Abstract
Sinorhizobium meliloti cells were engineered to overexpress Anabaena variabilis flavodoxin, a protein that is involved in the response to oxidative stress. Nodule natural senescence was characterized in alfalfa (Medicago sativa) plants nodulated by the flavodoxin-overexpressing rhizobia or the corresponding control bacteria. The decline of nitrogenase activity and the nodule structural and ultrastructural alterations that are associated with nodule senescence were significantly delayed in flavodoxin-expressing nodules. Substantial changes in nodule antioxidant metabolism, involving antioxidant enzymes and ascorbate-glutathione cycle enzymes and metabolites, were detected in flavodoxin-containing nodules. Lipid peroxidation was also significantly lower in flavodoxin-expressing nodules than in control nodules. The observed amelioration of the oxidative balance suggests that the delay in nodule senescence was most likely due to a role of the protein in reactive oxygen species detoxification. Flavodoxin overexpression also led to high starch accumulation in nodules, without reduction of the nitrogen-fixing activity.
Symbiotic nodules have a limited functional life that varies among different legume species. Nodule senescence is the sequence of structural, molecular, biochemical, and physiological events taking place in the process that a mature and functional nodule undergoes leading to the loss of the nitrogen-fixing activity and culminating in cell death of symbiotic tissue (Swaraj and Bishnoi, 1996; Puppo et al., 2005; Van de Velde et al., 2006).
Various models have been proposed to explain the mechanisms that trigger the process of natural or stress-induced nodule senescence. However, it is generally accepted that a senescence-inducing signal from the plant causes a decrease in antioxidant levels and thus an increase in reactive oxygen species (ROS) up to a point of no return. Numerous studies have shown that ROS and antioxidant systems are involved in natural (Lucas et al., 1998; Evans et al., 1999; Hernández-Jiménez et al., 2002; Puppo et al., 2005) as well as induced (Dalton et al., 1993; Becana et al., 2000; Hernández-Jiménez et al., 2002; Matamoros et al., 2003) nodule senescence. Nitrogen fixation is very sensitive to ROS, and nitrogenase activity drastically decreases during nodule senescence (Dalton et al., 1986).
Antioxidant systems that protect cells from oxidative damage have been described in symbiotic nodules (Dalton et al., 1986, 1993; Evans et al., 1999; Becana et al., 2000; Matamoros et al., 2003; Puppo et al., 2005). These include the enzymes superoxide dismutase (SOD), catalase, and peroxidase. Another enzymatic system associated with ROS detoxification is the ascorbate-glutathione pathway, which includes ascorbate peroxidase (APX), dehydroascorbate reductase (DHAR), monodehydroascorbate reductase (MDHAR), and glutathione reductase (GR; Dalton et al., 1986, 1992; Noctor and Foyer 1998; Becana et al., 2000). Ascorbate and reduced glutathione (GSH) in this pathway can also scavenge superoxide and hydrogen peroxide.
During nodule senescence, several ultrastructural alterations in the nodule tissues and cells have been observed (Lucas et al., 1998; Hernández-Jiménez et al., 2002; Puppo et al., 2005, and refs. therein; Van de Velde et al., 2006). Cytosol becomes electron dense, altered vesicles proliferate, and eventually the cytosol undergoes lysis. The number of peroxisomes increases, mitochondria form complex elongated structures, and symbiosomes change in size and shape and fuse during natural and induced senescence of nodules (Hernández-Jiménez et al., 2002). Damage of the symbiosome membrane is also detected (Puppo et al., 2005; Van de Velde et al., 2006).
A strategy of delayed nodule senescence could lead to increased nitrogen fixation and legume productivity. Delayed nodule senescence together with enhanced sustainability under field conditions are among the key aims of legume improvement programs (Puppo et al., 2005). An interesting approach proposed to achieve delayed senescence is to induce nodulation in legumes using rhizobial strains with modified redox capacity (Zahran, 2001).
The protein flavodoxin contains a FMN group acting as a redox center transferring electrons at low potentials (Pueyo et al., 1991; Pueyo and Gómez-Moreno, 1991). The FMN cofactor of flavodoxin can exist in three different redox states: oxidized, one-electron-reduced semiquinone, and two-electron-reduced hydroquinone. This property confers high versatility to flavodoxins in electron transport systems (Simondsen and Tollin, 1980; McIver et al., 1998). To date, flavodoxin has not been described in plants, as flavodoxin-encoding genes were lost during the transition of algae to plants (Zurbriggen et al., 2007) and, consequently, no homologs have been identified in the sequenced genome of Arabidopsis (Arabidopsis thaliana; Arabidopsis Genome Initiative, 2000). Flavodoxin is present as a constitutive or inducible protein in different microorganisms (Klugkist et al., 1986). In the nitrogen-fixing cyanobacterium Anabaena variabilis PCC 7119, flavodoxin is expressed under conditions of limited iron availability, replacing ferredoxin in the photosynthetic electron transport from PSI to NADP+ and in nitrogenase reduction (Sandmann et al., 1990). Reversible electron transfer from flavodoxin to NADP+ is catalyzed by ferredoxin NADP+ reductase in different pathways of oxidative metabolism (Arakaki et al., 1997). In its reduced state, flavodoxin might be able to react with ROS and revert to its original redox state in the presence of an appropriate electron source. This could probably occur without the associated molecular damage that metallic complexes in catalases or SODs suffer (Keyer et al., 1995). The presence of flavodoxin has not been documented to date in the symbiotic bacterium Sinorhizobium meliloti. In Escherichia coli, however, flavodoxin induction is linked to the oxidative stress-responsive regulon soxRS (Zheng et al., 1999). It has been suggested that flavodoxin and ferredoxin (flavodoxin) NADP+ reductase might be induced and have a role in reestablishing the cell redox balance under oxidative stress conditions (Liochev et al., 1994). The properties of flavodoxin suggest that its presence in the cell may have a facilitating effect on ROS detoxification. In fact, an increase in the amount of flavodoxin has been observed in some bacterial species subjected to oxidative stress (Zheng et al., 1999; Yousef et al., 2003; Singh et al., 2004), and transgenic tobacco (Nicotiana tabacum) plants expressing flavodoxin in chloroplasts show enhanced tolerance to a broad range of stresses related to oxidative damage (Tognetti et al., 2006, 2007a, 2007b).
In this work, Sinorhizobium meliloti was transformed with the A. variabilis flavodoxin gene and used to nodulate alfalfa (Medicago sativa) plants. The effects of flavodoxin expression on nodulation dynamics, on nodule development and senescence processes, and on nitrogen-fixing activity were analyzed. Mechanistic insights suggesting putative roles for flavodoxin in protection from ROS and the induced delay of nodule senescence are likewise discussed.
RESULTS
Flavodoxin Is Expressed in Alfalfa Nodules Elicited by S. meliloti Transformed with the A. variabilis Flavodoxin Gene
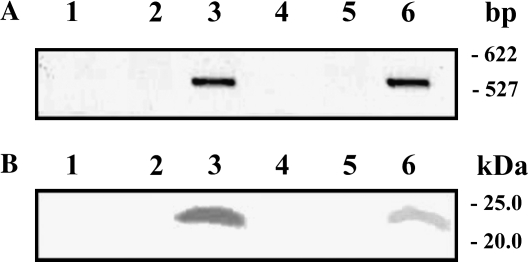
A. variabilis flavodoxin was cloned into the tetracycline-resistant expression plasmid pFAJ1709 (Dombrecht et al., 2001), and S. meliloti cells were transformed with the resulting plasmid pFAJ1709-Fld. Alfalfa plants were then nodulated by the transformed bacteria. We verified that the free-living transconjugant bacteria and derived bacteroids carried the flavodoxin gene and constitutively expressed the A. variabilis protein (Fig. 1). The characteristics of the expression plasmid, containing loci for plasmid stability during symbiosis, accounted for its permanence in the rhizobial cells in planta with no antibiotic selection, and the presence of the flavodoxin DNA was detected by PCR amplification in free-living bacteria and alfalfa nodule bacteroids harboring the pFAJ1709-Fld plasmid. The PCR product was a single band of the expected size (543 bp). No PCR product was obtained for bacteria carrying the pFAJ1079 plasmid or for untransformed bacteria (Fig. 1A).
Figure 1.
PCR amplification (A) and immunodetection (B) of flavodoxin in free-living bacteria and bacteroids of S. meliloti. Lane 1, Wild-type free-living S. meliloti cells; lane 2, free-living S. meliloti cells harboring expression plasmid pFAJ1709; lane 3, free-living S. meliloti cells transformed with plasmid pFAJ1079-Fld; lane 4, bacteroids isolated from alfalfa wild-type nodules; lane 5, S. meliloti bacteroids harboring expression plasmid pFAJ1709; lane 6, flavodoxin-expressing S. meliloti bacteroids.
An antibody raised against A. variabilis flavodoxin (Fillat et al., 1991) was used to confirm protein expression in both free-living bacteria and symbiotic forms. Immunoblot analysis after SDS-PAGE revealed one immunoreactive band of the expected size (23 kD) in flavodoxin-transformed S. meliloti bacteria and bacteroids (Fig. 1B), indicating that A. variabilis flavodoxin was expressed in S. meliloti transconjugants after differentiation to functional bacteroids and accumulated in bacteroids of alfalfa root nodules.
Nodulation kinetics, nodule number, and nodule weight were not affected by flavodoxin expression, as no significant differences were observed in those parameters when comparing plants inoculated with flavodoxin-expressing and control bacteria (data not shown).
Decline in Nitrogenase Activity Associated with Nodule Natural Senescence Is Delayed in Flavodoxin-Expressing Nodules
Results from our laboratory indicate that flavodoxin overexpression protects free-living bacteria from oxidative stress induced by oxidative agents such as hydrogen peroxide and methylviologen (F.J. Redondo, T. Coba de la Peña, M.M. Lucas, and J.J. Pueyo, unpublished data). To determine the potential antioxidant-protecting effect of flavodoxin on the senescence-associated decline of nitrogen fixation in symbiotic nodules, the nitrogen-fixing activity was estimated by acetylene reduction assay (ARA). Figure 2 shows the ARA results obtained for alfalfa nodules elicited by control bacteria (wild-type and bacteria harboring the plasmid pFAJ1709) and flavodoxin-expressing bacteria.
Figure 2.
Nitrogenase activity per gram fresh weight (FW) of nodule, measured by the ARA in nodules of alfalfa elicited by wild-type S. meliloti [control (wt); white bars], S. meliloti harboring the expression plasmid pFAJ1709 [control (plasmid); dark gray bars], and flavodoxin-expressing S. meliloti (Fld; light gray bars). Asterisks indicate significant differences. Values are means of three experiments, with n = 10 for each separate experiment.
No significant differences in nitrogenase activity were found between nodules formed by control wild-type bacteria and control bacteria harboring pFAJ1709. Nitrogenase activity increased from the earliest harvest date (10 d postinoculation [dpi]) until peaking at approximately 18 dpi and then declined gradually. No significant differences in nitrogenase activity were observed among plants infected by flavodoxin-expressing or control bacteria until 28 dpi. However, the decline in activity was much less marked for nodules expressing flavodoxin, and the capacity for nitrogen fixation remained intact at 28 dpi in flavodoxin-containing nodules and was 39% higher than that of control nodules. Although nitrogenase activity further declined with age, flavodoxin-containing nodules consistently displayed significantly higher nitrogenase activity values (51%, 83%, 91%, and 97% at 32, 36, 40, and 44 dpi, respectively) than control nodules of the same age. These results show that decline in nitrogenase activity is significantly delayed in alfalfa nodules elicited by flavodoxin-expressing S. meliloti.
Flavodoxin Overexpression Reduces Senescence-Related Structural and Ultrastructural Alterations in Alfalfa Nodules
To study the effect of flavodoxin expression on nodule development, nodules were examined at different ages (10, 30, and 44 dpi) by light microscopy and transmission electron microscopy.
No obvious structural or ultrastructural differences were observed in tissues of 10-dpi nodules elicited by flavodoxin-expressing bacteria when compared with control nodules (data not shown). In 30- and 44-dpi nodules, the size and structural and ultrastructural characteristics of the meristematic tissue (zone I; Vasse et al., 1990) and the invasion zone (zone II) were similar in both types of nodules. In zone II, the number of infection threads and the ratio of infected to uninfected cells were comparable in flavodoxin-expressing and control nodules (data not shown).
Histologically, some differences were found in both the mature zone (zone III) and the senescent zone (zone IV) between control and flavodoxin-expressing nodules. Differences were mainly related to the presence of large starch granules in the plastids of the nodule cells that we will present below in more detail, to the extension of the cell layers showing signs of senescence, and to the severity of the senescence symptoms (Figs. 3 and 4).
Figure 3.
Light micrographs showing the structural characteristics of control and flavodoxin-expressing nodules stained with toluidine blue. A, Longitudinal section of a 30-dpi control nodule showing the meristem (zI), the infection zone (zII), the nitrogen-fixing zone III (zIII), and an incipient senescent zone IV (star). Bar = 100 μm. B, A 30-dpi control nodule showing zII and the interzone II/III (zII/III) containing infected cells harboring bacteroids (b) and interstitial cells (ic). Note the starch granules (s) in zone II/III. Bar = 20 μm. C, Distal zone III in a 30-dpi control nodule. Bar = 20 μm. D, Image showing the proximal zone III with low starch accumulation in infected cells and the presence of infection threads (it). Bar = 25 μm. E, Longitudinal section of a 44-dpi control nodule showing an enlarged senescent zone IV (zIV). Bar = 100 μm. F, Senescent zones IV (zIV) and V (zV) in a 44-dpi control nodule. Remnant infection threads (it) and bacteria spreads (arrow) can be seen in fully senescent cells of zone V. Bar = 25 μm. G, Longitudinal section of a 30-dpi flavodoxin-expressing nodule. Bar = 100 μm. H, A 30-dpi flavodoxin-expressing nodule showing zII and the interzone II/III. Bar = 20 μm. I, Infected and interstitial cells in the distal zone III of a 30-dpi flavodoxin-expressing nodule. Bar = 20 μm. J, Detail of G showing starch accumulation (s) in basal infected and interstitial cells. Bar = 25 μm. K, Longitudinal section of a 44-dpi flavodoxin-expressing nodule showing reduced zone IV. Bar = 100 μm. L, Detail of K showing distal zone III and zone IV. Bar = 25 μm.
Figure 4.
Ultrastructural characteristics of infected and interstitial cells in control and flavodoxin-expressing nodules. A, Transmission electron microscopy image of zone II/III from a 30-dpi control nodule showing an infected cell with type 3 bacteroids (b3) and numerous mitochondria (m) and amyloplasts (a) with normal starch accumulation. B and C, Infected cells filled with nitrogen-fixing bacteroids (type 4) and interstitial cells (ic) in zone III of 30-dpi control nodules. Note the small size of the amyloplasts in both cell types. D, Image of zone III of a 30-dpi flavodoxin-expressing nodule. Infected cells harboring type 4 bacteroids (b4) display elongated starch granules in amyloplasts that surround the intercellular space (is). E, Image of zone III of a 44-dpi flavodoxin-expressing nodule. Infected cells harboring type 4 bacteroids (b4) display huge elongated starch granules in amyloplasts. F, Interstitial cell in the proximal zone III of a flavodoxin-expressing nodule (30 dpi) showing numerous amyloplasts with large spherical starch granules. G, Infected cells of the proximal zone III in a 30-dpi control nodule. Type 4 bacteroids are differentiating into type 5 (b4-5). H, Infected cells hosting type 5 bacteroid (b5) in the basal zone of a 30-dpi control nodule. I, Infected cell showing senescent bacteroids (arrow) in the basal zone of a 30-dpi control nodule. J, Degenerated symbiosomes with ghost bacteroids (arrow) and altered vacuole (v) in a host cell of zone IV in a 44-dpi control nodule. K and L, Infected cells hosting type 5 bacteroids (b5) in the basal zone of flavodoxin-expressing nodules (30 dpi). Note the elevated starch accumulation in amyloplasts (a), the elongated mitochondria (m), and the proliferation of peroxisomes (p). M, Interstitial cell with amyloplast and infected cell showing symbiosome fusion (x) in the basal zone in a 30-dpi flavodoxin-expressing nodule. N, Infected cell in zone IV in a 44-dpi flavodoxin-expressing nodule. Symbiosomes at different stages of senescence: apparently unaltered b5 (star), bacteroid with still dense cytosol, and bacteroid with cytosolic lysis (arrow). Bars = 2 μm.
In both types of nodules, bacteroids seemed to follow the normal differentiation pattern described by Vasse et al. (1990). As expected, type 3 bacteroids were found in interzone II/III cells of control nodules (Fig. 4A) and flavodoxin-expressing nodules (data not shown), and zone III displayed cells with type 4 bacteroids (Fig. 4, B–E).
In 30-dpi control nodules, infected cells were filled with bacteroids undergoing a clear differentiation process toward bacteroid type 5 (which showed a progressive loss of cytoplasmic heterogeneity) in proximal zone III (Fig. 4G). This indicated that the development of senescence zone IV was in progress. In some nodules, type 5 bacteroids (Fig. 4H) and more senescent bacteroids (Fig. 4I) were also observed. In 44-dpi control nodules, senescent zone IV was much more evident (Fig. 3E) than in the 30-dpi nodules (Fig. 3A). It included infected cells containing noneffective type 5 bacteroids and numerous degenerated cells with “ghost” bacteroid membranes surrounded by the symbiosome membrane (Fig. 4J). Cell organelles lost their location at the periphery of the cytoplasm, and vacuoles became irregular in shape (Fig. 4J). An incipient zone V (Timmers et al., 2000) with completely senescent cells and remnant infection threads was observed in some of the 44-dpi control nodules (Fig. 3F).
In contrast, in 30-dpi flavodoxin-containing nodules, the senescent zone IV was not evident (Fig. 3, G and J), and zone V was not developed in 44-dpi nodules (Fig. 3, K and L). Symbiosomes were clearly less altered than in control nodules. Type 5 bacteroids were found only in a small number of cells on the most basal part of the 30-dpi nodules (Fig. 4K). Following a pattern of senescence similar to that of control nodules, large peribacteroid spaces and fusion of symbiosomes were observed in senescent infected cells of flavodoxin-expressing nodules (Fig. 4, L and M). In zone IV of 44-dpi nodules, the most severe symptom of senescence was the presence of symbiosomes containing altered bacteroids (still presenting an electrodense cytosol), which coexisted with symbiosomes with a wide peribacteroid space and some type 5 bacteroids (Fig. 4N). These senescence features were only observed in approximately 10% of the cells in the proximal zone of flavodoxin-expressing nodules (at least three different nodules were analyzed, more than three sections per nodule and approximately 60–100 cells of the proximal nodule zone per section were observed). In contrast to control nodules, highly degenerated symbiosomes (collapsed ghost bacteroids) were not found in flavodoxin-containing nodules. Regarding cell organelles, no differences were observed in the peroxisomes and mitochondria when compared with control nodules. As observed in control nodules and previously described as features of nodule senescence (Hernández-Jiménez et al., 2002; Puppo et al., 2005), mitochondria formed elongated structures and the number of visible peroxisomes increased in zone IV (Fig. 4, K and L). Nevertheless, organelles maintained their location near the cell wall, and no important shape alterations were detected in the vacuoles of the most senescent cells. Considered jointly, these results indicate that in nodules induced by flavodoxin-expressing rhizobia, the senescence process had not reached the final stages found in control nodules at 44 dpi.
Overexpression of Flavodoxin Causes High Starch Accumulation
As reported previously (Vasse et al., 1990), in control nodules the maximum accumulation of starch was detected in the infected host cells of zone II/III (Figs. 3B and 4A). In infected and interstitial cells of zone III and zone IV, the amyloplasts had relatively small starch granules (Fig. 4, B, C, and G) that were hardly visible by light microscopy (Fig. 3).
Nodules formed by flavodoxin-expressing rhizobia showed atypical starch accumulation. In zone III of 30- and 44-dpi flavodoxin nodules, infected and interstitial cells displayed amyloplasts with enlarged starch granules that could be easily detected at the light microscopy level (compare Fig. 3, C and I). The number of starch granules was also much higher when compared with control nodules. Starch accumulation occurred in a similar manner in 30- and 44-dpi nodules with granules present from the most distal zone III to the basal nodule zone (Fig. 3, I and J). The ultrastructural study revealed that starch granules were located in the periphery of infected cells and showed an elongated shape (Fig. 4, D, E, and K–N). Amyloplasts of interstitial cells were larger than those of infected cells, occupied a considerable portion of the central zone of the cell, and harbored huge starch granules with spherical shapes (Fig. 4F).
Flavodoxin Induces Changes in Nodule Antioxidant Metabolism
Enzymatic activities of several antioxidant enzymes were determined in nodules (18, 32, and 44 dpi) of alfalfa plants inoculated with flavodoxin-expressing bacteria and compared with control nodules.
SOD activity increased in both types of nodules with nodule age (Fig. 5A). Although no significant differences were observed between nodules inoculated with either control or flavodoxin-expressing bacteria at any time, SOD appeared to increase earlier in control nodules. In flavodoxin-expressing nodules, the SOD activity was similar in 18- and 32-dpi nodules, and a significant increase could be appreciated only at 44 dpi.
Figure 5.
Antioxidant enzymes SOD (A) and catalase (B) and ascorbate-glutathione enzymes APX (C), MDHAR (D), DHAR (E), and GR (F) activities in control (white circles) and flavodoxin-expressing (black squares) nodules. Asterisks indicate significant differences. Values are means of three experiments, with n = 15 for each separate experiment.
Catalase enzymatic activity decreased appreciably with age in both types of nodules (Fig. 5B). This activity was lower in both types of 44-dpi nodules than in younger nodules (18 and 32 dpi). The 44-d-old nodules containing bacteroids that expressed flavodoxin displayed significantly lower activity than control nodules of the same age.
Activities of the ascorbate-glutathione cycle enzymes, APX, MDHAR, DHAR, and GR, were determined. APX activity decreased with nodule age in both types of nodules (Fig. 5C), but no significant differences were observed between the two types of nodules at the same given age. A slight decrease in MDHAR activity could be observed in 44-dpi nodules, but no significant differences were observed when comparing the two types of nodules of the same age (Fig. 5D). Nodule aging correlated with a moderate decrease in DHAR activity in both types of nodules (Fig. 5E). The decline was significantly less marked in 32-d-old flavodoxin-expressing nodules when compared with control nodules, whereas the DHAR activity levels were similar in 44-dpi nodules of both types. GR enzymatic activity was similar in 18- and 32-dpi nodules and increased in aged 44-dpi nodules (Fig. 5E). GR activity was higher in control nodules than in flavodoxin-expressing nodules at 32 dpi.
In general terms, it appears that antioxidant enzymatic activities followed similar trends in control and flavodoxin-expressing nodules. They varied in the same direction with nodule age and either increased or decreased in both types of nodules. However, variations between 18- and 32-dpi nodules were usually more marked in control nodules than in flavodoxin-containing nodules.
GSH and oxidized glutathione (GSSG) were quantified. Concentrations of the two metabolites increased with nodule age (Fig. 6, A and B) in both types of nodules. Increases between 18 and 32 dpi were more marked in the control than in flavodoxin-containing nodules. The GSH-GSSG ratio decreased with nodule age (Fig. 6C). This parameter, indicative of the oxidative balance in the nodule, was significantly higher in 18- and 32-dpi nodules containing flavodoxin-expressing bacteroids.
Figure 6.
Determination of GSH (A), GSSG (B), GSH-GSSG ratio (C), ascorbate (D), and total ascorbate (E) in control (white circles) and flavodoxin-expressing (black squares) nodules. Asterisks indicate significant differences. Values are means of three experiments, with n = 10 for each separate experiment.
The levels of reduced ascorbate (Asc) and total ascorbate (Asc + DHA) increased with nodule age (Fig. 6, D and E). No significant differences were detected between the two types of nodules for a given age.
Malondialdehyde (MDA), a product of membrane lipid peroxidation, was quantified by reaction with thiobarbituric acid. No significant differences between the two types of nodules were found at 18 dpi (Fig. 7). However, MDA content was significantly higher in 32-dpi control nodules, as compared with 32-dpi flavodoxin-expressing nodules. MDA content in both types of nodules was similar for 44-dpi nodules (Fig. 7).
Figure 7.
Determination of lipid peroxidation (MDA; nmol g−1 fresh weight [FW]) in control (white circles) and flavodoxin-expressing (black squares) nodules. The asterisk indicates a significant difference. Values are means of three experiments, with n = 6 for each separate experiment.
Carbon metabolism was investigated in the two types of nodules. Sucrose synthase (SS) and phosphoenolpyruvate carboxylase (PEPC) enzymatic activities were determined. Both enzymatic activities increased during nodule senescence. Activity values were significantly higher in 44-dpi nodules than in 18- and 32-dpi nodules, but no significant differences were observed between the two types of nodules (data not shown).
DISCUSSION
During the establishment of the Rhizobium-legume symbiosis, a complex signal interchange between the two partners, including ROS generation, regulates plant defense reactions and allows the successful achievement of symbiosis (Djordjevic et al., 1987; McKhann and Hirsch, 1994; Mithofer, 2002). ROS and antioxidants seem to play a critical role in signal transduction during the early events of symbiosis (Hérouart et al., 2002), and alteration of the bacterial antioxidant systems can inhibit or delay nodule formation (Santos et al., 2000; Harrison et al., 2005). In nodulated alfalfa plants, we observed that expression of flavodoxin in S. meliloti had no significant effects on nodulation kinetics and total number of nodules. Apparently, the presence of flavodoxin did not alter or destabilize the signaling mechanisms during nodule organogenesis.
No significant differences were detected between flavodoxin-expressing and control nodules regarding the maximum nitrogen-fixing activity values reached at 18 to 22 dpi, suggesting that expression of flavodoxin in the nodule did not affect nitrogenase activity. A senescence-associated decline in nitrogenase activity was observed from 22 dpi in control nodules; this decline was almost constant (r2 = 0.97, slope = −5,300). A similar activity decline (r2 = 0.99, slope = −5,900) occurred in flavodoxin-expressing nodules, but 1 week to 10 d later, starting at 28 to 32 dpi. This delay in the nitrogen-fixing activity decline observed in flavodoxin-containing nodules, considered jointly with the lesser degree of structural and ultrastructural alterations linked to the nodule senescence process, strongly suggest that overexpression of flavodoxin slows nodule senescence.
Tognetti et al. (2006) have observed that flavodoxin can interact with the phosphotransfer electron systems of chloroplasts in vitro. The supply of reducing power for nitrogenase is mediated by ferredoxin or flavodoxin, and effective electron transfer from pyruvic acid to nitrogenase through flavodoxin and the enzyme pyruvate-flavodoxin oxidoreductase has been established in Klebsiella pneumoniae (Deistung et al., 1985) and Rhodobacter capsulatus (Hallenbeck and Gennaro, 1998). Flavodoxin could increase the effectiveness of the nitrogenase complex because of its capacity to mediate electron transfer. However, the absence of differences in maximum activity between flavodoxin-containing and control nodules suggests that the main effect of flavodoxin might be that of enhancing detoxification systems.
SOD is the first line of defense against ROS, and mutants of S. meliloti defective in SOD activity induce premature senescence in nodules (Santos et al., 2000). However, expression of flavodoxin in alfalfa nodules had no effect on nodule SOD activity. In line with our results, significant decreases in catalase activity have been reported in senescent nodules of various legumes (Hernández-Jiménez et al., 2002; Groten et al., 2005). In nodules formed by mutants defective in catalase, a significant decrease in nitrogen-fixing activity was reported (Sigaud et al., 1999). Catalase-mediated detoxification may have been partly replaced by alternative mechanisms in flavodoxin-expressing senescent nodules (44 dpi), since catalase activity was significantly lower than in control nodules.
Glutathione and ascorbate are the most important redox buffers in the cell and the main antioxidants in legume nodules (Becana et al., 2000; Matamoros et al., 2003). Several works underscore the importance of the ascorbate-glutathione cycle and its components in the metabolism of the nodule (Frendo et al., 2005; Harrison et al., 2005). Groten et al. (2005) suggest that this cycle plays a regulation and signaling role rather than one of detoxification in nodule senescence. Variations in the amount of these antioxidants during nodule development and senescence could be involved in signal transduction, as has been described in senescent leaves (Pastori et al., 2003), and/or in counteracting oxidative stress. During natural senescence of lupin (Lupinus albus) nodules, Hernández-Jiménez et al. (2002) observed a decrease in APX, MDHAR, and DHAR activities and an increase in GR activity. They also observed a slight increase in the amount of GSH and GSSG and an increase in the GSH-GSSG ratio. In contrast, Groten et al. (2005) reported an increase in MDHAR activity and a decrease in APX and DHAR activities during natural senescence of pea (Pisum sativum) nodules, while ascorbate and total glutathione decreased and GR activity and the GSH-GSSG ratio remained constant. In senescent nodules of soybean (Glycine max), a decrease in ascorbate and total glutathione and an increase in the GSH-GSSG ratio were observed (Evans et al., 1999). In leghemoglobin-deficient nodules of Lotus japonicus, which present low ROS levels, Günther et al. (2007) reported increases in ascorbate levels and in MDHAR and DHAR enzymatic activities and decreases in APX and GR activities.
In alfalfa nodules, we observed a decrease in APX, MDHAR, and DHAR activities and an increase in GR activity during aging of both type of nodules. There was an increase in GSH, GSSG, and ascorbate levels and a decrease in the GSH-GSSG ratio. The glutathione balance in nodules expressing flavodoxin is more favorable than that in control nodules at 18 and 32 dpi. An increase in the effectiveness of the ascorbate-glutathione cycle (or a delay in its decay, as we report here for flavodoxin-containing nodules) could involve an increase in antioxidant activity in the nodule, as suggested previously (Becana et al., 2000).
Tognetti et al. (2006) observed that the enhanced tolerance to oxidative stress correlated with a decrease in ROS accumulation and oxidative damage in transgenic tobacco plants expressing a chloroplast-targeted flavodoxin. SOD, APX, and GR levels were similar in nonstressed transgenic and wild-type lines, and no induction was observed upon oxidative stress. GSH-GSSG ratio and ascorbate content under oxidative stress were significantly higher in transgenic plants expressing the chloroplast-targeted flavodoxin compared with wild-type plants. The protective effect of flavodoxin was proposed to be due to an involvement of flavodoxin in NADP+ photoreduction and in thioredoxin reduction in the chloroplasts and to a decrease in GSH oxidation (Tognetti et al., 2006).
The accumulation of oxidatively damaged lipids is a marker of oxidative stress in plant and animal tissues. These products arise, among other mechanisms, by the oxidative attack of lipids by ROS. MDA is a cytotoxic product of membrane lipid peroxidation, and its accumulation is an index of cellular damage (Halliwell and Gutteridge, 1999) and a senescence indicator (Hernández-Jiménez et al., 2002). Our results showing that lipid peroxidation is approximately twice as much in 32-dpi control nodules than in flavodoxin-containing nodules support our hypothesis that flavodoxin-induced changes in antioxidant metabolism are most likely the cause for delayed nodule senescence.
SS and PEPC activities are essential for nitrogen fixation (Gordon et al., 1999). Various works have reported variations in SS and PEPC activities and expression in nodules subjected to different stresses, but little is known about the activities of these enzymes during natural senescence. An increase in PEPC activity has been observed throughout the development and senescence of bean (Phaseolus vulgaris) nodules (Suganuma et al., 1997). We observed a significant increase in SS and PEPC with nodule aging. Apparently, the activities of these enzymes were not responsible for the differences observed in nitrogen fixation, as they were comparable in flavodoxin-containing and control nodules.
The presence of numerous amyloplasts has been described in the interzone II/III, while they are barely visible in other nodule zones (Vasse et al., 1990). A remarkable feature found in flavodoxin-expressing nodules was the high accumulation of starch in zone III. Atypical levels of starch accumulation have been described in nodules infected by different types of mutant bacteria. Nodules infected by bacteroids that are defective for the transport of dicarboxylic acids show a broad senescent zone IV and high levels of starch accumulation and undergo premature senescence (Finan et al., 1983). Pea nodules infected by bacteroids that are defective for Asp aminotransferase or for amino acid import/export through the bacteroid membrane also showed high starch accumulation (Lodwig et al., 2003). Alfalfa nodules with bacteroids that are defective in glutathione synthesis accumulated high levels of starch (Harrison et al., 2005). There is a common feature observed in all of these examples: nitrogen-fixing activity is severely reduced or even abolished. Bacteroids are not fully differentiated or their metabolism is altered in different pathways, presenting deficiencies in the import and processing of carbon skeletons and in the processing of amino acids and antioxidants. Carbon metabolism enzymes are usually altered, with a decrease in SS and PEPC activities. Photosynthates imported to the nodule are not metabolized in the symbiosomes and are stored as starch. During normal nodule development, an excess of photosynthates generates starch granules, and symbiosomes in zone III consume the starch accumulated in zones II and III (Thummler and Verma, 1987).
Alfalfa nodules infected by bacteroids expressing flavodoxin exhibited normal bacteroid development and differentiation and high nitrogen-fixing activity, indicating no defective metabolism. The putative involvement of flavodoxin in increasing detoxification or nitrogen-fixing efficiency did not apparently involve alterations in photosynthate requirements, as SS and PEPC activities were not affected. High starch accumulation could be the consequence of an increased supply of photosynthates to the nodule due to greater efficiency of nitrogen-fixing activity or to the redox state of the bacteroid. Starch accumulation could also be the consequence of increased production of ADP-Glc in nodule cells, the substrate for the synthesis of starch polymers. Flavodoxin can replace ferredoxin in thioredoxin reduction (Tognetti et al., 2006), and the ferredoxin/thioredoxin system has been identified as a starch biosynthesis regulator (Balmer et al., 2006). ADP-Glc pyrophosphorylase, a key regulatory enzyme of starch synthesis responsible for the synthesis of ADP-Glc in all plant organs (Smith et al., 1997), is activated by reduced thioredoxin (Geigenberger et al., 2005). ADP-Glc pyrophosphorylase is present in rhizobial cells (Lepek et al., 2002), where a steady provision of reduced thioredoxin favored by flavodoxin availability could lead to enhanced ADP-Glc synthesis in the bacteroid. It is unknown whether ADP-Glc can be exported to the cytosol, but it can be imported from the cytosol into the amyloplast (Smith et al., 1997).
A delay in nodule senescence could be expected to have a negative effect on other physiological processes, such as growth, fructification, or seed production, owing to the high energy cost of maintaining nitrogenase activity. Nevertheless, no significant differences in any of several morphometric parameters or in total nitrogen content were observed when comparing the two types of nodulated plants (data not shown). Some reports suggest that this extension in the period of high nitrogen-fixing activity has no negative effects on the reproductive period (Riggle et al., 1982), and it has been proposed that it could have a positive effect on biomass and seed production (Puppo et al., 2005). Overexpression of flavodoxin resulted in a delay in nodule senescence but not in an increase in plant biomass. Our results, however, do not exclude the possibility that other strategies to delay nodule senescence might have an effect on biomass production.
MATERIALS AND METHODS
Bacterial Strains, Plasmids, and Transformation
A nodulation-competent rifampicin-resistant Sinorhizobium meliloti 2011 was isolated from Vincent medium (Vincent, 1970) plates containing 100 μg mL−1 rifampicin. This S. meliloti strain is referred to as the wild type. Escherichia coli strain DH5α (Invitrogen) was grown in Luria-Bertani medium (Sambrook et al., 1989).
The flavodoxin gene of Anabaena variabilis PCC 7119 was PCR amplified using the pTrc99a-Fld plasmid (Fillat et al., 1991) as template. Primer sequences were 5′-GATCTAGATTCATAATGTCAAAG-3′ (forward) and 5′-ACTGGTACCTTTTTACAAACCAAAT-3′ (reverse). The final PCR product was 543 bp and carried XbaI and KpnI target sites flanking the coding region. Using both restriction enzymes (Amersham Pharmacia Biotech), the PCR product was inserted into the tetracycline-resistant expression plasmid pFAJ1709 (Dombrecht et al., 2001). The resulting recombinant plasmid carrying the flavodoxin gene was named pFAJ1709-Fld. E. coli DH5α was transformed with both expression vectors by electroporation using an E. coli Pulser Electroporator (Bio-Rad) by routine procedures (Sambrook et al., 1989).
Rifampicin-resistant S. meliloti carrying the expression plasmid FAJ1709 or pFAJ1709-Fld was obtained via triparental mating. E. coli DH5α carrying the mobilizing plasmid pRK2013 (Figurski and Helinski, 1979) was used as the helper strain. E. coli DH5α carrying pFAJ1709 or pFAJ1709-Fld was used as the donor strain. Bacteria were grown to reach an optical density at 600 nm of 0.8. Cultures were centrifuged at 7,000g for 5 min and resuspended in 0.9% NaCl. Recipient, donor, and helper strains (4:1:1) were grown together on triptone yeast plates. After 12 h of growth at 28°C, S. meliloti transconjugants were selected on triptone yeast plates in the presence of 100 μg mL−1 rifampicin and 20 μg mL−1 tetracycline.
Detection of flavodoxin DNA of A. variabilis PCC 7119 in transformed bacteria and bacteroids was done by PCR amplification using the primers mentioned above.
Plant Material and Growth Conditions
Alfalfa (Medicago sativa ‘Aragon R-1’ [Rocalba]) seeds were sterilized in 70% ethanol for 10 min, washed with sterile distilled water, and incubated in 0.1% Hg2Cl for 2 min. Seeds were washed again with sterile water, placed on 0.9% agar in petri dishes, and incubated for 36 h at 23°C in the dark to allow germination. Seedlings were transferred to growth pouches (CYG Seed Germination Pouches; Mega International) containing nitrogen-free nutrient solution (Hoagland and Arnon, 1938) and were grown under growth chamber conditions (180 μmol photons m−2 s−1, 23°C, 16/8-h photoperiod, 65% relative humidity). Bacterial inoculation was performed at sowing with wild-type S. meliloti, transconjugant S. meliloti carrying plasmid pFAJ1709 (referred to as control), or transconjugant S. meliloti carrying plasmid pFAJ1709-Fld, grown to exponential phase.
Immunoblot Analysis
Protein extracts were prepared from S. meliloti 2011 and transconjugants. Bacterial cultures were centrifuged at 8,000g and 4°C for 10 min. The pellet was resuspended in wash buffer (50 mm potassium phosphate, pH 7.5, 300 mm Suc, and 2 mm MgSO4) and centrifuged again under the same conditions. The pellet was resuspended in sonication buffer (20 mm potassium phosphate, pH 7.5, 1 mm EDTA, 5 mm MgCl2, 1 m glycerol, and 1 mm dithiothreitol [DTT]). Samples were subjected to 50-s sonication pulses (25 pulses separated by 30-s intervals) with a Vibra-Cell VC-375 sonicator (Sonics and Materials) set at 60% power. Sonicated bacteria were centrifuged at 12,000g and 4°C for 15 min, and the supernatant was collected.
Bacteroids were isolated from alfalfa nodules as follows. Nodules were ground on ice in a mortar with 50 mm potassium phosphate buffer, pH 7.5, 1 mm DTT, 10% (w/w) polyvinylpyrrolidone (PVP), 5 μg mL−1 leupeptin, and 5 μg mL−1 pepstatin. Samples were centrifuged at 2,000g and 4°C for 10 min. The pellet was resuspended in wash buffer, and protein extraction was performed as described above.
Protein content was estimated by means of the Bradford assay (Bio-Rad) using bovine serum albumin as the standard (Bradford, 1976).
Proteins (100 μg of total protein per lane) were subjected to denaturing PAGE on 15% acrylamide/bisacrylamide gels containing 6 m urea in a Laemmli buffer system (Laemmli, 1970). After electrophoresis, proteins were transferred onto a nitrocellulose membrane and visualized using a polyclonal rabbit antibody raised against A. variabilis flavodoxin (Fillat et al., 1991) at 1:750 dilution. Alkaline phosphatase-conjugated goat anti-rabbit IgG (Sigma) was used as a secondary antibody at 1:20,000 dilution. The reaction was detected by a colorimetric reaction (nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate; Sigma) according to the standard protocol. Ponceau Red staining was performed to ensure equal protein loading.
Nitrogenase Activity
Nitrogenase activity was determined by the ARA (Hardy et al., 1968) adapted by De Felipe et al. (1987). Nitrogen fixation measured as acetylene reduction activity was assayed on root systems enclosed in 100-mL tubes fitted with rubber stoppers. Six milliliters of air was removed from the tubes, and the same amount of acetylene was added. Gas samples were taken after 30 and 60 min and analyzed for ethylene and acetylene in a Konik model Cromatic KNK 2000 gas chromatograph equipped with a hydrogen flame ionization detector and with a column filled with Porapak R (80–100 mesh) using nitrogen as carrier gas at a flow rate of 20 mL min−1. Although the use of a “closed” system for measuring acetylene reduction can lead to an acetylene-induced decline in nitrogenase activity (Minchin et al., 1983), it is appropriate for comparative measurements, especially when the assay time is short.
Determination of Glutathione and Ascorbate
GSH and total glutathione (GSH + GSSG) were measured by means of the glutathione recycling assay (Anderson, 1985) modified by Floreani et al. (1997). GSH induces the reduction of 5,5′-dithio-bis(2-nitrobenzoic acid), resulting in 2-nitro-5-thiobenzoic acid and GSSG. GSSG is recycled to GSH in the presence of GSSG reductase and NADPH. 2-Nitro-5-thiobenzoic acid generation is monitored spectrophotometrically at 412 nm for 1 min at 25°C. The reaction mixture for the determination of total glutathione included 50 μL of nodule extract (40 μg), 5 mm EDTA, 1.5 mm 5,5′-dithio-bis(2-nitrobenzoic acid) (Sigma), 0.3 mm NADPH (Sigma), 0.01 mg of GR (Roche), 50 mm sodium phosphate buffer (pH 7.6), and 100 mm potassium phosphate buffer (pH 7.6) for neutralization. The final volume of the reaction mixture was 1 mL. After neutralization of the sample, GSH was eliminated by adding 4 μL of 2-vinyl-pyridine and incubation for 1 h. After two washes with diethylether, GSH was determined as the difference between total glutathione and GSSG.
Ascorbate was measured by the method described by Okamura (1980) modified by Law et al. (1983). This method is based on the ascorbate-dependent reduction of iron (III) to iron (II). Formation of the complex between iron (II) with 2,2′-dipyridyl is measured at 525 nm. Nodules were ground in a mortar with liquid nitrogen. Ascorbate was extracted from nodules (0.2 g) with 800 μL of 5% (w/v) metaphosphoric acid. After centrifugation (12,000g for 5 min at 4°C), 175-μL aliquots were neutralized with 35 μL of 1.5 m triethanolamine. Aliquots of 100 μL of neutralized supernatant were used for each assay. For determination of total ascorbate (ascorbate plus dehydroascorbate), 100 μL was reduced with 50 μL of 10 mm DTT and incubated for 15 min at room temperature; subsequently, 50 μL of 0.5% (w/v) N-ethylmaleimide was added. Dehydroascorbate was determined as the difference between total ascorbate and ascorbate.
Enzyme Activities
For determination of enzyme activities, nodules (200 mg mL−1 buffer) were ground in a mortar with liquid nitrogen and homogenized in 50 mm potassium phosphate buffer (pH 7.8) containing 0.1 mm EDTA, 1% (w/v) PVP-10, and 0.1% (v/v) Triton X-100. The homogenate was centrifuged (15,000 rpm, 4°C, 30 min), and the supernatant was used for the activity assay. Three separate experiments were performed, with six to 15 replicates.
Catalase (EC 1.11.1.6) activity was assayed spectrophotometrically by monitoring hydrogen peroxide decomposition at 240 nm (Aebi, 1984) for 1 min at 25°C. The volume of the reaction mixture was 0.5 mL and included 10 μL of nodule extract, 12 mm hydrogen peroxide, and 50 mm potassium phosphate buffer (pH 8.0).
SOD (EC 1.15.1.1) activity was determined spectrophotometrically by monitoring the inhibition of cytochrome c reduction in the presence of the xanthine-xanthine oxidase system at 550 nm (McCord and Fridovich, 1969) for 1 min at 25°C. One unit of activity was defined as the amount of enzyme that inhibits the rate of cytochrome c reduction by 50% at 25°C. The volume of the blank reaction mixture was 1 mL and included 50 mm potassium phosphate buffer (pH 7.8) containing 0.1 mm EDTA, 14 μm ferric cytochrome c, 0.1 mm xanthine, 5 μm KCN, and 16 μL of xanthine oxidase diluted solution. Diluted xanthine oxidase was prepared by adding 20 μL of stock solution (Sigma) to 800 μL of 50 mm potassium phosphate buffer, pH 7.8. For determination of activity in nodule extracts, 30 μL of homogenate was added to the reaction mixture.
APX (EC 1.11.1.11) activity was assayed by monitoring the disappearance of ascorbate at 290 nm (Asada, 1984) for 1 min at 25°C. The reaction mix contained 30 μL of nodule extract, 0.2 mm ascorbate, and 0.3 mm hydrogen peroxide in 50 mm HEPES-NaOH buffer (pH 7.6) in a final volume of 1 mL. APX-independent ascorbate oxidation was quantified in control reactions with the addition of 1 mm KCN, 0.5 mm p-chloromercuriphenylsulfonic acid, and boiled extract.
GR (EC 1.6.4.2) activity was assayed by monitoring NADPH oxidation at 340 nm (Dalton et al., 1986) for 1 min at 25°C. The volume of the reaction mixture was 1 mL and included 100 mm HEPES-NaOH buffer (pH 7.8) containing 0.1 mm EDTA, 3 mm MgCl2, 1 mm GSSG, 0.1 mm NADPH, and 40 μL of nodule extract.
MDHAR (EC 1.6.5.4) activity was assayed by monitoring NADH oxidation at 340 nm (Dalton et al., 1992) for 1 min at 25°C. The reaction mixture (1 mL) included 30 μL of nodule extract, 1 mm ascorbate, and 0.5 units of ascorbate oxidase in 50 mm Tris-HCl buffer (pH 7.8). MDHAR-independent NADH oxidation was quantified in the absence of the monodehydroascorbate generation system ascorbate-ascorbate oxidase.
DHAR (EC 1.8.5.1) activity was assayed by following the generation of ascorbate at 265 nm (Nakano and Asada, 1987) for 1 min at 25°C. The reaction mixture (0.5 mL) included 20 μL of nodule extract, 5 mm DHA, 50 mm GSH, and 0.1 mm EDTA in 50 mm potassium phosphate buffer (pH 6.5). A correction factor of 0.98 was applied in the assessment of enzyme activity to compensate for GSSG absorbance (Hernández-Jiménez et al., 2002).
SS (EC 2.4.1.13) activity was measured spectrophotometrically by monitoring NAD+ reduction at 340 nm (Morell and Copeland, 1985). The volume of the reaction mixture was 1 mL and included 50 μL of nodule extract (45–70 μg of total protein), 100 mm Suc, 2 mm UDP, 1.5 mm NAD+, and 250 μg of UDPG dehydrogenase in 100 mm Bicine-KOH (pH 8.5). Nodule extracts were prepared in 50 mm potassium phosphate buffer (pH 8.0) containing 1 mm EDTA, 20% (v/v) ethylene glycol, and 33% (w/fresh weight) PVP. The homogenate was centrifuged (15,000 rpm, 4°C, 30 min), and the supernatant was used for the activity assay.
PEPC (EC 4.1.1.31) activity was assayed spectrophotometrically by monitoring NADH oxidation at 340 nm (Vandercammen et al., 1989) as reported previously (Verdoy et al., 2006).
Lipid Peroxidation
Lipid peroxidation in nodules was assayed using the thiobarbituric acid method modified according to Singh et al. (2007). This test determines the MDA as an end product of the thiobarbituric acid reaction. Nodules (100 mg) were homogenized in 1 mL of 0.1% TCA solution using liquid nitrogen. The homogenate was centrifuged at 10,000g for 20 min, and 0.5 mL of the supernatant was added to 1 mL of 0.5% 2-thiobarbituric acid in 20% TCA. The mixture was heated at 95°C for 30 min, and the reaction was stopped by placing the reaction tubes in an ice bath. The samples were then centrifuged at 10,000g for 5 min, and the absorbance of the supernatant was recorded at 532 nm. The value for nonspecific absorption at 600 nm was subtracted. The amount of MDA is calculated using the extinction coefficient ɛ = 155 mm−1 cm−1.
Light and Transmission Electron Microscopy
Ten-, 30-, and 44-dpi nodules were collected and immediately fixed under vacuum for 2 h at 4°C in 5% glutaraldehyde and 4% paraformaldehyde in 100 mm Na-cacodylate buffer (pH 7.4) containing 25 mg mL−1 Suc. After a second fixation stage (1.5 h at 4°C), three 1-h washes with Suc-cacodylate buffer were performed. Tissues were postfixed in 1% osmium tetroxide in the same buffer (16 h at 4°C). Samples were dehydrated in an ethanol series, including incubation in 1% uranile in 70% ethanol for 24 h at 4°C. Samples were embedded in LR White resin (London Resin Co.) and polymerized for 24 h at 60°C. Alternatively, nodule samples were fixed in 0.5% glutaraldehyde and 1% paraformaldehyde in the same buffer, embedded in LR White resin, and polymerized under UV light at −20°C.
Semithin (1 μm) and ultrathin (70–90 nm) sections were cut with a Reichert Ultracut S ultramicrotome fitted with a diamond knife. Semithin sections for light microscopy were stained with 1% (w/v) toluidine blue in aqueous 1% sodium borate for direct observation with a Zeiss Axiophot photomicroscope (Oberkochen). Ultra-thin sections for transmission electron microscopy were processed as reported previously (González-Sama et al., 2004).
Statistical Analyses
Data were analyzed with the SPSS program version 13.0.1 (SPSS, Inc.) by one-way ANOVA, and significant differences among treatments were determined by lsd (P ≤ 0.05).
Acknowledgments
We thank Dr. M.F. Fillat for the kind gift of plasmid pTrc99a-Fld and F. Pinto and E. González for technical assistance.
This work was supported by the Comunidad de Madrid (fellowship to T.C.d.l.P. and grant to J.J.P.), the Consejo Superior de Investigaciones Científicas and the European Social Fund (fellowship to F.J.R. and postdoctoral contract to T.C.d.l.P.), and the Spanish Ministry of Education and Science (grant to J.J.P.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: José J. Pueyo (pueyo@ccma.csic.es).
References
- Aebi H (1984) Catalase in vitro. Methods Enzymol 105 121–126 [DOI] [PubMed] [Google Scholar]
- Anderson ME (1985) Determination of glutathione and glutathione disulphide in biological samples. Methods Enzymol 113 548–555 [DOI] [PubMed] [Google Scholar]
- Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408 796–815 [DOI] [PubMed] [Google Scholar]
- Arakaki AK, Ceccarelli EA, Carrillo N (1997) Plant-type ferredoxin-NADP+ reductases: a basal structural framework and a multiplicity of functions. FASEB J 11 133–140 [DOI] [PubMed] [Google Scholar]
- Asada K (1984) Chloroplasts: formation of active oxygen and its scavenging. Methods Enzymol 105 422–429 [Google Scholar]
- Balmer Y, Vensel WH, Cai N, Manieri W, Schurmann P, Hurkman WJ, Buchanan BB (2006) A complete ferredoxin/thioredoxin system regulates fundamental processes in amyloplasts. Proc Natl Acad Sci USA 103 2988–2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becana M, Dalton DA, Moran JF, Iturbe-Ormaetxe I, Matamoros MA, Rubio MC (2000) Reactive oxygen species and antioxidants in legume nodules. Physiol Plant 109 372–381 [Google Scholar]
- Bradford MM (1976) Rapid and sensitive methods for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72 248–254 [DOI] [PubMed] [Google Scholar]
- Dalton DA, Langeberg L, Robbins M (1992) Purification and characterization of monodehydroascorbate reductase from soybean root nodules. Arch Biochem Biophys 292 281–286 [DOI] [PubMed] [Google Scholar]
- Dalton DA, Langeberg L, Treneman NC (1993) Correlations between the ascorbate-glutathione pathway and effectiveness in legume root nodules. Plant Physiol 87 365–370 [Google Scholar]
- Dalton DA, Russel SA, Hanus FJ, Pascoe GA, Evans HJ (1986) Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc Natl Acad Sci USA 83 3811–3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felipe MR, Fernández-Pascual MM, Pozuelo JM (1987) Effects of herbicides Lindex and Simazine on chloroplasts and nodule development, nodule activity and grain yield in Lupinus albus L. cv. Multolupa. Plant Soil 101 99–105 [Google Scholar]
- Deistung J, Cannon FC, Cannon MC, Hill S, Thorneley RNF (1985) Electron transfer to nitrogenase in Klebsiella pneumoniae: nifF gene cloned and the gene product, a flavodoxin, purified. Biochem J 231 743–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic MA, Gabriel DW, Rolfe BG (1987) Rhizobium: the refined parasite of legumes. Annu Rev Phytopathol 25 145–168 [Google Scholar]
- Dombrecht B, Vanderleyden J, Michiels J (2001) Stable RK2-derived cloning vectors for the analysis of gene expression and gene function in gram-negative bacteria. Mol Plant Microbe Interact 14 426–430 [DOI] [PubMed] [Google Scholar]
- Evans PJ, Gallesi D, Mathieu C, Hernandez MJ, de Felipe MR, Halliwell B, Puppo A (1999) Oxidative stress occurs during soybean nodule senescence. Planta 208 73–79 [Google Scholar]
- Figurski DH, Helinski DR (1979) Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA 76 1648–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillat MF, Borrias WE, Weisbeek PJ (1991) Isolation and overexpression in Escherichia coli of the flavodoxin gene from Anabaena PCC 7119. Biochem J 280 187–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan TM, Wood JM, Jordan DC (1983) Symbiotic properties of C4-dicarboxylic acid transport mutants of Rhizobium leguminosarum. J Bacteriol 154 1403–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floreani M, Petrone M, Debetto P, Palatini P (1997) A comparison between different methods for the determination of reduced and oxidized glutathione in mammalian tissues. Free Radic Res 26 449–455 [DOI] [PubMed] [Google Scholar]
- Frendo P, Harrison J, Norman C, Jimenez MJH, Van de Sype G, Gilabert A, Puppo A (2005) Glutathione and homoglutathione play a critical role in the nodulation process of Medicago truncatula. Mol Plant Microbe Interact 18 254–259 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Kolbe A, Tiessen A (2005) Redox regulation of carbon storage and partitioning in response to light and sugars. J Exp Bot 56 1469–1479 [DOI] [PubMed] [Google Scholar]
- González-Sama A, Lucas MM, De Felipe MR, Pueyo JJ (2004) An unusual infection mechanism and nodule morphogenesis in white lupin (Lupinus albus). New Phytol 163 371–380 [DOI] [PubMed] [Google Scholar]
- Gordon AJ, Minchin FR, James CL, Komina O (1999) Sucrose synthase in legume nodules is essential for nitrogen fixation. Plant Physiol 120 867–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groten K, Vanacker H, Dutilleul C, Bastian F, Bernard S, Carzaniga R, Foyer CH (2005) The roles of redox processes in pea nodule development and senescence. Plant Cell Environ 28 1293–1304 [Google Scholar]
- Günther C, Schlereth A, Udvardi M, Ott T (2007) Metabolism of reactive oxygen species is attenuated in leghemoglobin-deficient nodules of Lotus japonicus. Mol Plant Microbe Interact 20 1596–1603 [DOI] [PubMed] [Google Scholar]
- Hallenbeck PC, Gennaro G (1998) Stopped-flow kinetic studies of low potential electron carriers of the photosynthetic bacterium Rhodobacter capsulatus: ferredoxin I and NifF. Biochim Biophys Acta 1365 435–442 [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC (1999) Free Radicals in Biology and Medicine, Ed 3. Oxford University Press, Oxford
- Hardy RWF, Holsten RD, Jackson EK, Burns RC (1968) The acetylene-ethylene assay for N2 fixation: laboratory and field evaluation. Plant Physiol 43 1185–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J, Jamet A, Muglia CI, Van de Sype G, Aguilar OM, Puppo A, Frendo P (2005) Glutathione plays a fundamental role in growth and symbiotic capacity of Sinorhizobium meliloti. J Bacteriol 187 168–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Jiménez MJ, Lucas MM, de Felipe MR (2002) Antioxidant defense and damage in senescing lupin nodules. Plant Physiol Biochem 40 645–657 [Google Scholar]
- Hérouart D, Baudouin E, Frendo P, Harrison J, Santos R, Jamet A, Van de Sype G, Touati D, Puppo A (2002) Reactive oxygen species, nitric oxide and glutathione: a key role in the establishment of the legume-Rhizobium symbiosis? Plant Physiol Biochem 40 619–624 [Google Scholar]
- Hoagland DR, Arnon DI (1938) The Water Culture Method for Growing Plants without Soil. Agricultural Experimental Station Circular 347. University of California, Berkeley, CA
- Keyer K, Gort AS, Imlay JA (1995) Superoxide and the production of oxidative DNA damage. J Bacteriol 177 6782–6790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugkist J, Voorberg J, Haaker H, Veeger C (1986) Characterization of 3 different flavodoxins from Azotobacter vinelandii. Eur J Biochem 155 33–44 [DOI] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 680–685 [DOI] [PubMed] [Google Scholar]
- Law MY, Charles SA, Halliwell B (1983) Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts: the effect of hydrogen peroxide and of paraquat. Biochem J 210 899–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepek VC, D'Antuono AL, Tomatis PE, Ugalde JE, Giambiagi S, Ugalde RA (2002) Analysis of Mesorhizobium loti glycogen operon: effect of phosphoglucomutase (pgm) and glycogen synthase (g/gA) null mutants on nodulation of Lotus tenuis. Mol Plant Microbe Interact 15 368–375 [DOI] [PubMed] [Google Scholar]
- Liochev SI, Hausladen A, Beyer WF, Fridovich I (1994) NADPH:ferredoxin oxidoreductase acts as a paraquat diaphorase and is a member of the soxRS regulon. Proc Natl Acad Sci USA 91 1328–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodwig EM, Hosie AHF, Bordes A, Findlay K, Allaway D, Karunakaran R, Downie JA, Poole PS (2003) Amino acid cycling drives nitrogen fixation in the legume-Rhizobium symbiosis. Nature 422 722–726 [DOI] [PubMed] [Google Scholar]
- Lucas MM, Van de Sype G, Hérouart D, Hernández MJ, Puppo A, de Felipe MR (1998) Immunolocalization of ferritin in determinate and indeterminate legume root nodules. Protoplasma 204 61–70 [Google Scholar]
- Matamoros MA, Dalton DA, Ramos J, Clemente MR, Rubio MC, Becana M (2003) Biochemistry and molecular biology of antioxidants in the rhizobia-legume symbiosis. Plant Physiol 133 499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JM, Fridovich I (1969) Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244 6049–6055 [PubMed] [Google Scholar]
- McIver L, Leadbeater C, Campopiano DJ, Baxter RL, Daff SN, Chapman SK, Munro AW (1998) Characterisation of flavodoxin NADP+ oxidoreductase and flavodoxin: key components of electron transfer in Escherichia coli. Eur J Biochem 257 577–585 [DOI] [PubMed] [Google Scholar]
- McKhann HI, Hirsch AM (1994) Does Rhizobium avoid the host response? Curr Top Microbiol Immunol 192 139–162 [DOI] [PubMed] [Google Scholar]
- Minchin FR, Witty JF, Sheehy JE, Müller M (1983) A major error in the acetylene reduction assay: decreases in nodular nitrogenase activity under assay conditions. J Exp Bot 34 641–649 [Google Scholar]
- Mithofer A (2002) Suppression of plant defence in rhizobia-legume symbiosis. Trends Plant Sci 7 440–444 [DOI] [PubMed] [Google Scholar]
- Morell M, Copeland L (1985) Sucrose synthase of soybean nodules. Plant Physiol 78 149–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Asada K (1987) Purification of ascorbate peroxidase in spinach chloroplasts: its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol 28 131–140 [Google Scholar]
- Noctor N, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49 249–279 [DOI] [PubMed] [Google Scholar]
- Okamura M (1980) An improved method for determination of L-ascorbic acid and L-dehydroascorbic acid in blood plasma. Clin Chim Acta 103 259–268 [DOI] [PubMed] [Google Scholar]
- Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH (2003) Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell 15 939–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueyo JJ, Gómez-Moreno C (1991) Characterization of the cross-linked complex formed between ferredoxin-NADP+ reductase and flavodoxin from Anabaena PCC 7119. Biochim Biophys Acta 1059 149–156 [Google Scholar]
- Pueyo JJ, Gómez-Moreno C, Mayhew SG (1991) Oxidation-reduction potentials of ferredoxin NADP+ reductase and flavodoxin from Anabaena PCC7119 and of their electrostatic and covalent complexes. Eur J Biochem 202 1065–1071 [DOI] [PubMed] [Google Scholar]
- Puppo A, Groten K, Bastian F, Carzaniga R, Soussi M, Lucas MM, De Felipe MR, Harrison J, Vanacker H, Foyer CH (2005) Legume nodule senescence: roles for redox and hormone signalling in the orchestration of the natural aging process. New Phytol 165 683–701 [DOI] [PubMed] [Google Scholar]
- Riggle BD, Wiebold WJ, Kenworth WJ (1982) Effect of photosynthate source-sink manipulation on dinitrogen fixation of male-fertile and male-sterile soybean isolines. Crop Sci 24 5–8 [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Sandmann G, Peleato ML, Fillat MF, Lazaro MC, Gómez-Moreno C (1990) Consequences of the iron-dependent formation of ferredoxin and flavodoxin on photosynthesis and nitrogen-fixation on Anabaena strains. Photosynth Res 26 119–125 [DOI] [PubMed] [Google Scholar]
- Santos R, Hérouart D, Puppo A, Touati D (2000) Critical protective role of bacterial superoxide dismutase in Rhizobium-legume symbiosis. Mol Microbiol 38 750–759 [DOI] [PubMed] [Google Scholar]
- Sigaud S, Becquet V, Frendo P, Puppo A, Hérouart D (1999) Differential regulation of two divergent Sinorhizobium meliloti genes for HPII-like catalases during free-living growth and protective role of both catalases during symbiosis. J Bacteriol 181 2634–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simondsen RP, Tollin G (1980) Structure-function relations in flavodoxins. Mol Cell Biochem 33 13–24 [DOI] [PubMed] [Google Scholar]
- Singh AK, Li H, Sherman LA (2004) Microarray analysis and redox control of gene expression in the cyanobacterium Synechocystis sp. PCC 6803. Physiol Plant 120 27–35 [DOI] [PubMed] [Google Scholar]
- Singh MP, Singh DK, Rai M (2007) Assessment of growth, physiological and biochemical parameters and activities of antioxidative enzymes in salinity tolerant and sensitive basmati rice varieties. J Agron Crop Sci 193 398–412 [Google Scholar]
- Smith AM, Denyer K, Martin C (1997) The synthesis of the starch granule. Annu Rev Plant Physiol Plant Mol Biol 48 67–87 [DOI] [PubMed] [Google Scholar]
- Suganuma N, Okada Y, Kanayama Y (1997) Isolation of a cDNA for nodule-enhanced phosphoenolpyruvate carboxylase from pea and its expression in effective and plant-determined ineffective pea nodules. J Exp Bot 48 1165–1173 [Google Scholar]
- Swaraj K, Bishnoi NR (1996) Physiological and biochemical basis of nodule senescence in legumes: a review. Plant Physiol Biochem 23 105–116 [Google Scholar]
- Thummler F, Verma DPS (1987) Nodulin-100 of soybean is the subunit of sucrose synthase regulated by the availability of free heme in nodules. J Biol Chem 262 14730–14736 [PubMed] [Google Scholar]
- Timmers ACJ, Soupene E, Auriac MC, de Billy F, Vasse J, Boistard P, Truchet G (2000) Saprophytic intracellular rhizobia in alfalfa nodules. Mol Plant Microbe Interact 13 1204–1213 [DOI] [PubMed] [Google Scholar]
- Tognetti VB, Monti MR, Valle EM, Carrillo N, Smania A (2007. a) Detoxification of 2,4-dinitrotoluene by transgenic plants expressing a bacterial flavodoxin. Environ Sci Technol 41 4071–4076 [DOI] [PubMed] [Google Scholar]
- Tognetti VB, Palatnik JF, Fillat MF, Melzer M, Hajirezael MR, Valle EM, Carrillo N (2006) Functional replacement of ferredoxin by a cyanobacterial flavodoxin in tobacco confers broad-range stress tolerance. Plant Cell 18 2035–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognetti VB, Zurbriggen MD, Morandi EN, Fillat MF, Valle EM, Hajirezael MR, Carrillo N (2007. b) Enhanced plant tolerance to iron starvation by functional substitution of chloroplast ferredoxin with a bacterial flavodoxin. Proc Natl Acad Sci USA 104 11495–11500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Velde W, Pérez Guerra JC, De Keyser A, De Rycke R, Rombauts S, Maunoury N, Mergaert P, Kondorosi E, Holsters M, Goormachtig S (2006) Aging in legume symbiosis. A molecular view on nodule senescence in Medicago truncatula. Plant Physiol 141 711–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandercammen A, Francois J, Hers HG (1989) Characterization of trehalose-6-phosphate synthase and trehalose-6- phosphate phosphatase of Saccharomyces cerevisiae. Eur J Biochem 182 613–620 [DOI] [PubMed] [Google Scholar]
- Vasse J, de Billy F, Camut S, Truchet G (1990) Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J Bacteriol 172 4295–4306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoy D, Coba de la Peña T, Redondo FJ, Lucas MM, Pueyo JJ (2006) Transgenic Medicago truncatula plants that accumulate proline display nitrogen-fixing activity with enhanced tolerance to osmotic stress. Plant Cell Environ 29 1913–1923 [DOI] [PubMed] [Google Scholar]
- Vincent JM (1970) A Manual for the Practical Study of Root Nodule Bacteria. Blackwell Scientific Publications, Oxford
- Yousef N, Pistorius EK, Michel KP (2003) Comparative analysis of idiA and isiA transcription under iron starvation and oxidative stress in Synechococcus elongatus PCC 7942 wild-type and selected mutants. Arch Microbiol 180 471–483 [DOI] [PubMed] [Google Scholar]
- Zahran HH (2001) Rhizobia from wild legumes: diversity, taxonomy, ecology, nitrogen fixation and biotechnology. J Biotechnol 91 143–153 [DOI] [PubMed] [Google Scholar]
- Zheng M, Doan B, Schneider TD, Storz G (1999) OxyR and SoxRS regulation of fur. J Bacteriol 181 4639–4643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurbriggen MD, Tognetti VB, Carrillo N (2007) Stress-inducible flavodoxin from photosynthetic microorganisms: the mystery of flavodoxin loss from the plant genome. IUBMB Life 59 1–6 [DOI] [PubMed] [Google Scholar]