various genes, including FCA (for FLOWERING TIME CONTROL PROTEIN α, β, β, δ), FLC is also repressed by an autonomous pathway that induces flowering independently of environmental cues (Quesada et al., 2003; Boss et al., 2004). Finally, GA promotes flowering of Arabidopsis under SD (Wilson et al., 1992; Blazquez et al., 1998; Eriksson et al., 2006). These four flowering pathways converge on the transcriptional regulation of FT and SUPPRESSOR OF CONSTANS1, which promote LFY expression to confer floral identity on developing floral primordia (Corbesier and Coupland, 2005).
Flowering time can also be influenced by other factors. Plants exhibit accelerated flowering in response to shade, drought, low nutrients, decreased light quality, heat, and general oxidative stress (Halliday et al., 1994; Martinez-Zapater et al., 1994; Levy and Dean, 1998; Miller et al., 2007). Furthermore, early work suggested a role of ascorbic acid (AA) in the control of flowering (Chinoy et al., 1957; Hillman, 1962; Bharti and Garg, 1970). Recently, AA-deficient Arabidopsis mutants have been reported to exhibit an altered flowering phenotype. Four AA-deficient mutants, vtc1, vtc2, vtc3, and vtc4, were originally isolated by Conklin and coworkers (1996, 2000). These mutants greatly aided in the identification of enzymes that catalyze steps in the predominant AA biosynthetic pathway, which was elucidated by Wheeler et al. (1998). The VTC1 gene encodes a GDP-Man pyrophosphorylase (Conklin et al., 1999), and the VTC4 gene encodes an l-Gal-1-P phosphatase (Laing et al., 2004; Conklin et al., 2006). The VTC2 gene has been cloned (Jander et al., 2002) and has recently been identified as a GDP-l-Gal phosphorylase/l-Gal guanylyl-transferase (Laing et al., 2007; Linster et al., 2007; Smirnoff et al., 2007). The VTC3 gene has not yet been identified. The point mutations in these VTC genes result in the formation of the respective proteins with decreased enzyme activity. Therefore, the vtc mutants contain between 20% and 50% of the wild-type AA content (Conklin, 2001). The AA-deficient mutant vtc1 was reported to flower and senesce before the wild type when grown under LD (Barth et al., 2004; Conklin and Barth, 2004). Interestingly, this mutant and vtc2 were shown to exhibit delayed flowering and senescence when grown under SD (Pavet et al., 2005). Further support that perturbation of AA levels affects flowering time comes from the observation that artificially increasing the AA content delays flowering in LD-grown Arabidopsis (Attolico and De Tullio, 2006) and Brassica rapa (Daniela and De Tullio, 2007) but causes accelerated flowering in B. rapa when grown under SD (Daniela and De Tullio, 2007). Based on these reports and current knowledge about mechanisms regulating flowering, several hypotheses were formulated to explain the contrasting flowering phenotypes when AA levels are altered (Barth et al., 2006). However, experimental support for these hypotheses is still lacking.
In order to understand how AA influences flowering time, we exposed the four AA-deficient Arabidopsis mutant lines vtc1-1, vtc2-1, vtc3-1, and vtc4-1 (Conklin et al., 2000) to SD and LD conditions and assessed their flowering phenotypes. We examined the expression of key regulatory genes in the photoperiodic, autonomous, and GA pathways and generated double mutants of vtc1-1 and photoperiodic as well as autonomous flowering pathway mutants to investigate whether AA specifically affects any of the known pathways.
RESULTS
AA Deficiency Promotes Flowering and Senescence under Both SD and LD
To assess the flowering phenotype of the wild type and the vtc mutants, plants were grown under SD (10 h of light/14 h of dark) and LD (16 h of light/8 h of dark). Vegetative and reproductive growth was assessed over a period of 8 to 11 weeks. Under SD, vtc1-1, vtc3-1, and vtc4-1 mutants started flowering at 7 weeks after sowing, whereas the wild type and vtc2-1 started to produce flowers at 9 weeks after sowing (Fig. 1A). Under LD, all vtc mutants started to produce inflorescences about 3 weeks after sowing, whereas the wild type started flowering 4 weeks after sowing (Fig. 1A). It is well established that late-flowering plants form more leaves (Koornneef et al., 1991). Indeed, the early-flowering phenotype of the vtc mutants correlated with an approximately 20% decreased number of rosette leaves in comparison with the wild type when grown under SD (Fig. 1, B and C) and LD (Fig. 1, B and D), with the exception of vtc2-1 under SD. The vtc2-1 mutant produced significantly fewer leaves than the wild type but more leaves than the other vtc mutants under SD (Fig. 1B). However, flower buds appeared at the same time as in the wild type. In addition to the vtc1-1, vtc2-1, vtc3-1, and vtc4-1 mutants, we also examined the flowering phenotype of the vtc2-3 mutant and of vtc mutants that were not yet backcrossed to the wild type. These mutant plants also showed early flowering (Supplemental Fig. S1), demonstrating that this phenotype cosegregated with AA deficiency. Based on the early-flowering phenotype of the vtc mutants, we hypothesized that the mutants enter senescence before the wild type. In fact, senescence of rosette leaves occurs earlier in the vtc mutants than in the wild type irrespective of the photoperiod (Fig. 1, C and D).
Figure 1.
Flowering and senescence phenotypes of wild-type and vtc mutant plants. A, Col wild-type (Col WT) and vtc mutant plants were grown under SD and LD, and photographs were taken at 10 and 4 weeks after sowing. B, Number of rosette leaves when inflorescences were 1 cm in length. Means ± se of 10 individual plants are shown. Significant differences in comparison with the wild type are indicated with asterisks: *** P < 0.001, by Student's t test. C and D, Number of leaves and senescence phenotypes of the wild-type and vtc mutant plants grown under SD and LD.
In total, our data suggest that AA deficiency causes significantly early flowering and senescence under both SD and LD. It is known that various stress conditions can promote flowering and senescence (Bernier et al., 1993; Martinez-Zapater et al., 1994; Scholl et al., 1998; Kus et al., 2002, Miller et al., 2007). According to the free radical theory of aging, low levels of antioxidants promote the formation of reactive oxygen species (ROS) and thus advance aging and senescence (Harman, 1956). Therefore, we tested whether the vtc mutants suffer from oxidative stress by measuring the hydrogen peroxide (H2O2) content.
AA Deficiency Results in Slightly Elevated Levels of H2O2
Rosette leaves of 3-week-old wild-type and mutant plants grown under SD did not show significant differences in the endogenous H2O2 content. However, when plants were 7 weeks old, H2O2 levels were about 40% to 90% higher in vtc1-1 and vtc2-1 than in the wild type but were unchanged in vtc3-1 and vtc4-1 (Supplemental Fig. S2A). In LD-grown mutants, the H2O2 content was typically more elevated than in SD-grown mutants, with vtc1-1 and vtc2-1 generally exhibiting higher H2O2 levels than vtc3-1 and vtc4-1, which correlated with the endogenous amount of leaf AA in the mutants (Supplemental Fig. S3B; Conklin et al., 2000; Conklin, 2001). Note that all vtc mutants started flowering at about 3 and 7 weeks after sowing when grown under LD and SD, respectively (except for vtc2-1). However, H2O2 levels were not significantly elevated in vtc3-1 and vtc4-1 compared with the wild type in SD and for vtc4-1 in LD at the time of flowering, suggesting that the early-flowering phenotype is not caused just by elevated oxidative stress in the vtc mutants. To better define the role of AA deficiency in the control of flowering time, we investigated whether the vtc mutants exhibit altered expression of key regulatory genes controlling known flowering pathways.
AA Deficiency Promotes the Expression of Genes Controlling the Circadian Clock and the Photoperiodic Flowering Pathway
The photoperiodic pathway regulates flowering in long photoperiods in the facultative LD plant Arabidopsis (Komeda, 2004; Corbesier and Coupland, 2005; Imaizumi and Kay, 2006). Since the vtc mutants had an early-flowering phenotype under LD, we investigated whether transcript levels of circadian clock and photoperiodic pathway genes are heightened in the vtc mutants under LD and whether the expression of photoperiodic genes is altered under SD.
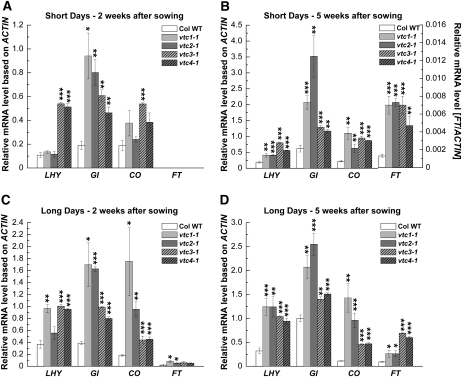
Transcription levels were determined in 2- and 5-week old rosette leaves of plants grown under both SD and LD. Neither the wild type nor the vtc mutants flowered at these two developmental stages under SD. Under LD, the 2-week-old plants represent the vegetative state, whereas all genotypes were at the reproductive state when they were 5 weeks old (Fig. 1). Transcript levels of LHY, a component of the circadian oscillator (Corbesier and Coupland, 2005; Allen et al., 2006), and genes in the photoperiodic pathway, including GI, CO, and FT, are in many cases more than 2-fold up-regulated in the vtc mutants under both SD (Fig. 2, A and B) and LD (Fig. 2, C and D). Under the PCR conditions used here, expression of FT was not detected in 2-week-old SD-grown plants (Fig. 2A) and expression was low in 5-week-old plants under SD (see different scale on the right y axis in Fig. 2B). This was expected, as vtc mutants started flowering only 7 weeks after sowing (see above). However, note that mRNA levels in vtc mutants are generally elevated at an early developmental stage (2-week-old seedlings), suggesting that AA deficiency constitutively alters the expression of these genes irrespective of the photoperiod and that these flowering time genes act early in development (Araki and Komeda, 1993; Simon et al., 1996).
Figure 2.
Relative transcript levels, based on ACTIN, of circadian clock and photoperiodic pathway genes in Col wild-type (Col WT) and vtc mutant plants. Plants were grown under SD (A and B) and LD (C and D). Transcript levels of the circadian clock gene LHY and the photoperiodic flowering pathway genes GI, CO, and FT were determined by reverse transcription-PCR from leaf tissue of 2- and 5-week-old plants harvested at 4 h after lights were turned on. Note the different scale to visualize FT expression in B. Expression data ± se of three individual samples per genotype are shown. Significant differences in comparison with the wild type are indicated with asterisks: * P < 0.05, ** P < 0.01, *** P < 0.001, by Student's t test.
In summary, the early-flowering phenotype of the vtc mutants is associated with significantly higher mRNA levels of circadian clock and photoperiodic pathway genes when plants are grown under either LD or SD. Since the circadian clock regulates GI and CO transcription, we investigated whether AA deficiency influences circadian rhythms.
AA Deficiency Increases the Amplitude of Transcription of Circadian Oscillator Genes But Does Not Affect Circadian Period
To determine whether AA affects circadian rhythms, we entrained the wild-type and vtc mutant plants in day-neutral conditions (12 h of light/12 h of dark). Three weeks after sowing, plants were shifted to constant light. Starting in the last 12-h-light/12-h-dark cycle, expression levels of LHY, TOC1, GI, and CO were determined for an additional 48 h under constant light. The oscillator establishes the rhythm of CO gene expression, which is mediated by GI (Mizoguchi et al., 2005). We investigated whether AA deficiency alters circadian clock period.
In comparison with the wild type, the amplitude of the gene expression rhythm was higher in vtc1-1 and vtc3-1, with the trough level of expression remaining constant but the peak level increased. This expression pattern was most pronounced under free-running (i.e. constant light) conditions (Supplemental Fig. S4). Thus, our data indicate that LHY, TOC1, GI, and CO are constitutively up-regulated when endogenous AA levels are low. However, there is no effect on period. Therefore, these data raised the question of what factors would drive this up-regulation. Since light is an important external factor that entrains and maintains circadian rhythms, we investigated whether light input mediated through photoreceptors is altered in the vtc mutants compared with the wild type.
AA Deficiency Alters the Expression of Phytochromes and Cryptochromes
Light is perceived by phytochromes and cryptochromes. PHYA, CRY1, and CRY2 positively affect flowering, whereas PHYB represses flowering (Cerdan and Chory, 2003; El-Din El-Assal et al., 2003; Liu et al., 2008). We examined whether vtc mutants contain altered transcript levels of PHYA, CRY1, CRY2, and PHYB.
Leaves harvested from 5-week-old vtc mutants grown under SD and LD generally had significantly higher levels of PHYA, CRY1, and CRY2, whereas PHYB mRNA levels were lower in comparison with the wild type (Supplemental Fig. S5). However, differences in transcript levels were in most cases less than 2-fold. This might explain why we did not observe altered hypocotyl growth when plants were grown under dark, white, red, or blue light (Supplemental Fig. S6).
To further investigate a possible effect of AA on PHYB, we tested whether artificially increasing the AA content in the early-flowering phyB-9 mutant (Columbia-0 [Col] background) by spraying plants with the AA precursor l-Gal would alter flowering time. Although leaf AA levels were significantly elevated in l-Gal-sprayed compared with water-treated phyB-9 plants (Supplemental Fig. S7A), flowering was not affected (Supplemental Fig. S7, B and C), suggesting that functional PHYB is required for delayed flowering through degradation of CO, as expected (Cerdan and Chory, 2003; El-Din El-Assal et al., 2003). However, this experiment also suggests that early flowering in phyB mutants is AA independent. This is supported by the fact that phyB mutants are not AA-deficient. The AA content decreases more rapidly in phyB mutants than in the wild type with increasing age, an expected result given that phyB mutants are early flowering and senesce after flowering (Supplemental Fig. S7D).
Taken together, our results show that AA deficiency has a modest effect on transcription of red and blue light receptors that influence the expression of genes in the photoperiodic flowering pathway. However, we cannot rule out that AA deficiency influences other flowering pathways, which could contribute to the early-flowering phenotype of the vtc mutants.
AA Deficiency Alters the Expression of FLC
To test whether the autonomous pathway affects flowering in the vtc mutants, we assessed transcript levels of the floral repressor FLC, a key integrator in the autonomous pathway. High levels of FLC suppress flowering (Michaels and Amasino, 1999; Sheldon et al., 1999). Therefore, we investigated whether FLC mRNA levels are lower in vtc mutants compared with the wild type. Since FCA is one of several autonomous pathway genes that regulate FLC, we also evaluated FCA mRNA levels in vtc mutants.
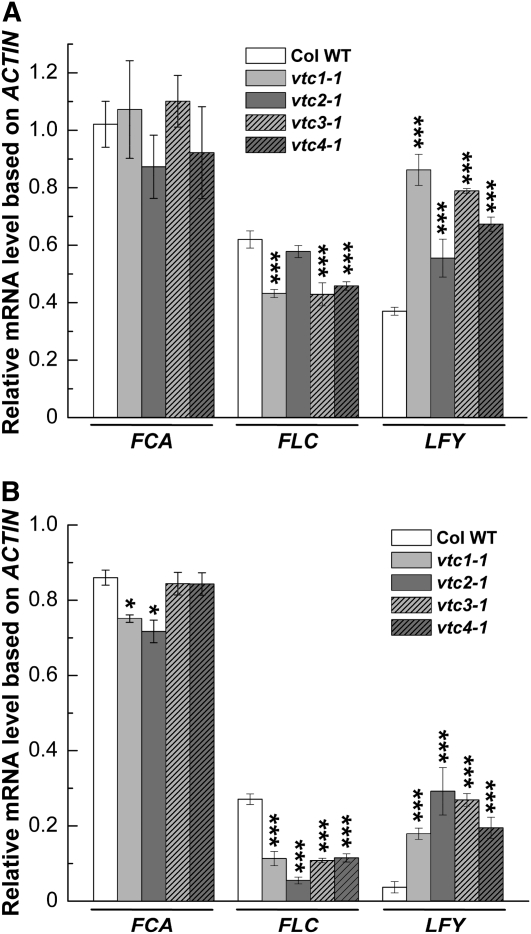
The FCA transcript is alternatively spliced, resulting in the formation of the four different transcripts, α, β, β, and δ (Macknight et al., 1997). We used primers that allowed us to amplify a cDNA product generated from the α, β, and δ transcripts. FCA-β is the only form encoding functional FCA (Macknight et al., 2002). FCA mRNA levels were not altered in the vtc mutants compared with the wild type when plants were grown under SD (Fig. 3A). FCA transcripts were slightly decreased in vtc1-1 and vtc2-1, but not in vtc3-1 and vtc4-1, when grown under LD (Fig. 3B). However, FLC transcript levels were approximately 30% lower in SD-grown vtc1-1, vtc3-1, and vtc4-1 mutants compared with the wild type, whereas FLC mRNA levels were similar in vtc2-1 and the wild type (Fig. 3A). In contrast, FLC transcript levels were more than 2-fold lower (P < 0.001) in all vtc mutants than in the wild type under LD (Fig. 3B). Under SD, LFY mRNA levels were approximately two times higher in the vtc mutants than in the wild type, with the exception of vtc2-1 (Fig. 3A), which is in line with the observed flowering phenotype of the mutants (compare with Fig. 1). Even higher transcript levels of LFY were observed under LD (Fig. 3B).
Figure 3.
Expression analysis of FCA, FLC, and LFY in Col wild-type (Col WT) and vtc mutant plants. Relative transcript levels of FCA and FLC, which are key regulatory genes in the autonomous pathway, and the floral meristem identity gene LFY in inflorescences of 11-week-old plants grown under SD (A) and 5-week-old plants grown under LD (B) are shown. Inflorescence tissues were harvested at 4 h after lights were turned on. Transcript levels were assessed in inflorescences of three individual plants and normalized to ACTIN. Means ± se of three independent replicates are shown. Significant differences in comparison with the wild type are indicated with asterisks: * P < 0.05, *** P < 0.001, by Student's t test.
Under noninductive SD conditions, flower initiation depends on the plant hormone GA. The vtc1 mutant has been reported to contain decreased levels of GA1 and GA4 and altered expression of GA biosynthetic genes (Kiddle, 2004; Foyer et al., 2007). We determined expression levels of three AA-dependent GA biosynthetic genes (gibberellin 20-oxidase 1 [GA20OX1], gibberellin 3-oxidase 2 [GA3OX2], and gibberellin 3-oxidase 4 [GA3OX4]; Supplemental Table S2) but could not detect significant differences in expression between the vtc mutants and the wild type. Only transcript levels of GA3OX4, which could convert GA20 into GA1 and/or GA4 (Lester et al., 2005; Eriksson et al., 2006), were higher in vtc1-1 in comparison with the wild type under SD (Supplemental Fig. S8A) but not under LD (Supplemental Fig. S8B).
In summary, although GA levels were not measured directly, our results indicate that the GA pathway may not or may only partially contribute to the early-flowering phenotype in the vtc mutants. To further investigate the role of AA on flowering time, we analyzed the flowering phenotype and expression of genes in the photoperiodic and autonomous pathways in plants containing artificially elevated levels of AA.
Artificially Increasing the Level of AA Delays Flowering and Senescence
We presented evidence that low levels of AA confer early flowering that correlates with an up-regulation of circadian clock and photoperiodic pathway genes and a down-regulation of FLC mRNA. Conversely, we asked whether flowering and senescence can be delayed when we artificially increase the endogenous AA content and whether genes that are up-regulated in the vtc mutants in comparison with the wild type are down-regulated in plants containing elevated levels of AA. To elevate AA levels, plants were sprayed with l-Gal, an intermediate in the AA biosynthetic pathway (Wheeler et al., 1998).
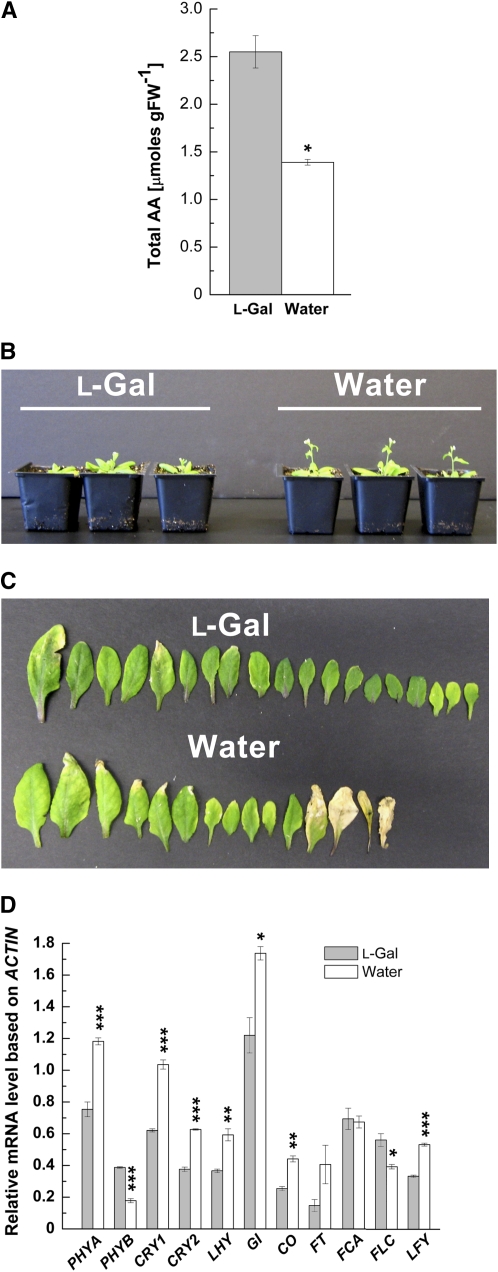
In comparison with plants sprayed with water, l-Gal treatment resulted in an approximately 2-fold increase in the total AA content in LD-grown plants (Fig. 4A). Flowering of l-Gal-sprayed plants grown under LD was delayed by about 5 d in comparison with plants sprayed with water (Fig. 4B). This is supported by the fact that l-Gal-sprayed plants developed 17 ± 0.4 (se) rosette leaves, whereas water-sprayed plants formed only 13 ± 0.4 rosette leaves (n = 16 in both cases; Fig. 4C). The delayed-flowering phenotype was accompanied by delayed senescence (Fig. 4C). In comparison with the water-sprayed plants, we detected lower mRNA levels of PHYA, CRY1, CRY2, LHY, GI, CO, FT, and LFY in l-Gal-sprayed plants, whereas transcript levels of PHYB and FLC were increased and those of FCA were not changed (Fig. 4D). Similar results were found in l-Gal- and water-treated wild-type plants grown under SD (Supplemental Fig. S9).
Figure 4.
Effects of l-Gal treatment on flowering time in LD-grown plants. A, Total AA content in 5-week-old Col wild-type plants sprayed with l-Gal or water. FW, Fresh weight. B, Flowering phenotype of l-Gal- and water-treated plants. C, Number of leaves and senescence phenotype of l-Gal- and water-sprayed plants. D, Expression analysis of photoreceptor (PHYA, PHYB, CRY1, and CRY2), circadian clock (LHY), photoperiodic pathway (GI, CO, and FT), autonomous (FCA and FLC), and floral meristem identity (LFY) genes in plants treated with l-Gal or water. Leaf and inflorescence tissues of 5-week-old plants were harvested at 4 h after lights were turned on. To assess the expression of FT, 33 PCR amplification cycles were run. Transcript levels were based on ACTIN. Results represent means ± se of three independent replicates. Significant differences between l-Gal and water treatments are indicated with asterisks: * P < 0.05, ** P < 0.01, *** P < 0.001, by Student's t test.
In summary, the results presented in Figure 4 and Supplemental Figure S9 are in accordance with data obtained for the AA-deficient vtc mutants. Furthermore, these data support our observation that alterations in the leaf AA content significantly affect the onset of flowering that correlates with transcriptional changes of genes in the photoperiodic, autonomous, and light perception pathways. Low levels of AA promote flowering and senescence, whereas high levels of AA delay flowering and senescence irrespective of the photoperiod. The data presented above suggest that AA acts upstream of pathways that control flowering. To test this, we crossed the early-flowering mutant vtc1-1 to photoperiodic and autonomous pathway mutants, which are delayed in flowering.
vtc1-1 gi-1, vtc1-1 co-2, vtc1-1 ft-1, and vtc1-1 fca-1 Double Mutants Exhibit Delayed Flowering Despite Their AA Deficiency
To test whether AA plays a specific role in the photoperiodic and/or the autonomous pathways, we crossed vtc1-1 to the late-flowering photoperiodic pathway mutants gi-1, co-2, and ft-1 and to the autonomous pathway mutant fca-1, which is also delayed in flowering (Koornneef et al., 1991; Putterill et al., 1995; Macknight et al., 1997; Fowler et al., 1999; Suárez-López et al., 2001). If AA specifically acts in the autonomous pathway, vtc1-1 gi-1, vtc1-1 co-2, and vtc1-1 ft-1 double mutants should exhibit a promotion of flowering compared with gi-1, co-2, and ft-1 single mutants due to the activity of the autonomous pathway in vtc1-1 (Fig. 3). Likewise, vtc1-1 fca-1 double mutants are expected to exhibit earlier flowering than fca-1 single mutants due to the enhanced activity of the photoperiodic pathway measured in vtc1-1 (Fig. 2; Supplemental Fig. S4).
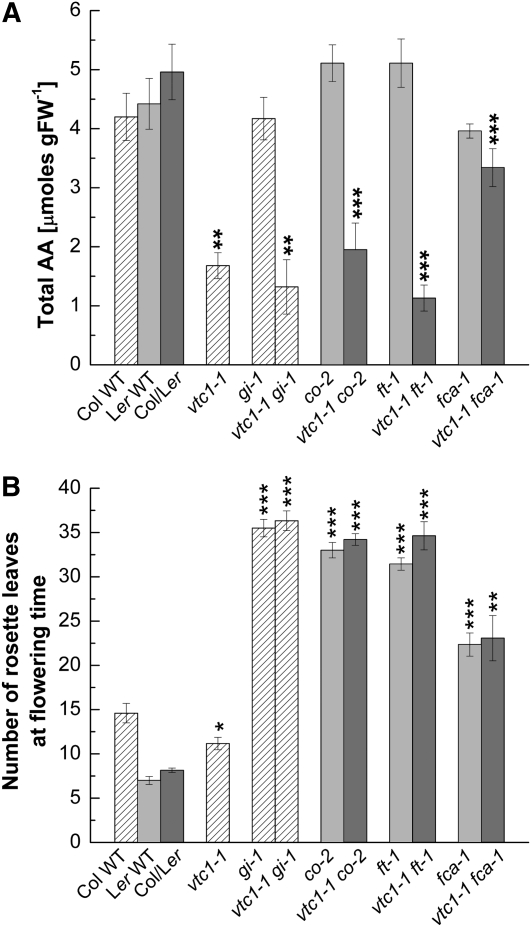
All double mutants displayed a delayed-flowering phenotype despite their AA deficiency (Fig. 5A). Note that vtc1-1 fca-1 has approximately 70% of the Col/Landsberg erecta (Ler) wild-type AA content, suggesting that fca-1 can partially suppress the vtc1-1 mutation. All homozygous double mutants had a similar number of rosette leaves as the gi-1, co-2, ft-1, and fca-1 single mutants, whereas vtc1-1 produced fewer rosette leaves than the Col wild type under LD (Fig. 5B) and SD (Supplemental Fig. S10). Note that the se bars for both the AA content and flowering time data in the Col/Ler wild type and in the double mutants of two different backgrounds are comparable to the se bars of the respective wild-type controls, suggesting low genetic variability in the pools of plants used for the experiments.
Figure 5.
Effects of the vtc1-1 mutation on flowering time and AA content in the background of photoperiodic and autonomous pathway mutants. Plants were grown under LD. A, Total AA content in 3-week-old wild-type (WT) controls and single and double mutants. Means ± se of three to six independent replicates per genotype are shown. FW, Fresh weight. B, Total rosette leaf number of all genotypes at flowering time. Results depict means ± se of nine to 17 independent plants per genotype. Shading patterns indicate plants of the same genetic background, allowing for easier statistical comparison of single and double mutants with their respective wild-type controls. Asterisks denote significant differences: * P < 0.05, ** P < 0.01, *** P < 0.001, by Student's t test.
In summary, our genetic analysis demonstrates that the gi-1, co-2, ft-1, and fca-1 mutations are all epistatic to vtc1-1, suggesting that GI, CO, FT, and FCA play roles in the promotion of flowering of vtc1-1. However, AA does not appear to have a specific role in any of the known flowering pathways.
DISCUSSION
Recent publications reported contrasting flowering and senescence phenotypes of the vtc1 and vtc2 mutants, exhibiting delayed and early flowering/senescence under SD (Pastori et al., 2003; Pavet et al., 2005) and LD (Barth et al., 2004; Conklin and Barth, 2004), respectively. In order to understand how AA influences flowering time, we examined the flowering phenotype of the wild type in comparison with four AA-deficient mutant lines grown under SD and LD.
We found that all four vtc mutants, with the exception of vtc2-1 grown under SD, exhibit early flowering irrespective of the photoperiod (Fig. 1). This is in contrast to findings by Pastori et al. (2003) and Pavet et al. (2005). Investigation of vtc mutants, which were not yet backcrossed to the wild type, also revealed an early-flowering phenotype (Supplemental Fig. S1), suggesting that the observed differences are not due to the presence of additional mutations and are related to AA deficiency. Therefore, the reason for the observed contrasting flowering phenotypes is unclear at present. Growth conditions, including nutrient supply and light intensity, may alter flowering time and could account for the differences in the time of flowering. For example, the lower light intensity used in the experiments reported here (160 μmol photons m−2 s−1) under both SD and LD conditions compared with the higher one used by the Pastori and Pavet groups (250 μmol photons m−2 s−1) under SD could differentially affect AA pool size and redox status. Higher light intensities increase leaf ascorbate concentration (Smirnoff, 2000; Bartoli et al., 2006; Dowdle et al., 2007), whereas changes in photoperiods only affect AA pool size slightly or not at all (Queval et al., 2007; Supplemental Fig. S3). Thus, one would expect delayed flowering in plants grown at higher light intensities. It would hence be worthwhile to investigate the flowering time of vtc mutants grown under different light intensities.
Finally, based on rosette leaf number and transcript levels of photoperiodic pathway genes (Fig. 2, A and B), vtc2-1 would be considered early flowering under SD. However, flower buds emerged at the same time as in the wild type, which correlates with the expression of the autonomous pathway gene FLC (Fig. 3). The reason for the different response of vtc2-1 under SD is unclear at present. Nevertheless, we provide evidence that alterations in the AA content have a significant effect on flowering time.
AA Influences Flowering Time through a Mechanism That Is Independent of Its Antioxidant Activity
Early flowering in AA-deficient vtc mutants (Fig. 1) and late flowering in plants with artificially elevated AA levels (Fig. 4; Supplemental Fig. S9) could simply be explained by the antioxidant function of AA to scavenge ROS. Low levels of AA, as in the vtc mutants (Supplemental Fig. S3; Conklin et al., 2000), would be expected to result in elevated levels of ROS. Indeed, the vtc mutants contain elevated H2O2 levels (Supplemental Fig. S2), suggesting that they are suffering some oxidative stress under optimal growth conditions, ultimately resulting in early flowering/senescence. However, our data suggest that the early-flowering phenotype cannot be solely attributed to increased oxidative stress in the vtc mutants. This conclusion is supported by the following facts. First, oxidative stress in the vtc mutants compared with the wild type is generally higher under LD than SD, with little effect on vtc3-1 and vtc4-1 grown at SD. However, the flowering phenotype of the vtc mutants does not correlate with the endogenous H2O2 content (compare Fig. 1 and Supplemental Fig. S2). For example, the vtc3-1 and vtc4-1 mutants generally exhibit lower or similar levels of H2O2 than the wild type (Supplemental Fig. S2), but the mutants still exhibit an early-flowering phenotype (Fig. 1). Second, in comparison with the wild type, the difference in leaf number produced in the vtc mutants is similar under SD and LD (vtc mutants formed approximately 20% fewer leaves than the wild type under SD and LD, except for vtc2-1 grown under SD; Fig. 1B). Third, Arabidopsis mutants lacking cytosolic ascorbate peroxidase and exhibiting constitutive accumulation of H2O2 under optimal growth conditions show delayed flowering under LD and constant light (Pnueli et al., 2003). It should be mentioned, however, that some double mutants deficient in cytosolic and thylakoid ascorbate peroxidase suffering oxidative stress exhibit early flowering (Miller et al., 2007). Fourth, double mutants of vtc1-1 and flowering time mutants are AA deficient (Fig. 5A) but have delayed flowering (Fig. 5B). In summary, our data demonstrate that AA influences flowering in a photoperiod-independent manner through factors (discussed in detail below) other than or perhaps in addition to ROS and/or redox changes. Therefore, expression and genetic analyses were conducted to establish whether AA specifically acts in known flowering time pathways.
AA Does Not Seem to Act Specifically in Any of the Known Flowering Pathways and May Play a General Role in Responding to Environmental Signals
Data presented in this study show that AA has a significant effect on flowering time and that alterations in the AA content confer differential gene expression of all genes in known pathways. The alterations in gene expression patterns are consistent with the observed flowering phenotypes (Figs. 2–4; Supplemental Figs. S4, S5, S8, and S9). In fact, the expression patterns in the vtc mutants and in wild-type plants with artificially altered AA levels would fit the external coincidence model (Yanovsky and Kay, 2003; Hayama and Coupland, 2004; Putterill et al., 2004; Saunders, 2005; Imaizumi and Kay, 2006; Kobayashi and Weigel, 2007) as well as the function of phytochromes and cryptochromes in the regulation of CO (Reed et al., 1993; Guo et al., 1998; Mockler et al., 1999, 2003; Yang et al., 2000; Cerdan and Chory, 2003; Valverde et al., 2004). AA deficiency, however, does not affect the circadian clock despite enhancing the expression of clock genes, including LHY (Fig. 2; Supplemental Fig. S4). Early flowering may be promoted through enhanced expression of GI, which promotes the expression of LHY/CCA1, CO, and FT, conferring early flowering. Such a phenomenon has been reported recently (Oda et al., 2004). Oda and coworkers (2004) suppressed the PHYTOCHROME INTERACTING FACTOR3 gene, resulting in higher CO and FT mRNA levels, and thus in early flowering, under LD without affecting circadian period.
Taken together, our expression and genetic analyses do not support a specific role of AA in the known flowering pathways (Fig. 5, B and C). The mechanism through which AA influences flowering time, therefore, remains unclear. Instead, the picture that emerges is that alterations in the AA content confer perturbations in plant metabolism and gene expression that indirectly affect flowering. The observed gene expression differences cause a variety of pleiotropic effects in the vtc mutants. This may be explained by the fact that, in addition to its function as an antioxidant, AA also serves as an essential cofactor for a variety of enzymes. For example, AA is required for the biosynthesis of the plant hormones abscisic acid, GA, and ethylene (Arrigoni and De Tullio, 2000, 2002). Previous studies have demonstrated a high content of abscisic acid (Pastori et al., 2003; S.O. Kotchoni and C. Barth, unpublished data) but low levels of GA (Kiddle, 2004; Foyer et al., 2007) in vtc1. Therefore, one would expect the late-flowering phenotype of the vtc mutants (Barth et al., 2006), as reported by Pastori et al. (2003) and Pavet et al. (2005).
In addition to changes in the abscisic acid and GA contents, low levels of AA promote the accumulation of the phytoalexin camalexin (Colville and Smirnoff, 2008) and salicylic acid (Barth et al., 2004; M. Mukherjee and C. Barth, unpublished data), which has been demonstrated to also influence flowering time (Martinez et al., 2004). It was also shown that de novo AA synthesis increases in response to methyl jasmonate (Sasaki-Sekimoto et al., 2005; Wolucka et al., 2005). Glutathione (Pavet et al., 2005) and α-tocopherol (Kanwischer et al., 2005) have been reported to be slightly elevated in vtc1 or unchanged in vtc mutants (Colville and Smirnoff, 2008). Consequently, alterations in the AA pool size result in hormonal changes, conferring altered gene expression, leading to a variety of phenotypes in the vtc mutants. These include altered flowering (Pastori et al., 2003; Conklin and Barth, 2004; this study), enhanced resistance to virulent pathogens (Barth et al., 2004; Pavet et al., 2005), altered primary root development (Olmos et al., 2006), changes in energy status (Wormuth et al., 2006), altered heat tolerance (Larkindale et al., 2005), increased sensitivity to salt stress (Huang et al., 2005), altered cell wall biosynthesis (Lukowitz et al., 2001), and sensitivity to ozone (Conklin et al., 1996, 2000). Whereas some of those phenotypes can clearly be attributed to the lack of antioxidant capacity of the vtc mutants, some phenotypes cannot simply be explained by elevated ROS levels and altered redox status in the mutants. In fact, the ascorbate oxidation state does not vary in the vtc mutants (Conklin et al., 2000; Colville and Smirnoff, 2008; M. Mukherjee and C. Barth, unpublished data). This study provides evidence that the AA pool size affects the regulation of growth and developmental and defense responses. Thus, it is possible that AA levels serve as an internal signal that helps properly poise plants to respond to environmental signals and facilitates the adjustment of plant development, including the transition from the vegetative to the reproductive phase. To evaluate this possibility, future experiments will have to address how plants perceive AA, how the endogenous AA content is regulated, and how plants translate alterations in the AA pool size into developmental adjustments.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) wild-type ecotype Col and previously described Arabidopsis mutants vtc1-1, vtc2-1, vtc3-1, and vtc4-1 (kindly provided by P. Conklin; Conklin et al., 1996, 2000; Conklin, 2001) were grown in a growth chamber (Percival). Mutant plants were backcrossed to Col wild type four times (vtc1-1), three times (vtc3-1), or two times (vtc2-1 and vtc4-1). The flowering time mutants gi-1 (CS3123), co-2 (CS175 and CS55), ft-1 (CS56), and fca-1 (CS167) and the wild type Ler (CS20) were obtained from the Arabidopsis Biological Resource Center Stock Center. Plants were grown on soil (Metromix 360; BFG Supplies) in replicate flats containing 32 inserts with wild-type controls and mutants always present on the same flat. Temperature in the chamber was 23°C at day and at night. Plants were grown under LD (16 h of light/8 h of dark, growth chamber lights turned on at 6:00 am and turned off at 10:00 pm) and SD (10 h of light/14 of h dark, growth chamber lights turned on at 6:00 am and turned off at 4:00 pm) at a light intensity of 160 μmol photons m−2 s−1 (fluorescent bulbs).
Two-week-old plants were harvested by pooling aboveground tissue of seedlings. When plants were 3 weeks old, rosette leaves of similar age were marked using a soft marker. Rosette leaves were pooled from two to four individual plants. Plants were only used once for tissue harvest to avoid gene expression alterations due to wound effects. In all cases, plant tissue was collected at 4 h after growth chamber lights were turned on, immediately frozen in liquid nitrogen, and stored at −80°C until further analysis.
Generation and Identification of Double Mutants
The vtc1-1 mutant (Col background; Conklin et al., 2000) was crossed with gi-1 (Col background; Fowler et al., 1999), co-2 (Ler background; Putterill et al., 1995), ft-1 (Ler background; Kardailsky et al., 1999), and fca-1 (Ler background; Macknight et al., 1997). F1 progeny of the crosses were allowed to self. F2 progeny were screened for AA deficiency (Conklin et al., 2000), and DNA was extracted from progeny that scored as AA deficient. Per plant, two PCR procedures were carried out to identify homozygous vtc1-1 gi-1, vtc1-1 co-2, vtc1-1 ft-1, and vtc1-1 fca-1 double mutants using the primers listed in Supplemental Table S1. F3 seeds from homozygous double mutants were used for experiments. The individual Col and Ler wild types and the Col × Ler crosses were used as controls. To minimize genetic variability, resulting from crossing two different backgrounds, seeds were pooled from 12 different crossing events of the Col and Ler wild-type controls (Col × Ler; Miller et al., 2007), and at least three independent double mutants (except for vtc1-1 co-2) were evaluated for flowering time.
Measurement of H2O2 Content
Freshly harvested rosette leaves (approximately 0.1 g) were incubated by shaking (250 rpm) in 3 mL of 25 mm phosphate buffer, pH 7.0, containing 0.05% guaiacol (Sigma) and 2.5 units mL−1 horseradish peroxidase (Sigma) in the dark at 25°C for 2 h. Absorbance of the solution was measured at 450 nm as described (Von Tiedemann, 1997). H2O2 concentrations were determined using a H2O2 standard curve, containing 5, 10, 25, 50, 75, 100, or 150 μm H2O2 (Sigma) as described (Kotchoni et al., 2006).
Determination of Flowering Time
Flowering time was assessed by counting the number of rosette leaves when flower bolts were 1 cm in length or when floral buds were visible at the center of the rosette.
Circadian Rhythm Experiments
Wild-type and vtc mutant plants were entrained in day-neutral (12 h of light/12 h of dark) conditions for 3 weeks and then subjected to constant light for 48 h. Rosette leaves were harvested starting in the last 12-h-light/12-h-dark cycle (chamber lights turned on at 6:00 am and turned off at 6:00 pm), beginning 1 h after lights were turned on. Tissue was harvested every 4 h in the last dark/light cycle, and tissue collection continued for another 48 h under constant light. Temperature in the growth chamber was 23°C throughout the experiment. The Percival growth chambers used for these experiments were programmed for temperature and photoperiod. A 12-h-light/12-h-dark photoperiod program with constant temperature was created for the first 3 weeks of growth. The program was then switched to constant light for another 48 h.
Exogenous Application of l-Gal
Wild-type plants were grown under SD and LD as described above. Plants were sprayed every other day with either water or 10 mm l-Gal. Spraying started when plants were 8 d old and continued until plants finished their life cycle. Tissue of 5- and 13-week-old plants grown under SD and LD was harvested for gene expression analyses.
Measuring AA Content
Leaf AA content was determined in whole 3-week-old rosettes using the ascorbate oxidase assay (Conklin et al., 1997) and the iron reduction assay (Dowdle et al., 2007).
RNA Isolation, cDNA Synthesis, and Gene Expression Analysis
Total RNA from rosette leaves and inflorescences was extracted using Tri-Reagent (Molecular Research Center). Five microliters of total RNA was subjected to reverse transcription using a first-strand cDNA synthesis kit (Invitrogen) and 10 pg of oligo(dT) primers. Two micrograms of cDNA was utilized for PCR using gene-specific primers (Supplemental Table S2), running 20 or 25 amplification cycles (linear range of amplification) unless otherwise noted. The linear range of amplification was determined by running increasing cycle numbers and analyzing the amount of cDNA fragments. PCR fragments were separated on 1% agarose gels containing ethidium bromide. Band intensities were quantified with ImageQuant 5.0 (Amersham Biosciences). A cDNA fragment generated from ACTIN served as an internal control.
Statistical Analyses
Data are expressed as mean values ± se. Experiments were repeated at least three times. P values were determined by Student's t test analysis.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Flowering phenotype of Col wild-type and vtc mutant plants under LD.
Supplemental Figure S2. H2O2 content in Col wild-type and vtc mutant plants grown under SD and LD.
Supplemental Figure S3. Total AA content in Col wild-type and vtc mutant plants grown under SD and LD.
Supplemental Figure S4. Circadian rhythms of clock and photoperiod pathway genes in Col wild-type and vtc mutant plants.
Supplemental Figure S5. Expression analysis of phytochrome and cryptochrome genes in Col wild-type and vtc mutant plants.
Supplemental Figure S6. Hypocotyl length in Col wild type, Ler wild type, vtc, and phytochrome and cryptochrome mutants.
Supplemental Figure S7. Effect of l-Gal on flowering time in phyB-9 and developmental changes in the AA content in wild-type, vtc1-1, and phyB plants grown under LD.
Supplemental Figure S8. Expression analysis of GA 3-oxidase 4 in Col wild-type and vtc mutant plants.
Supplemental Figure S9. Effect of l-Gal on flowering time in SD-grown plants.
Supplemental Figure S10. Flowering phenotype of wild-type controls, vtc1-1, and flowering time single mutants and double mutants with vtc1-1 under SD.
Supplemental Table S1. Sequences of oligonucleotide primers used for mutant identification.
Supplemental Table S2. Sequences of oligonucleotide primers used for gene expression analysis.
Supplementary Material
Acknowledgments
We thank Patricia Conklin for providing seeds of the vtc mutants and Georg Jander as well as Dale Karlson for helpful comments on the manuscript.
This work was supported by a National Aeronautics and Space Administration West Virginia EPSCoR Research Seed Grant (grant no. 10002987R) and a West Virginia University Summer Undergraduate Research Experience stipend.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Carina Barth (carina.barth@mail.wvu.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T (2005) FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309 1052–1056 [DOI] [PubMed] [Google Scholar]
- Alabadi D, Oyama T, Yanovsky MJ, Harmon FG, Mas P, Kay SA (2001) Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 293 880–883 [DOI] [PubMed] [Google Scholar]
- Allen T, Koustenis A, Theodorou G, Somers DE, Kay SA, Whitelam GC, Devlin PF (2006) Arabidopsis FHY3 specifically gates phytochrome signaling to the circadian clock. Plant Cell 18 2506–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Komeda Y (1993) Analysis of the role of the late-flowering locus, Gl, in the flowering of Arabidopsis thaliana. Plant J 3 231–239 [Google Scholar]
- Arrigoni O, De Tullio MC (2000) The role of ascorbic acid in cell metabolism: between gene-directed functions and unpredictable chemical reactions. J Plant Physiol 157 481–488 [Google Scholar]
- Arrigoni O, De Tullio MC (2002) Ascorbic acid: much more than just an antioxidant. Biochim Biophys Acta 1569 1–9 [DOI] [PubMed] [Google Scholar]
- Attolico AD, De Tullio MC (2006) Increased ascorbate content delays flowering in long-day grown Arabidopsis thaliana (L.). Heynh. Plant Physiol Biochem 44 462–466 [DOI] [PubMed] [Google Scholar]
- Barth C, De Tullio M, Conklin PL (2006) The role of ascorbic acid in the control of flowering time and the onset of senescence. J Exp Bot 57 1657–1665 [DOI] [PubMed] [Google Scholar]
- Barth C, Moeder W, Klessig DF, Conklin PL (2004) The timing of senescence and response to pathogens is altered in the ascorbate-deficient Arabidopsis mutant vitamin c-1. Plant Physiol 134 1784–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli CG, Yu J, Gomez F, Fernandez L, McIntosh L, Foyer CH (2006) Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. J Exp Bot 57 1621–1631 [DOI] [PubMed] [Google Scholar]
- Bernier G, Havelange A, Houssa C, Petitjean A, Lejeune P (1993) Physiological signals that induce flowering. Plant Cell 5 1147–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti S, Garg OP (1970) Changes in the ascorbic acid content of the lateral buds of soybean in relation to flower induction. Plant Cell Physiol 11 723–727 [Google Scholar]
- Blazquez MA (2005) Plant science. The right time and place for making flowers. Science 309 1024–1025 [DOI] [PubMed] [Google Scholar]
- Blazquez MA, Green R, Nilsson O, Sussman MR, Weigel D (1998) Gibberellins promote flowering of Arabidopsis by activating the LEAFY promoter. Plant Cell 10 791–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss PK, Bastow RM, Mylne JS, Dean C (2004) Multiple pathways in the decision to flower: enabling, promoting, and resetting. Plant Cell (Suppl) 16 S18–S31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdan PD, Chory J (2003) Regulation of flowering time by light quality. Nature 423 881–885 [DOI] [PubMed] [Google Scholar]
- Chen M, Ni M (2006) RFI2, a RING-domain zinc finger protein, negatively regulates CONSTANS expression and photoperiodic flowering. Plant J 46 823–833 [DOI] [PubMed] [Google Scholar]
- Chinoy JJ, Nanda KK, Garg OP (1957) Effect of ascorbic acid on growth and flowering of Trigonella foenum-graecum and Brassica chinensis. Physiol Plant 10 869–876 [Google Scholar]
- Colville L, Smirnoff N (2008) Antioxidant status, peroxidase activity, and PR protein transcript levels in ascorbate-deficient Arabidopsis thaliana vtc mutants. J Exp Bot 59 3857–3868 [DOI] [PubMed] [Google Scholar]
- Conklin PL (2001) Recent advances in the role and biosynthesis of ascorbic acid in plants. Plant Cell Environ 24 383–394 [Google Scholar]
- Conklin PL, Barth C (2004) Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant Cell Environ 27 959–971 [Google Scholar]
- Conklin PL, Gatzek S, Wheeler GL, Dowdle J, Raymond MJ, Rolinski S, Isupov M, Littlechild JA, Smirnoff N (2006) Arabidopsis thaliana VTC4 encodes l-galactose-1-P phosphatase, a plant ascorbic acid biosynthetic enzyme. J Biol Chem 281 15662–15670 [DOI] [PubMed] [Google Scholar]
- Conklin PL, Norris SR, Wheeler GL, Williams EH, Smirnoff N, Last RL (1999) Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proc Natl Acad Sci USA 96 4198–4203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Pallanca JE, Last RL, Smirnoff N (1997) l-Ascorbic acid metabolism in the ascorbate-deficient Arabidopsis mutant vtc1. Plant Physiol 115 1277–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Saracco SA, Norris SR, Last RL (2000) Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics 154 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Williams EH, Last RL (1996) Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc Natl Acad Sci USA 93 9970–9974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbesier L, Coupland G (2005) Photoperiodic flowering of Arabidopsis: integrating genetic and physiological approaches to characterization of the floral stimulus. Plant Cell Environ 28 54–66 [Google Scholar]
- Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, et al (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316 1030–1033 [DOI] [PubMed] [Google Scholar]
- Daniela AA, De Tullio M (2007) Contrasting effects of increased ascorbate content on growth and development of long-day and short-day grown Brassica rapa. Caryologia 60 185–187 [Google Scholar]
- Dowdle J, Ishikawa T, Gatzek S, Rolinski S, Smirnoff N (2007) Two genes in Arabidopsis thaliana encoding GDP-l-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J 52 673–689 [DOI] [PubMed] [Google Scholar]
- El-Din El-Assal S, Alonso-Blanco C, Peeters AJ, Wagemaker C, Weller JL, Koornneef M (2003) The role of cryptochrome 2 in flowering in Arabidopsis. Plant Physiol 133 1504–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson S, Bohlenius H, Moritz T, Nilsson O (2006) GA4 is the active gibberellin in the regulation of LEAFY transcription and Arabidopsis floral initiation. Plant Cell 18 2172–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler S, Lee K, Onouchi H, Samach A, Richardson K, Morris B, Coupland G, Putterill J (1999) GIGANTEA: a circadian clock-controlled gene that regulates photoperiodic flowering in Arabidopsis and encodes a protein with several possible membrane-spanning domains. EMBO J 18 4679–4688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Kiddle G, Verrier P (2007) Transcriptional profiling approaches to understanding how plants regulate growth and defence: a case study illustrated by analysis of the role of vitamin C. EXS 97 55–86 [DOI] [PubMed] [Google Scholar]
- Garner WW, Allard HA (1920) Effect of the relative length of day and night and other factors of the environment on growth and reproduction in plants. J Agric Res 18 553–606 [Google Scholar]
- Garner WW, Allard HA (1923) Further studies on photoperiodism, the response of plants to relative length of day and night. J Agric Res 23 871–920 [Google Scholar]
- Guo H, Yang H, Mockler TC, Lin C (1998) Regulation of flowering time by Arabidopsis photoreceptors. Science 279 1360–1363 [DOI] [PubMed] [Google Scholar]
- Halliday KJ, Koornneef M, Whitelam GC (1994) Phytochrome B and at least one other phytochrome mediate the accelerated flowering response of Arabidopsis thaliana L. to low red/far-red ratio. Plant Physiol 104 1311–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerontol 11 298–300 [DOI] [PubMed] [Google Scholar]
- Hayama R, Coupland G (2004) The molecular basis of diversity in the photoperiodic flowering responses of Arabidopsis and rice. Plant Physiol 135 677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman WS (1962) Experimental control of flowering in Lemna. IV. Inhibition of photoperiodic sensitivity by copper. Am J Bot 49 892–897 [Google Scholar]
- Huang C, He W, Guo J, Chang X, Su P, Zhang L (2005) Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J Exp Bot 56 3041–3049 [DOI] [PubMed] [Google Scholar]
- Imaizumi T, Kay SA (2006) Photoperiodic control of flowering: not only by coincidence. Trends Plant Sci 11 550–558 [DOI] [PubMed] [Google Scholar]
- Imaizumi T, Schultz TF, Harmon FG, Ho LA, Kay SA (2005) FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science 309 293–297 [DOI] [PubMed] [Google Scholar]
- Imaizumi T, Tran HG, Swartz TE, Briggs WR, Kay SA (2003) FKF1 is essential for photoperiodic-specific light signalling in Arabidopsis. Nature 426 302–306 [DOI] [PubMed] [Google Scholar]
- Jack T (2004) Molecular and genetic mechanisms of floral control. Plant Cell (Suppl) 16 S1–S17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jander G, Norris SR, Rounsley SD, Bush DF, Levin IM, Last RL (2002) Arabidopsis map-based cloning in the post-genome era. Plant Physiol 129 440–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Marchal V, Panigrahi KC, Wenkel S, Soppe W, Deng XW, Valverde F, Coupland G (2008) Arabidopsis COP1 shapes the temporal pattern of CO accumulation conferring a photoperiodic flowering response. EMBO J 27 1277–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwischer M, Porfirova S, Bergmuller E, Dormann P (2005) Alterations in tocopherol cyclase activity in transgenic and mutant plants of Arabidopsis affect tocopherol content, tocopherol composition, and oxidative stress. Plant Physiol 137 713–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D (1999) Activation tagging of the floral inducer FT. Science 286 1962–1965 [DOI] [PubMed] [Google Scholar]
- Kiddle G (2004) The role of ascorbate in plant defence and development. PhD thesis. University of Bristol, Bristol, UK
- Klebs G (1913) Über das Verhältnis der Aussenwelt zur Entwicklung der Pflanze. Heidelberger Academie der Wissenschaften 5 1–47 [Google Scholar]
- Kobayashi Y, Weigel D (2007) Move on up, it's time for change: mobile signals controlling photoperiod-dependent flowering. Genes Dev 21 2371–2384 [DOI] [PubMed] [Google Scholar]
- Komeda Y (2004) Genetic regulation of time to flower in Arabidopsis thaliana. Annu Rev Plant Biol 55 521–535 [DOI] [PubMed] [Google Scholar]
- Koornneef M, Hanhart CJ, van der Veen JH (1991) A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol Gen Genet 229 57–66 [DOI] [PubMed] [Google Scholar]
- Kotchoni SO, Kuhns C, Ditzer A, Kirch HH, Bartels D (2006) Over-expression of different aldehyde dehydrogenase genes in Arabidopsis thaliana confers tolerance to abiotic stress and protects plants against lipid peroxidation and oxidative stress. Plant Cell Environ 29 1033–1048 [DOI] [PubMed] [Google Scholar]
- Kus JV, Zaton K, Sarkar R, Cameron RK (2002) Age-related resistance in Arabidopsis is a developmentally regulated defense response to Pseudomonas syringae. Plant Cell 14 479–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing WA, Bulley S, Wright M, Cooney J, Jensen D, Barraclough D, MacRae E (2004) A highly specific l-galactose-1-phosphate phosphatase on the path to ascorbate biosynthesis. Proc Natl Acad Sci USA 101 16976–16981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing WA, Wright MA, Cooney J, Bulley SM (2007) The missing step of the l-galactose pathway of ascorbate biosynthesis in plants, an l-galactose guanyltransferase, increases leaf ascorbate content. Proc Natl Acad Sci USA 104 9534–9539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Hall JD, Knight MR, Vierling E (2005) Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol 138 882–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester DR, Phillips A, Hedden P, Andersson I (2005) Purification and kinetic studies of recombinant gibberellin dioxygenases. BMC Plant Biol 5 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy YY, Dean C (1998) The transition to flowering. Plant Cell 10 1973–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster CL, Gomez TA, Christensen KC, Adler LN, Young BD, Brenner C, Clarke SG (2007) Arabidopsis VTC2 encodes a GDP-l-galactose phosphorylase, the last unknown enzyme in the Smirnoff-Wheeler pathway to ascorbic acid in plants. J Biol Chem 282 18879–18885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LJ, Zhang YC, Li QH, Sang Y, Mao J, Lian HL, Wang L, Yang HQ (2008) COP1-mediated ubiquitination of CONSTANS is implicated in cryptochrome regulation of flowering in Arabidopsis. Plant Cell 20 292–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowitz W, Nickle TC, Meinke DW, Last RL, Conklin PL, Somerville CR (2001) Arabidopsis cyt1 mutants are deficient in a mannose-1-phosphate guanylyltransferase and point to a requirement of N-linked glycosylation for cellulose biosynthesis. Proc Natl Acad Sci USA 98 2262–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknight R, Bancroft I, Page T, Lister C, Schmidt R, Love K, Westphal L, Murphy G, Sherson S, Cobbett C, et al (1997) FCA, a gene controlling flowering time in Arabidopsis, encodes a protein containing RNA-binding domains. Cell 89 737–745 [DOI] [PubMed] [Google Scholar]
- Macknight R, Duroux M, Laurie R, Dijkwel P, Simpson G, Dean C (2002) Functional significance of the alternative transcript processing of the Arabidopsis floral promoter FCA. Plant Cell 14 877–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez C, Pons E, Prats G, Leon J (2004) Salicylic acid regulates flowering time and links defence responses and reproductive development. Plant J 37 209–217 [DOI] [PubMed] [Google Scholar]
- Martinez-Zapater JM, Coupland G, Dean C, Koornneef M (1994) The transition to flowering in Arabidopsis. In EM Meyerowitz, CR Somerville, eds, Arabidopsis. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 403–411
- Michaels SD, Amasino RM (1999) FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11 949–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Suzuki N, Rizhsky L, Hegie A, Koussevitzky S, Mittler R (2007) Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant Physiol 144 1777–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi T, Wright L, Fujiwara S, Cremer F, Lee K, Onouchi H, Mouradov A, Fowler S, Kamada H, Putterill J, et al (2005) Distinct roles of GIGANTEA in promoting flowering and regulating circadian rhythms in Arabidopsis. Plant Cell 17 2255–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockler T, Yang H, Yu X, Parikh D, Cheng YC, Dolan S, Lin C (2003) Regulation of photoperiodic flowering by Arabidopsis photoreceptors. Proc Natl Acad Sci USA 100 2140–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockler TC, Guo H, Yang H, Duong H, Lin C (1999) Antagonistic actions of Arabidopsis cryptochromes and phytochrome B in the regulation of floral induction. Development 126 2073–2082 [DOI] [PubMed] [Google Scholar]
- Oda A, Fujiwara S, Kamada H, Coupland G, Mizoguchi T (2004) Antisense suppression of the Arabidopsis PIF3 gene does not affect circadian rhythms but causes early flowering and increases FT expression. FEBS Lett 557 259–264 [DOI] [PubMed] [Google Scholar]
- Olmos E, Kiddle G, Pellny T, Kumar S, Foyer C (2006) Modulation of plant morphology, root architecture, and cell structure by low vitamin C in Arabidopsis thaliana. J Exp Bot 57 1645–1655 [DOI] [PubMed] [Google Scholar]
- Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH (2003) Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell 15 939–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavet V, Olmos E, Kiddle G, Mowla S, Kumar S, Antoniw J, Alvarez ME, Foyer CH (2005) Ascorbic acid deficiency activates cell death and disease resistance responses in Arabidopsis. Plant Physiol 139 1291–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pnueli L, Liang H, Rozenberg M, Mittler R (2003) Growth suppression, altered stomatal responses, and augmented induction of heat shock proteins in cytosolic ascorbate peroxidase (Apx1)-deficient Arabidopsis plants. Plant J 34 187–203 [DOI] [PubMed] [Google Scholar]
- Putterill J, Laurie R, Macknight R (2004) It's time to flower: the genetic control of flowering time. Bioessays 26 363–373 [DOI] [PubMed] [Google Scholar]
- Putterill J, Robson F, Lee K, Simon R, Coupland G (1995) The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 80 847–857 [DOI] [PubMed] [Google Scholar]
- Quesada V, Macknight R, Dean C, Simpson GG (2003) Autoregulation of FCA pre-mRNA processing controls Arabidopsis flowering time. EMBO J 22 3142–3152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queval G, Issakidis-Bourguet E, Hoeberichts FA, Vandorpe M, Gakiere B, Vanacker H, Miginiac-Maslow M, Van Breusegem F, Noctor G (2007) Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2-induced cell death. Plant J 52 640–657 [DOI] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J (1993) Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki-Sekimoto Y, Taki N, Obayashi T, Aono M, Matsumoto F, Sakurai N, Suzuki H, Hirai MY, Noji M, Saito K, et al (2005) Coordinated activation of metabolic pathways for antioxidants and defence compounds by jasmonates and their roles in stress tolerance in Arabidopsis. Plant J 44 653–668 [DOI] [PubMed] [Google Scholar]
- Saunders DS (2005) Erwin Bunning and Tony Lees, two giants of chronobiology, and the problem of time measurement in insect photoperiodism. J Insect Physiol 51 599–608 [DOI] [PubMed] [Google Scholar]
- Sawa M, Nusinow DA, Kay SA, Imaizumi T (2007) FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science 318 261–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer R, Ramsay N, Samach A, Corden S, Putterill J, Carre IA, Coupland G (1998) The late elongated hypocotyl mutation of Arabidopsis disrupts circadian rhythms and the photoperiodic control of flowering. Cell 93 1219–1229 [DOI] [PubMed] [Google Scholar]
- Scholl R, Rivero-Lepinckas L, Crist D (1998) Growth of plants and preservation of seeds. In JM Martinez-Zapater, J Salinas, eds, Arabidopsis Protocols. Humana Press, Totowa, NJ, pp 3–5
- Sheldon CC, Burn JE, Perez PP, Metzger J, Edwards JA, Peacock WJ, Dennis ES (1999) The FLF MADS box gene: a repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell 11 445–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R, Igeno MI, Coupland G (1996) Activation of floral meristem identity genes in Arabidopsis. Nature 384 59–62 [DOI] [PubMed] [Google Scholar]
- Smirnoff N (2000) Ascorbate biosynthesis and function in photoprotection. Philos Trans R Soc Lond B Biol Sci 355 1455–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N, Dowdle J, Ishikawa T (2007) The role of VTC2 in vitamin C biosynthesis in Arabidopsis thaliana. Comp Biochem Physiol A 146 S250 [Google Scholar]
- Strayer C, Oyama T, Schultz TF, Raman R, Somers DE, Mas P, Panda S, Kreps JA, Kay SA (2000) Cloning of the Arabidopsis clock gene TOC1, an autoregulatory response regulator homolog. Science 289 768–771 [DOI] [PubMed] [Google Scholar]
- Suárez-López P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G (2001) CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 410 1116–1120 [DOI] [PubMed] [Google Scholar]
- Tournois J (1912) Influence de la lumière sur la floraison du houblon japonais et du chanvre déterminées par des semis haitifs. C R Acad Sci Ser III Sci Vie 155 297–300 [Google Scholar]
- Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G (2004) Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 303 1003–1006 [DOI] [PubMed] [Google Scholar]
- Von Tiedemann A (1997) Evidence for a primary role of active oxygen species in induction of host cell death during infection of bean leaves with Botrytis cinerea. Physiol Mol Plant Pathol 50 151–166 [Google Scholar]
- Wang ZY, Tobin EM (1998) Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell 93 1207–1217 [DOI] [PubMed] [Google Scholar]
- Wheeler GL, Jones MA, Smirnoff N (1998) The biosynthetic pathway of vitamin C in higher plants. Nature 393 365–369 [DOI] [PubMed] [Google Scholar]
- Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D (2005) Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309 1056–1059 [DOI] [PubMed] [Google Scholar]
- Wilson RN, Heckman JW, Somerville CR (1992) Gibberellin is required for flowering in Arabidopsis thaliana under short days. Plant Physiol 100 403–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolucka BA, Goossens A, Inze D (2005) Methyl jasmonate stimulates the de novo biosynthesis of vitamin C in plant cell suspensions. J Exp Bot 56 2527–2538 [DOI] [PubMed] [Google Scholar]
- Wormuth D, Baier M, Kandlbinder A, Scheibe R, Hartung W, Dietz KJ (2006) Regulation of gene expression by photosynthetic signals triggered through modified CO2 availability. BMC Plant Biol 6 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HQ, Wu YJ, Tang RH, Liu D, Liu Y, Cashmore AR (2000) The C termini of Arabidopsis cryptochromes mediate a constitutive light response. Cell 103 815–827 [DOI] [PubMed] [Google Scholar]
- Yanovsky MJ, Kay SA (2003) Living by the calendar: how plants know when to flower. Nat Rev Mol Cell Biol 4 265–275 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.