Abstract
There is now general agreement that nitric oxide (NO) is an important and almost universal signal in plants. Nevertheless, there are still many controversial observations and opinions on the importance and function of NO in plants. Partly, this may be due to the difficulties in detecting and even more in quantifying NO. Here, we summarize major pathways of NO production in plants, and briefly discuss some methodical problems.
Key Words: chemiluminescence, DAF-fluorescence, mitochondria, nitrate reductase, nitric oxide, nitric oxide synthase, NO detection, NO signaling
Introduction
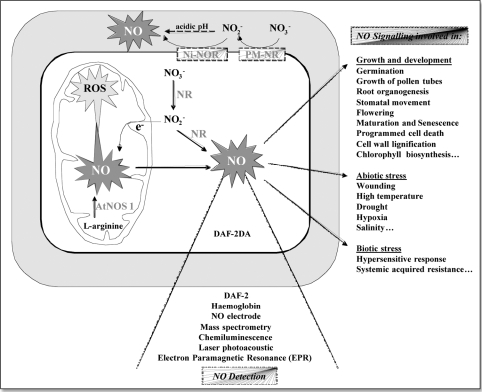
The last decade has brought many new insights into pathways of NO production and NO functions in plants, and to date the role of NO in plants appears even more multi-faceted than in animals. Plant borne NO seems involved in controlling cell differentiation and lignification, root and shoot development, flowering, growth and reorientation of pollen tubes, senescence and maturation, stomatal movement, plant-pathogen interactions and programmed cell death, and many others (see Fig. 1). There are a number of excellent recent reviews on the roles of NO in plants,1–8 and in principle there is little doubt that NO is an important second messenger. However, many details of the NO story are still questionable. We do not know with certainty how NO is synthesized in specific situations, how NO concentrations in plants are regulated and where exactly NO exerts its effects in various signalling processes. This lack of knowledge is partially due to the technical difficulties in measuring and quantifying NO. In the following, we give a brief summary of reactions in plants that may contribute to NO production, and we discuss some open questions and obvious discrepancies in plant NO research.
Figure 1.
Schematic diagram summarizing reactions and locations where NO is produced, methods to detect NO and major reactions where NO has been suggested to be involved as a signal. For further summaries on NO signalling compare.1–4,6–7,9
A Quick Look on Methods
NO detection.
Free radicals are often difficult to detect and to quantify due to their high reactivity and short half-life. However, at the very low concentrations (pM to nM) found in plants, NO is relatively stable. NO production by plants, plant cells, purified mitochondria or purified nitrate reductase (NR) has been demonstrated and partially quantified with a remarkably large number of methods, including chemiluminescence, DAF-fluorescence and fluorescence imaging, electron paramagnetic resonance spectroscopy (EPR), oxyhemoglobin/methemoglobin, laser photoacoustics, NO electrodes and mass-spectrometry (compare Fig. 1).
It is important to be aware that all methods, with the exception of DAF-fluorescence, depend either on NO out-diffusion from cells and tissues, or they require rapidly prepared cell extracts to be mixed e.g., with EPR-spin traps or with hemoglobin. Out-diffusion of NO is indeed facilitated by the moderate water solubility and high diffusibility of the small molecule. Chemiluminescence, mass spectrometry and laser photoacoustics depend on a diffusion of NO not only out of cells but also out of the aqueous into a gas phase (headspace) or through a gas-diffusible membrane. Results obtained with different methods are often in conflict. For example, laser photoacoustics10 as well as hemoglobin,11–13 EPR14–15 and mass-spectrometry16 consistently indicated that infection of cells or leaves with avirulent bacterial strains or with elicitors lead to NO emission, although reported kinetics and quantities were often remarkably different. In contrast, measurement of NO emission by chemiluminescence from tobacco cell suspensions or leaves treated with the fungal elicitor cryptogein17 or with avirulent Pseudomonas syringae pv. tomato DC 3000 (Planchet and Kaiser, unpublished) gave no or only a weak and late NO emission that was absent in nitrate reductase-free plants and apparently not causally related to NO emission. Such conflicting results obtained with the above methods cannot be traced back to internal scavenging of NO, because they all rely on NO out-diffusion and emission, as already stated. Whether and why one or the other detection system may give controversial results in specific situations is not known yet.
A special problem: diaminofluoresceins.
These frequently and widely used fluorescence indicators are available in cell impermeable forms and as cell permeable diacetates (DAF-2DA and DAF-FM DA), which are hydrolyzed inside cells and thereby trapped. Upon NO production, DAF-2 is nitrosated to give the highly fluorescing and relatively stable DAF-2 triazole (DAF-2T). Compared to the other methods, DAF fluorescence has the advantage to be closest to the potential sites of NO production inside living cells, and in addition provides the possibility for fluorescence imaging which—at least in theory—permits cellular and subcellular localization of NO. Increased DAF-fluorescence in plant cells has been reported in response to pathogens,18–21 in closing stomata,22–25 in xylem differentiation and lignification,26 in context with cytokinin signalling27–28 to name just a few examples out of many. This widespread use of DAF-2 and its derivatives is actually astonishing for several reasons:
DAF actually does not react directly with NO, but with the more oxidized forms such as NO+ or N2O329–30 which can be produced by NO autoxidation in presence of oxygen, or by reaction of NO with NO2,30 or with reactive oxygen species (ROS).31 In addition, DAF-2 itself can be oxidized by ROS into a free radical intermediate that directly reacts with NO.32 This implies that changes in DAF-fluorescence occur not only in response to NO production, as generally thought, but in response to changes in ROS with subsequent NO or DAF oxidation. This is especially important, because many cellular processes result in ROS (mitochondrial and chloroplastic electron transport, photorespiration, plasma-membrane-NADH oxidases and others), or require ROS (e.g., programmed cell death, lignification and others). A so-called “oxidative burst” is a common observation in plants subjected to stress, and it has been suggested that the two events (NO and ROS production) have to occur in parallel.11,33–34 On the other hand, as shown above, one compound might actually prevent the detection of the other.
Other potential interfering agents are ascorbic acid and dehydroascorbic acid, which react with DAF to give products with fluorescence emission profiles similar to that of DAF-2T, as deduced from capillary electrophoresis with laser-induced fluorescence detection and electro spray mass spectrometry.35 Again, this is potentially important because the redox state of the ascorbate/dehydroascorbate system of plants is also responsive to stress.
The “pharmaceutical approach”: NO scavengers, NO donors and NOS-inhibitors.
At large part of our pictures on the function of NO in plants is based on the use of chemicals, mainly provided by medical research on NO. Examples for NO scavengers are 2-phenyl-4,4,5-tetramethylimidazole-1-oxyl 3-oxide (PTIO) and its derivatives (carboxy-PTIO (cPTIO) or trimethylammonio-PTIO). These relatively unstable compounds are thought to be cell impermeable,30 and when used with intact cell or tissues, they should only react with NO or its derivatives after diffusion out of cells. This is an important, but often neglected property of these NO scavengers. When PTIO reacts with NO in aqueous solution according to equations (2 to 4), nitrite is formed via NO2 and N2O3.
| 2 |
| 3 |
| 4 |
These intermediate redox forms of NO, specifically N2O3, can react with amines, thiols or hydroxyl groups to form NO adducts (see ref. 36 and literature cited). The specificity of cPTIO as NO scavenger has been questioned,37 and our own work has shown that the product of the reaction of NO with cPTIO, cPTI, still prevented the induction of a hypersensitive response in tobacco leaves, although it did not scavenge NO.17 Thus, the presence or absence of PTIO effects on a given plant response is no unequivocal proof for a participation of NO.
Frequently used NO donors are sodium nitroprusside (SNP) and diethylamine/NO (DEA/NO; NaEt2NN(O)NO). The kinetics of NO production in aqueous solutions are very different for the two donors (Planchet E, Kaiser WM, unpublished): DEA/NO produces a rapid (seconds to minutes) and transient NO burst, whereas SNP gives a continuous, long-lasting NO production—again this is an often neglected but important difference. Further, in a recent study on breakage of seed dormancy by NO, it was shown that SNP produces a number of other gaseous compounds in addition to NO,38 specifically HCN. Cyanide, like NO, can also break seed dormancy and the effect is also prevented by cPTIO. This study38 highlights some interpretation problems associated with the “pharmacological approach”.
At least for nitric oxide synthase (NOS, see below), a number of inhibitors (AET, L-NAME, L-NMMA, L-NIL) are available that are all structural analogues of the substrate, L-arginine. Thus, if a reaction can be blocked by one of these inhibitors, the conclusion would be that NOS is involved as NO source. In plants, very high concentrations of the inhibitors (up to 300 mM) were occasionally required in order to see effects,39–40 and such concentrations are more than 1000-fold above those used in animal NO research. Partly, this may be due to low uptake of the inhibitors. On the other hand, concentrations of the natural substrate of NOS, L-arginine, are highly variable and may become very high in plants, e.g., under low K+ and/or high NH4+ nutrition. One other problem associated with the use of NOS inhibitors lies in the fact that other cellular reactions also use L-arginine as substrate (e.g., arginine decarboxylase involved in polyamine biosynthesis) and will therefore respond to the inhibitors.
Sources for No
Nitrite-dependent NO production.
Several plant systems use nitrite as a substrate, according to the basic reaction (5):
| 5 |
These are (also compare Fig. 1):
Cytosolic NR (cNR)
A plasma membrane-bound NR (PM-NR) associated with a PM-nitrite: NO reductase.
Mitochondrial electron transport
Xanthine dehydrogenase/oxidase
Nonenzymatic NO formation at acidic pH.
Assimilatory nitrate reductase (NR). NR has long been known as a source for NO,41–43 although it's NO producing capacity has been occasionally overestimated. Meanwhile, it has been shown that the capacity of NR to produce NO is about 1% of its normal nitrate reduction capacity.44–45 The involvement of NR in NO production has been further established by work with nia mutants and with plants made NR-free by growth conditions (ammonium as sole N source and/or tungstate supply instead of molybdate to give a nonfunctional NR).45–47 The enzyme, which normally reduces nitrate (+5) to nitrite (+3) at the expense of NAD(P)H, also catalyzes a 1-electron transfer from NAD(P)H to nitrite resulting in NO (+2) formation. With purified NR in solution and chemiluminescence-gas phase detection in a headspace, NO production by NR was found to be only slightly higher in pure nitrogen (or argon) than in air,45 which indicates that in such a cell-free system, loss of NO by autoxidation or by reaction with ROS produced on the same enzyme48–49 is usually ≥ 50% of the total NO produced.45 Modulation of NR activity by reversible serine phosphorylation has been shown to modulate NO production,44 and plants constitutively expressing a NR where the regulatory serine was exchanged against aspartic acid50 had a diurnal NO emission pattern opposite to the wild type (low in light, high in the dark).51 All this highlights the importance of NR in NO production and its regulation (reviewed in ref. 8).
PM-NR/Ni:NOR. Plant roots contain a PM-bound NR which appears associated with a nitrite: NO oxidoreductase. PM-NR reduces nitrate to nitrite in the apoplastic space, and at least part of that may be further reduced to NO by the latter enzyme. Whether the two enzymes are inducible or constitutive is not known yet.52–53
Mitochondria. Suspension cells of the tobacco nia-double mutant (or ammonium-grown WT cells) gave no chemiluminescence signal and hardly any increase in DAF-fluorescence, neither in air nor in nitrogen.17,45 When supplied with nitrite (≤ 0.5 mM) under nitrogen, however, the NR-deficient cells produced as much NO as NR-expressing WT cells.45,54 The high anoxic NO production was completely blocked by inhibitors of respiratory electron transport,45,54–55 suggesting that plant mitochondria, just like mitochondria from animals, contain a nitrite:NO reductase activity. This was confirmed with mitochondria purified from various plant sources, which produced NO under nitrogen when supplied with NADH and nitrite.54 As for NR, NO production rates under anoxia (1 to 10 nmoles NO mg−1 protein h−1 measured by chemiluminescence) were only about 1/1000 of the respiratory electron transport capacity. In air, purified mitochondria produced no NO detectable with chemiluminescence.45,54 The conclusion is that reduction of nitrite to NO by mitochondrial electron transport is very sensitive to (competitive) inhibition by oxygen54 and accordingly does not function in air. On the other hand, if NO would be rapidly oxidized e.g., to N2O3 (+3), at least part of it should be detectable by DAF-fluorescence (compare data on the mitochondrial location of AtNOS1 described below ref. 56). Recent chemiluminescence measurements suggested that the ability for NO production was restricted to mitochondria from roots, whereas leaf mitochondria did not produce NO, neither in vitro nor in vivo.54 On the other hand, Modolo et al.15 using EPR, showed that mitochondrial electron transport does produce NO also in leaves. The cause for the discrepancy is not known yet.
Xanthine oxidase/dehydrogenase (XDH). XDH has also been occasionally suggested as a source for NO using nitrite and xanthine as a substrate.57–58 However, our own experiments in cooperation with R. Mendel, Braunschweig, using recombinant XDH, gave no evidence for NO production by the enzyme itself.
Nonenzymatic NO production. according to equation (6) should occur only at pH below 4.5, since the pKa of nitrous acid is about 3.2.
| 6 |
Conditions favouring nonenzymatic NO formation may occasionally exist in the apoplast of plant cells.59
L-Arginine dependent NO production.
However, in addition to nitrite-based NO production, and in analogy to animals, plants appear to posses an enzyme, nitric oxide synthase (NOS), that is completely independent of nitrite and converts L-arginine into L-citrulline and NO using NADPH and O2 (reaction 7) and requires Ca2+/calmodulin.
| 7 |
One very important difference between NO production based on nitrite to that based on L-arginine and NOS becomes immediately obvious: The former is optimal in the absence of oxygen, whereas the presence of oxygen is obligatory for NOS-catalyzed NO production.
Much of the original literature suggesting a NOS-like activity as a source for NO in plants was based on pharmacological evidence (see below). Inhibition of reactions by chemical analogs of L-arginine was (and still is) taken as a strong indication that the reaction was triggered by NOS-derived NO. Immunological evidence was obtained with antibodies against animal NOS,60–63 but those antibodies proved to be rather unspecific.64–65 In addition, no Arabidopsis gene or protein homolog to the large and complex animal protein has been found. More recently, a new horizon in NO research was opened by the finding of the Crawford group5,25,56 that Arabidopsis contains a gene with sequence similarity to a gene from Helix pomatia that is implicated in NO synthesis. The gene encodes a 60kDa protein, which, when expressed in E. coli, increased NO synthesis in cell extracts. When the corresponding gene (AtNOS1) was knocked out in Arabidopsis, the resulting mutant had reduced NO production in roots (measured with DAF-2DA). Contrary to animal NOS (about 140 kDA), the much smaller AtNOS1 requires no flavin or tetrahydrobiopterin, but only Ca2+, CaM and NADPH. AtNOS1 seems constitutively expressed. It is part of the signalling pathway involved in ABA-induced stomatal closure, germination, root and shoot growth, seed fertility,25 control of flower timing,66 senescence56 and seems also involved in NO production during plant-pathogen interactions, as derived from experiments with DAF-FM DA and EPR.67 Also, in the atnos1 knock out mutant, induction of defence related genes by Pseudomonas syringae was suppressed compared to the wild type.67 All these data suggest very convincingly that AtNOS1 could be the ‘real’ plant NOS with properties and functions complementary to animal NOS, but without the requirement for tetrahydrobiopterin as cofactor.
Very recent work by Crawford's group indicates that AtNOS1 is located in the mitochondria.56 This is surprising, because mitochondria are one major source for ROS which are thought to very rapidly react with NO,68–69 and this view is consistent with the finding that purified mitochondria gave no chemiluminescence signal in air, but only under nitrogen.54 But how is it possible that purified mitochondria producing NO via AtNOS1 show increased DAF-fluorescence in air?56 And how could NO be a signal to react with extra-mitochondrial constituents, if it is so rapidly oxidized within the mitochondria? Or is the actual signal not NO itself but its oxidized forms? The questions are still open.
NR-derived NO versus AtNOS-derived NO.
As shown above, the various plant systems that produce NO from nitrite are relatively inactive under aerobic conditions yet much more active under hypoxia or anoxia; whereas L-arginine-dependent NOS requires oxygen. Actually, the two systems could cooperate and even replace each other, perhaps depending on oxygenation of plant tissues.70 Interestingly, in a recent study where NO production in crude extracts from Sorghum embryonic axes by NR and by NOS was quantified by EPR, NO production capacity of NOS was 10% of NR.71 Our own data with tobacco leaf extract gave a mean NOS-capacity of about 0.5 to 1 nmole g−1 FW h−1, and a mean nitrite:NO reductase activity of cNR of 50 to 100 nmole g−1 FW h−1, both at substrate saturation. Thus, the capacity of NR to produce NO appears always higher than that of NOS, at least in plants well fertilized with nitrate.
If plants that do not reduce nitrate—for whatever reason—would not be able to activate signalling chains for their development or defence reactions in which NO is an obligatory intermediate, they would not survive. But the hypersensitive response and expression of defence proteins (pathogenesis-related proteins, PR-1) were triggered in nia double mutants that did not emit NO, and on the other hand also in plants highly overproducing NO (Nitrite reductase (NiR)-deficient transformants).17 Nia double mutants, WT plants and NiR-deficient NO-overproducers show some phenotypic differences, but when supplied with ammonium-N their seeds germinate, the seedlings grow almost normally, their stomata appear functional and they complete their life cycle, at least under good growth conditions (Kaiser WM, unpublished; also compare ref. 22). Whether phenotypic differences in root systems often described under nitrate versus ammonium nutrition are actually due to NO is not known. A conclusion out of these considerations is that either a NOS-like activity is an obligatory and universal NO source in plants. Or if neither NR nor NOS are obligatory for plants to grow, the ‘global’ necessity for NO in plants would be a fiction.
Presently, however, it appears that both, NR and AtNOS1 contribute NO. The above-described properties of knock out mutants of AtNOS125,56,66–67 appear to confirm that AtNOS1 can provide NO for developmental signalling as well as for defence, and that it is more important than nitrite-derived NO. But how can that be on the background of a much higher capacity of NR to produce NO? And how is it possible that nitrite-derived NO can be so easily quantified by chemiluminescence, whereas NOS-derived NO was not detected?17 Certainly, there are still a number of controversial observations and unsolved problems with respect to the endogenous sources for NO in plants.
Other Factors Affecting No Levels
Regulation of NO levels by hemoglobins?
Many cellular components may react with NO and its derivatives, including thiols and hemes. In context with a possible regulation of NO levels in plants, hemoglobins have recently gained special attention. At least part of cellular NO scavenging has been attributed to the so-called ‘non symbiotic hemoglobins’ (ns-Hbs), which are ubiquitous in plants and induced by nitrate, nitrite and NO.72 Ns-Hbs have been suggested to act as NAD(P)H-dependent dioxygenases to convert NO to nitrate.73–75 Interestingly, most of the NO dioxygenase activity has been located in the cytosol, not in the mitochondria.76 It seems, therefore, very improbable that aerobic NO formation by purified mitochondria is so extremely low because of a ns-Hb dioxygenase activity. Rates of NO scavenging by crude extracts from alfalfa root cultures were up to 300 µmol g−1 FW h−1 at an estimated NO concentration of 1 µM.73 These rates are about 3 × 103-fold higher than rates of NO production by NR and 3 × 105-fold higher than the potential NO production by NOS (on a FW-basis). One reason for those extraordinary high rates of NO scavenging may be that the applied NO concentrations were several orders of magnitude above those thought to exist in plants cells.45 On the other hand, expression of hemoglobin affected NO levels produced by alfalfa root cultures,77 and in transgenic rice strong underexpression of ns-Hb increased NO emission from rice leaves, whereas overexpression decreased NO emission measured by chemiluminescence (Ohwaki Y, Kaiser WM, unpublished). Thus, expression of ns-Hb may well have a regulatory function for NO-levels. To what extent all this happens at realistic NO concentrations, has to be examined yet.
How can endogenous NO be a signal when plants are sinks for exogenous NO?
Plant root systems—at least under some conditions (e.g., soil flooding)—may receive some nitrite produced by soil micro-organisms. Shoots and leaves of rosette plants clinging to the soil surface may also directly receive dissolved or gaseous NO from soil micro-organisms, which may actually produce much more NO than the roots or the whole plants. For example, a single illuminated Arabidopsis plant growing in a plastic pot (5 cm upper diameter) containing about 100 mL garden soil contributed less than 1/1000 to the total NO emitted (pot plus plant, Kaiser WM, unpublished). Inevitably, high microbial activity in N-fertilized soils will lead to a massive NO influx into roots systems, but also into soil-covering leaves.
Anthropogenic emission can also cause high local atmospheric NO concentrations, where plants than become sinks for NO, not sources. Wildt and colleagues78 found a NO compensation point of about 1 ppb. But in cases where roots or whole plants are NO sinks, how can NO signalling function? The answer is usually, that the above-described plant systems, and specifically AtNOS1, are able to create high local NO concentrations, far above those resulting from NO diffusion into the plant. Clear quantitative experimental evidence for that, however, is still missing.
Conclusions and Perspectives
We have tried to show that in spite of years of intense research, there are still a number of very important questions, both basic ones and methodical ones, waiting for answers:
To what extent is NO quantification outside cells (by chemiluminescence, laser photoacoustics, mass spectrometry, EPR) reflecting rates of NO production?
As DAF reacts with oxidized NO, is an increase in DAF-fluorescence reflecting a change in NO production or a change in NO oxidation?
Extractable NOS activity is only 1% of NO production by NR. How can such a small NOS activity create a NO signal on the much higher background of NR-NO?
How can NO be a signal in root systems growing in soils that emit NO at fluxes two to three orders of magnitude above those emitted by the plant itself?
How can NO create measurable signals in organelles (e.g., mitochondria) where ROS is simultaneously produced, probably at much higher rates?
Future work certainly requires a concerted action of laboratories where different methods for NO and ROS measurements are established, using plant material and conditions as similar as possible. Results will be the more reliable the more different approaches are used. In addition, extensive use of mutants and transformants (Arabidopsis, tobacco) with modified NR, NOS and Hb-expression (many are already available) will certainly help to assess, whether NO really is the all-purpose signalling molecule that it is believed to be.
Acknowledgements
This work was supported by the DFG Ka 456/1–3, and the SFB 567.
Abbreviations
- AtNOS
arabidopsis thaliana nitric oxide synthase
- cNR
cytosolic nitrate reductase
- cPTI
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl
- cPTIO
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- DAF-2
4,5-diaminofluorescein
- DAF-2DA
4,5-diaminofluorescein diacetate
- DAF-2T
diaminofluorescein triazole
- DAF-FM DA
4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate
- DEA/NO
diethylamine/nitric oxide
- EPR
electron paramagnetic resonance
- Ni-NOR
nitrite-nitric oxide reductase
- NiR
nitrite reductase
- NO
nitric oxide
- NR
nitrate reductase
- Ns-Hb
non-symbiotic hemoglobin
- PM-NR
plasma membrane-bound nitrate reductase
- PTI
2-phenyl-4,4,5,5-tetramethylimidazole-1-oxyl
- PTIO
2-phenyl-4,4,5,5-tetramethylimidazole-1-oxyl 3-oxide
- ROS
reactive oxygen species
- SNP
sodium nitroprusside
- XDH
xanthine oxidase/dehydrogenase
Footnotes
Previously published onlinse as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=2435
References
- 1.Lamattina L, García-Mata C, Graziano M, Pagnussat G. Nitric oxide: The versatility of an extensive signal molecule. Annu Rev Plant Biol. 2003;54:109–136. doi: 10.1146/annurev.arplant.54.031902.134752. [DOI] [PubMed] [Google Scholar]
- 2.Neill SJ, Desikan R, Hancock JT. Nitric oxide signalling in plants. New Phytol. 2003;159:11–35. doi: 10.1046/j.1469-8137.2003.00804.x. [DOI] [PubMed] [Google Scholar]
- 3.Romero-Puertas MC, Perazzolli M, Zago ED, Delledonne M. Nitric oxide signalling functions in plant-pathogen interactions. Cell Microbiol. 2004;6:795–803. doi: 10.1111/j.1462-5822.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- 4.Wendehenne D, Durner J, Klessig DF. Nitric oxide: A new player in plant signalling and defence responses. Curr Opin Plant Biol. 2004;7:449–455. doi: 10.1016/j.pbi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Crawford NM, Guo FQ. New insights into nitric oxide metabolism and functions. Trends Plant Sci. 2005;259:1360–1385. doi: 10.1016/j.tplants.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Delledonne M. NO news is good news for plants. Curr Opin Plant Biol. 2005;8:390–396. doi: 10.1016/j.pbi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Lamotte O, Courtois C, Barnavon L, Pugin A, Wendehenne D. Nitric oxide in plants: The biosynthesis and cell signalling properties of a fascinating molecule. Planta. 2005;221:1–4. doi: 10.1007/s00425-005-1494-8. [DOI] [PubMed] [Google Scholar]
- 8.Meyer C, Lea US, Provan F, Kaiser WM, Lillo C. Is nitrate reductase a major player in the plant NO (nitric oxide) game? Photosynth Res. 2005;83:181–189. doi: 10.1007/s11120-004-3548-3. [DOI] [PubMed] [Google Scholar]
- 9.del Río LA, Corpas FJ, Barroso JB. Nitric oxide and nitric oxide synthase activity in plants. Phytochemistry. 2004;65:783–792. doi: 10.1016/j.phytochem.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Mur LAJ, Santosa IE, Laarhoven LJ, Holton NJ, Harren FJM, Smith AR. Laser photoacoustic detection allows in plants detection of nitric oxide in tobacco following challenge with avirulent and virulent Pseudomonas syringae pathovars. Plant Physiol. 2005;138:1247–1258. doi: 10.1104/pp.104.055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delledonne M, Xia Y, Dixon RA, Lamb C. Nitric oxide as a signal in plant defense resistance. Nature. 1998;394:585–588. doi: 10.1038/29087. [DOI] [PubMed] [Google Scholar]
- 12.Clarke A, Desikan R, Hurst RD, Hancock JT, Neill SJ. NO way back: Nitric oxide and programmed cell death in Arabidopsis thaliana suspension cultures. Plant J. 2000;24:667–677. doi: 10.1046/j.1365-313x.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- 13.Xu MJ, Dong JF, Zhu MY. Nitric oxide mediates the fungal elicitor-induced hypericin production of Hypericum perforatum cell suspension cultures through a jasmonic-acid-dependent signal pathway. Plant Physiol. 2005;139:991–998. doi: 10.1104/pp.105.066407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo P, Cao Y, Li Z, Zhao B. Role of an endogenous nitric oxide burst in the resistance of wheat to stripe rust. Plant Cell Env. 2004;27:473–477. [Google Scholar]
- 15.Modolo LV, Augusto O, Almeida IMG, Magalhaes JR, Salgado I. Nitrite as the major source of nitric oxide production by Arabidopsis thaliana in response to Pseudomonas syringae. FEBS Lett. 2005;579:3814–3820. doi: 10.1016/j.febslet.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 16.Conrath U, Amoroso G, Kölhe H, Sültemeyer DF. Noninvasive online detection of nitric oxide from plants and some others organisms by mass spectrometry. Plant J. 2004;38:1015–1022. doi: 10.1111/j.1365-313X.2004.02096.x. [DOI] [PubMed] [Google Scholar]
- 17.Planchet E, Sonoda M, Zeier J, Kaiser WM. Nitric oxide (NO) as an intermediate in the cryptogein-induced hypersensitive response - A critical reevaluation. Plant Cell Env. 2005;29:59–69. doi: 10.1111/j.1365-3040.2005.01400.x. [DOI] [PubMed] [Google Scholar]
- 18.Foissner I, Wendehenne D, Langebartels C, Durner J. In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. Plant J. 2000;23:817–824. doi: 10.1046/j.1365-313x.2000.00835.x. [DOI] [PubMed] [Google Scholar]
- 19.Krause M, Durner J. Harpin inactivates mitochondria in Arabidopsis suspension cells. Mol Plant-Microbe Interact. 2004;17:131–139. doi: 10.1094/MPMI.2004.17.2.131. [DOI] [PubMed] [Google Scholar]
- 20.Lamotte O, Gould K, Lecourieux D, Sequeira-Legrand A, Lebrun-Garcia A, Durner J, Pugin A, Wendehenne D. Analysis of nitric oxide signaling functions in tobacco cells challenged by the elicitor cryptogein. Plant Physiol. 2004;135:516–529. doi: 10.1104/pp.104.038968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto A, Katou S, Yoshioka H, Doke N, Kawakita K. Involvement of nitric oxide generation in hypersensitive cell death induced by elicitin in tobacco cell suspension culture. J Gen Plant Pathol. 2004;70:85–92. [Google Scholar]
- 22.Desikan R, Griffiths R, Hancock J, Neill S. A new role for an old enzyme: Nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc Natl Acad Sci. 2002;99:16314–16318. doi: 10.1073/pnas.252461999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.García-Mata C, Lamattina L. Nitric oxide and abscisic acid cross talk in guard cells. Plant Physiol. 2002;128:790–792. doi: 10.1104/pp.011020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neill S, Desikan R, Clarke A, Hancock JT. Nitric oxide is a novel component of abscisic acid signaling in stomatal guard cells. Plant Physiol. 2002;128:13–16. [PMC free article] [PubMed] [Google Scholar]
- 25.Guo FQ, Okamoto M, Crawford NM. Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science. 2003;302:100–104. doi: 10.1126/science.1086770. [DOI] [PubMed] [Google Scholar]
- 26.Gabaldón C, Gómez Ros LV, Pedreno MA, Ros Barceló A. Nitric oxide production by the differentiating xylem of Zinnia elegans. New Phytol. 2005;165:121–130. doi: 10.1111/j.1469-8137.2004.01230.x. [DOI] [PubMed] [Google Scholar]
- 27.Tun NN, Holk A, Scherer GFE. Rapid increase of nitric oxide (NO) release in plant cell cultures induced by cytokinin. FEBS Lett. 2001;509:174–176. doi: 10.1016/s0014-5793(01)03164-7. [DOI] [PubMed] [Google Scholar]
- 28.Carimi F, Zottini M, Costa A, Cattelan I, De Michele R, Terzi M, Lo Schiavo. NO signalling in cytokinin-induced programmed cell death. Plant Cell Env. 2005;28:11718. [Google Scholar]
- 29.Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: Diaminofluoresceins. Anal Chem. 1998;70:2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- 30.Espey MG, Miranda KM, Thomas DD, Wink DA. Distinction between nitrosating mechanisms within human cells and aqueous solution. J Biol Chem. 2001;276:30085–30091. doi: 10.1074/jbc.M101723200. [DOI] [PubMed] [Google Scholar]
- 31.Balcerczyk A, Soszynski M, Bartosz G. On the specificity of 4-amino-5-methylamino-2′,7′-difluorofluorescein as a probe for nitric oxide. Free Rad Biol Med. 2005;39:327–335. doi: 10.1016/j.freeradbiomed.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 32.Jourd'Heuil D. Increased nitric-oxide dependent nitrosylation of 4,5-diaminofluorescein by oxidants: Implications for the measurement of intracellular nitric oxide. Free Rad Biol Med. 2002;33:676–684. doi: 10.1016/s0891-5849(02)00955-3. [DOI] [PubMed] [Google Scholar]
- 33.Delledonne M, Zeier J, Marocco C, Lamb C. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA. 2001;98:13454–13459. doi: 10.1073/pnas.231178298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desikan R, Cheung MK, Bright J, Henson D, Hancock JT, Neill SJ. ABA, hydrogen peroxide and nitric oxide signalling in stomatal guards. J Exp Bot. 2004;55:205–212. doi: 10.1093/jxb/erh033. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Kim WS, Hatcher N, Potgieter K, Moroz LL, Gillette R, Sweedler JV. Interfering with nitric oxide measurements. 4,5-diaminofluorescein reacts with dehydroascorbic acid and ascorbic acid. J Biol Chem. 2002;277:48472–48478. doi: 10.1074/jbc.M209130200. [DOI] [PubMed] [Google Scholar]
- 36.Espey MG, Miranda KM, Thomas DD, Xavier S, Citrin D, Vitek MP, Wink DA. A chemical perspective on the interplay between NO, reactive oxygen species, and reactive nitrogen species. Ann NY Acad Sci. 2002;962:195–206. doi: 10.1111/j.1749-6632.2002.tb04068.x. [DOI] [PubMed] [Google Scholar]
- 37.Pfeiffer S, Leopold E, Hemmens B, Schmidt K, Werner ER, Mayer B. Interference of carboxy-PTIO with nitric oxide-and peroxynitrite-mediated reactions. Free Rad Biol Med. 1997;22:787–794. doi: 10.1016/s0891-5849(96)00407-8. [DOI] [PubMed] [Google Scholar]
- 38.Bethke PC, Libourel IGL, Reinöhl V, Jones RL. Sodium nitroprusside, cyanide, nitrite, and nitrate break Arabidopsis seed dormancy in a nitric oxide-dependent manner. Planta. 2005 doi: 10.1007/s00425-005-0116-9. In press. [DOI] [PubMed] [Google Scholar]
- 39.Mackerness SAH, John CF, Jordan B, Thomas B. Early signalling components in ultraviolet-B responses: Distinct roles for different reactive oxygen species and nitric oxide. FEBS Lett. 2001;489:237–242. doi: 10.1016/s0014-5793(01)02103-2. [DOI] [PubMed] [Google Scholar]
- 40.Lum HK, Butt YKC, Lo SCL. Hydrogen peroxide induces a rapid production of nitric oxide in Mung Bean (Phaseolus aureus) Nitric Oxide: Biol Chem. 2002;6:205–213. doi: 10.1006/niox.2001.0395. [DOI] [PubMed] [Google Scholar]
- 41.Harper JE. Evolution of nitrogen oxide(s) during in vivo nitrate reductase assay of soybean leaves. Plant Physiol. 1981;68:1488–1493. doi: 10.1104/pp.68.6.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dean JV, Harper JE. Nitric oxide and nitrous oxide production by soybean and winged bean during the in vivo nitrate reductase assay. Plant Physiol. 1986;82:718–723. doi: 10.1104/pp.82.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dean JV, Harper JE. The conversion of nitrite to nitrogen oxide(s) by the constitutive NAD(P)H-nitrate reductase enzyme from soybean. Plant Physiol. 1988;88:389–395. doi: 10.1104/pp.88.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rockel P, Strube F, Rockel A, Wildt J, Kaiser WM. Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J Exp Bot. 2002;53:103–110. [PubMed] [Google Scholar]
- 45.Planchet E, Gupta KJ, Sonoda M, Kaiser WM. Nitric oxide emission from tobacco leaves and cell suspensions: Rate limiting factors and evidence for the involvement of mitochondrial electron transport. Plant J. 2005;41:732–743. doi: 10.1111/j.1365-313X.2005.02335.x. [DOI] [PubMed] [Google Scholar]
- 46.Magalhaes JR, Silva FLIM, Salgado I, Ferrarese-Filho O, Rockel P, Kaiser WM. Nitric oxide and nitrate reductase in higher plants. Physiol Mol Biol Plants. 2002;8:11–17. [Google Scholar]
- 47.Sakihama Y, Nakamura S, Yamasaki H. Nitric oxide production mediated by nitrate reductase in the green alaga Chlamydomonas reinhardtii: An alternative NO production pathway in photosynthetic organisms. Plant Cell Physiol. 2002;43:290–297. doi: 10.1093/pcp/pcf034. [DOI] [PubMed] [Google Scholar]
- 48.Ruoff P, Lillo C. Molecular oxygen as electron acceptor in the NADH-nitrate reductase system. Biochem Biophys Res Com. 1990;172:1000–1005. doi: 10.1016/0006-291x(90)91545-4. [DOI] [PubMed] [Google Scholar]
- 49.Yamasaki H, Sakihama Y. Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: In vitro evidence for the NR-dependent formation of active nitrogen species. FEBS Lett. 2000;468:89–92. doi: 10.1016/s0014-5793(00)01203-5. [DOI] [PubMed] [Google Scholar]
- 50.Lillo C, Lea US, Leydecker MT, Meyer C. Mutation of the regulatory phosphorylation site of tobacco nitrate reductase results in constitutive activation of the enzyme in vivo and nitrite accumulation. Plant J. 2003;35:566–573. doi: 10.1046/j.1365-313x.2003.01828.x. [DOI] [PubMed] [Google Scholar]
- 51.Lea US, ten Hoopen F, Provan F, Kaiser WM, Meyer C, Lillo C. Mutation of the regulatory phosphorylation site of tobacco nitrate reductase results in high nitrite excretion and NO emission from leaf and root tissue. Planta. 2004;219:59–65. doi: 10.1007/s00425-004-1209-6. [DOI] [PubMed] [Google Scholar]
- 52.Stöhr C, Strube F, Marx G, Ullrich WR, Rockel P. A plasma membrane-bound enzyme of tobacco roots catalyses the formation of nitric oxide from nitrite. Planta. 2001;212:835–841. doi: 10.1007/s004250000447. [DOI] [PubMed] [Google Scholar]
- 53.Meyer C, Stöhr C. Soluble and plasma membrane-bound enzymes involved in nitrate and nitrite metabolism. In: Foyer CH, Noctor G, editors. Photosynthetic nitrogen assimilation and associated carbon and respiratory metabolism. Dordecht: The Nederlands: Kluwer Academic Publishers; 2003. pp. 49–62. [Google Scholar]
- 54.Gupta KJ, Stoimenova M, Kaiser WM. In higher plants, only root mitochondria, but not leaf mitochondria reduce nitrite to NO, in vitro and in situ. J Exp Bot. 2005;56:2601–2609. doi: 10.1093/jxb/eri252. [DOI] [PubMed] [Google Scholar]
- 55.Tischner R, Planchet E, Kaiser WM. Mitochondrial electron transport as a source for nitric oxide in the unicellular green alga Chlorella sorokiniana. FEBS Lett. 2004;576:151–155. doi: 10.1016/j.febslet.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Guo FQ, Crawford NM. Arabidopsis nitric oxide synthase1 is targeted to mitochondria and protects against oxidative damage and dark-induced senescence. Plant Cell. 2005;17:3436–3450. doi: 10.1105/tpc.105.037770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, Blake DR. Xanthine oxidoreductase catalyses the reduction of nitrate and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998;427:225–228. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- 58.Godber BLJ, Doel JJ, Sapkota GP, Blake DR, Stevens CR, Eisenthal R, Harrison R. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J. Biol Chem. 2000;275:7757–7763. doi: 10.1074/jbc.275.11.7757. [DOI] [PubMed] [Google Scholar]
- 59.Bethke PC, Badger MR, Jones RL. Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell. 2004;16:332–341. doi: 10.1105/tpc.017822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuo WN, Ku TW, Jones DL, Baptiste J. Nitric oxide synthase immunoreactivity in baker's yeasts, lobster and wheat germ. Biochem Arch. 1995;11:73–78. [Google Scholar]
- 61.Sen S, Cheema IR. Nitric oxide synthase and calmodulin immunoreactivity in plant embryonic tissue. Biochem Arch. 1995;11:221–227. [Google Scholar]
- 62.Barroso JB, Corpas FJ, Carreras A, Sandalio LM, Valderrama R, Palma JM, Lupianez JA, del Río LA. Localization of nitric-oxide synthase in plant peroxisomes. J Biol Chem. 1999;274:36729–36733. doi: 10.1074/jbc.274.51.36729. [DOI] [PubMed] [Google Scholar]
- 63.Ribiero EA, Cunha FQ, Tamashino WMSC, Martins IS. Growth phase-dependent subcellular localization of nitric oxide synthase in maize cells. FEBS Lett. 1999;445:283–286. doi: 10.1016/s0014-5793(99)00138-6. [DOI] [PubMed] [Google Scholar]
- 64.Lo DCL, Butt YKC, Chan YSG. False nitric oxide synthase immunoreactivity in Asparagus Bean (Vigna sesquipdalis) Nitric Oxide: Biol Chem. 2000;4:175. doi: 10.1006/niox.2000.0249. [DOI] [PubMed] [Google Scholar]
- 65.Butt YKC, Lum JHK, Lo DCL. Proteomic identification of plant proteins probed by mammalian nitric oxide synthase antibodies. Planta. 2003;216:762–771. doi: 10.1007/s00425-002-0926-y. [DOI] [PubMed] [Google Scholar]
- 66.He Y, Tang RH, Hao Y, Stevens RD, Cook CW, Ahn SM, Jing L, Yang Z, Chen L, Guo F, Fiorani F, Jackson RB, Crawford NM, Pei ZM. Nitric oxide represses the Arabidopsis floral transition. Science. 2004;305:1968–1971. doi: 10.1126/science.1098837. [DOI] [PubMed] [Google Scholar]
- 67.Zeidler D, Zähringer U, Gerber I, Bubery I, Hartung T, Bors W, Hutzler P, Durner J. Innate immunity in Arabidopsis thaliana: Lipopolysaccharides activate nitric oxide synthase (NOS) and induce defense genes. Proc Natl Acad Sci USA. 2004;101:15811–15816. doi: 10.1073/pnas.0404536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 69.Wink DA, Mitchell JB. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Rad Biol Med. 1998;25:434–456. doi: 10.1016/s0891-5849(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 70.Neill S, Desikan R, Bright J, Hancock J. Nitric oxide as a mediator of abscisic acid signalling in guard cells. In: Magalhaes J, Singh RP, Passos LP, editors. Nitric oxide signalling in higher plants. Focus on Plant Molecular Biology. 1. Houston: Studium Press; 2004. pp. 131–148. [Google Scholar]
- 71.Simontacchi M, Jasid S, Puntarulo S. Nitric oxide generation during early germination of sorghum seeds. Plant Sci. 2004;167:839–847. [Google Scholar]
- 72.Ohwaki Y, Kawagishi-Kobayashi M, Wakasa K, Fujihara S, Yonemama T. Induction of Class-1 nonsymbiotic hemoglobin genes by nitrate, nitrite and nitric oxide in cultured rice cells. Plant Cell Physiol. 2005;46:324–331. doi: 10.1093/pcp/pci030. [DOI] [PubMed] [Google Scholar]
- 73.Igamberdiev AU, Seregelyes C, Manac'h N, Hill RD. NADH-dependent metabolism of nitric oxide in alfalfa root cultures expressing barley hemoglobin. Planta. 2004;219:95–102. doi: 10.1007/s00425-003-1192-3. [DOI] [PubMed] [Google Scholar]
- 74.Perazzolli M, Dominici P, Romero-Puertas MC, Zago E, Zeier J, Sonoda M, Lamb C, Delledonne M. Arabidopsis nonsymbiotic hemoglobin Ahb1 modulates nitric oxide bioactivity. Plant Cell. 2004;16:2785–2794. doi: 10.1105/tpc.104.025379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seregelyes C, Igamberdiev AU, Maassen A, Hennig J, Dudits D. NO-degradation by alfalfa class 1 hemoglobin (Mhb1): A possible link to PR-1a gene expression in Mhb1-overproducing tobacco plants. FEBS Lett. 2004;571:61–66. doi: 10.1016/j.febslet.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 76.Igamberdiev AU, Hill RD. Nitrate, NO and haemoglobin in plant adaptation to hypoxia: An alternative to classic fermentation pathways. J Exp Bot. 2004;55:2473–2482. doi: 10.1093/jxb/erh272. [DOI] [PubMed] [Google Scholar]
- 77.Dordas C, Hasinoff BB, Igamberdiev AU, Manac'h N, Rivoal J, Hill RD. Expression of a stress-induced hemoglobin affects NO levels produced by alfalfa root cultures under hypoxic stress. Plant J. 2003;35:763–770. doi: 10.1046/j.1365-313x.2003.01846.x. [DOI] [PubMed] [Google Scholar]
- 78.Wildt J, Kley D, Rockel P, Rockel A, Segschneider HJ. Emission of NO from several higher plant species. J Geophys Res. 1997;102:5919–5927. [Google Scholar]