Abstract
The dynamic actin cytoskeleton has been proposed to be linked to gravity sensing in plants but the mechanistic understanding of these processes remains unknown. We have performed detailed pharmacological analyses of the role of the dynamic actin cytoskeleton in gravibending of maize (Zea mays) root apices. Depolymerization of actin filaments with two drugs having different mode of their actions, cytochalasin D and latrunculin B, stimulated root gravibending. By contrast, drug-induced stimulation of actin polymerization and inhibition of actin turnover, using two different agents phalloidin and jasplakinolide, compromised the root gravibending. Importantly, all these actin drugs inhibited root growth to similar extents suggesting that high actin turnover is essential for the gravity-related growth responses rather than for the general growth process. Both latrunculin B and cytochalasin D treatments inhibited root growth but restored gravibending of the decapped root apices, indicating that there is a strong potential for effective actin-mediated gravity sensing outside the cap. This elusive gravity sensing outside the root cap is dependent not only on the high rate of actin turnover but also on weakening of myosin activities, as general inhibition of myosin ATPases induced stimulation of gravibending of the decapped root apices. Collectively, these data provide evidence for the actin turnover-mediated gravity sensing outside the root cap.
Key Words: actin cytoskeleton, gravisensing, graviresponding, root cap
Introduction
Traditionally, the gravisensitivity of growing root apices is associated with their root caps which cover the extreme tips of root apices and protect them from mechanical damage due to soil particles.1,2 It is undisputable that root cap statocytes located in the center of root caps are critical for the high gravisensitivity of root apices. Ever since Nemec identified sedimenting starch-enriched statoliths located specifically within root cap statocytes,3 the starch-statolith theory has become the most influential concept to shape our ideas on this important topic of plant cell biology.2,4–7 Although it is obvious that statoliths are closely related to those elusive cytological processes which allow gravisensing in lower and higher plants,2,8–10 we are far from identifying the molecules and the processes in which they participate to accomplish the perception of gravity. We need unconventional concepts and new attitudes in order to overcome the current obstacles to the elucidation of these urgent questions of plant cell biology. One such novel concept was introduced by Andreas Sievers and colleagues who implicated the actin cytoskeleton in this mechano-perception and mechano-transduction puzzle.2,11,12 According to the original version of this concept, long actin filaments (AFs or F-actin) are providing direct structural links between surfaces of statocytes and the plasma membrane.
However, at variance with the original version of this concept,12 several studies13–17 have reported just the opposite response to what would be expected if long F-actin elements should interconnect statolith surfaces with the plasma membrane. Actually, growing root apices of maize, rice and cress, when treated with two anti-F-actin drugs, cytochalasin D and latrunculin B, having different modes of their actions,18 were shown to be unaffected or even stimulated in both sensing of gravity and performance of gravity oriented growth.13,14 Similarly, depolymerization of F-actin stimulated gravibending also in above-ground organs of Arabidopsis,15 indicating that this is a rather general phenomenon. These unexpected findings, confirmed also in the present study, leave us with a dilemma as to what the actin cytoskeleton actually does in root cap statocytes in relation to gravity sensing.
It is quite apparent that root cap statocytes are distinct from all other root cells due to the absence of prominent AFs and actin bundles which are ordinarily recognizable at the light microscope level.14,19–21 Typically, the root cap statocytes seem to be depleted of actin when compared with cells of the lateral root cap.14,19,20,22 However, the actin cytoskeleton of root cap statocytes is prone to massive polymerization, as shown by labeling methods which are based on cross-linking of existing AFs and phalloidin-induced polymerization of permeabilized living cells before their fixation.22,23 The unique organization of the actin cytoskeleton in root cap statocytes helps explain effective sedimentation of the amyloplasts (statoliths). This occurs not only on account of the mass of the statoliths but also because the statoliths are free of any relevant constraint due to the absence of a robust cytoskeleton which might otherwise control the positioning of such large organelles.19,20 In fact, such cytoskeleton-unrestrained20 mobility of statoliths, also known as sedimentation, can be further enhanced via disintegration of the actin cytoskeleton.8,24,25 Importantly, root cap statocytes represent an unique plant cell type as they lack ER elements deeper in their cytoplasm.22,25–28 Such ER elements are characteristic of all other plant cells and their mobility requires intact actin cytoskeleton and myosin-based forces (e.g., ref. 29). Nevertheless, data gained from in vivo observations show that tubular ER elements are mobile at the cell periphery of root cap statocytes.22 Moreover, sedimenting statoliths are not static organelles but perform continuous up-and-down movements along the gravity vector.30,31 All these observations suggest that the surfaces of statoliths and the peripheral cytoplasm are well-equipped with a cytoskeleton which supports a vigorous, but spatially restricted, motility.
The above findings suggest an attractive possibility that the high sensitivity of statocytes to gravity is based on rather weak but highly dynamic actin cytoskeleton assembled from short but interconnected AFs. Recently, we confirmed this unique actin status of root cap statocytes also in vivo.32. In contrast to all other plant cells, this unique actin cytoskeleton of the statocytes fails to secure actomyosin-dependent positioning of its large amyloplast-based statoliths, and these then are free to move and accumulate at the cellular bottom, in accordance with the gravity vector.19,31 If this is the case, then the immediate prediction is that drug-mediated stabilization of F-actin and inhibition of actin turnover in statocytes should specifically interfere with the gravibending of roots. In contrast, an additional weakening of the actin cytoskeleton integrity, using F-actin disintegrating drugs, should enhance the gravisensitivity of root apices. Our present data confirm these predictions: the stabilization of F-actin in cells of root caps blocks the root gravibending, whereas disintegration of F-actin and inhibition of myosin ATPases stimulate the gravibending of root apices. A surprising additional finding is that anti-actomyosin drugs almost restore a gravibending of root apices devoid of their root caps. These latter findings suggest that the root cap is not the sole site for the gravisensing in a root apex, and that gravity can be sensed at locations outside the root cap if the dynamic actin cytoskeleton is sufficiently weakened with actomyosin drugs.
Materials and Methods
Plant material and inhibitor treatments.
Caryopses of Zea mays L. cv. Gritz (Maïsadour semences, France) were soaked overnight in aerated tap water and placed between damp paper towels in Petri dishes. Dishes were maintained in the vertical position and incubated at 26°C for 48 h. Young seedlings with roots 5–7 cm long were selected. For pharmacological treatments, growing root portions were submerged in one of the following solutions: latrunculin-B (10 µM) for 2 h, cytochalasin D (10 µM) for 2 h, phalloidin (100 µM) for 1 h, jasplakinolide (100 µM and 500 µM) for 1 h, 2,3-butanedione monoxime (1 mM) for 4 h. In jasplakinolide and phalloidin treatments, only the root cap was treated. All experiments were performed at room temperature. Jasplakinolide was obtained from Molecular Probes (Eugene, OR) and latrunculin B from Calbiochem (La Jolla, CA). All the other chemicals were obtained from Sigma chemicals (St. Louis, MO).
Measurements of root growth and curvature kinetics.
To assess the effect of the various inhibitors on the root gravitropism, the time course and magnitude of the curvature were continuously monitored. Petri dishes containing the roots were mounted vertically and imaged using a horizontally mounted dissecting microscope (Leisegang, Germany). Roots were gravistimulated by rotating the Petri dishes 90°. Images of curving roots were captured at 10-min intervals for a total period of 8 h using a high resolution digital camera (Pixera 120ES, Los Gatos, CA). Roots that deviated more than 10° from the vertical prior to the 90° reorientation were ignored.
All curvature and growth measurements were analysed from the digitized images using image analysis software UTHSCSA Image Tool program, developed at the Texas University Health Science Center, San Antonio, TX (http://ddsdx.uthscsa.edu/dig/itdesc.html).
Decapping of root apices.
The root caps were removed from root apices using a laser microdissection system (Leica AS LMD, Germany). The contact area between the root cap and epidermis was gently scraped with a scalpel blade until the edge of the cap under the action of the laser beam began to separate from the root. The point of the blade was then used to accompany the cap and pull it from the root apex. This procedure did not significantly perturb root function (measured as rate of root elongation). The microdissection system was based on a nitrogen laser producing nanosecond pulses with a wavelength of 337.1 nm and a peak power of 75 kW.
Results
In order to probe the importance of dynamic actin cytoskeleton for both gravisensing and gravitropism of maize root apices, we have taken advantage of several well-characterized anti-actin drugs which either prevent actin turnover and stabilize F-actin (phalloidin and jasplakinolide), or disintegrate F-actin (cytochalasin D and latrunculin B).18 To decipher the elusive role of the actin cytoskeleton in root cap statocytes, we combined these treatments with experimental removal of root caps from maize root apices without affecting their viability and growth rates.33–35 The dynamic actin cytoskeleton and high actin turnover were shown to be absolutely essential for root gravibending as both phalloidin and jasplakinolide inhibited root gravicurvature. By contrast, both cytochalasin D and latrunculin B failed to inhibit the gravibending, even though these drugs significantly diminished root growth. In fact, these actin drugs significantly promoted root gravicurvatures (Table 1).
Table 1.
Growth rates, final angles, and bending rates of intact, decapped, and drug-treated maize root apices during 8 h after their treatments
| Treatments | Roots | Growth Rate (mm h−1) | Final Angle (degrees) |
| No treatment | Intact | 1.73 ± 0.31 | 70.15 ± 3.60 |
| Decapped | 1.69 ± 0.36 | 24.22 ± 3.40 | |
| JAS | Intact | 1.05 ± 0.28 | 17.82 ± 1.95 |
| (10 µM for 1 h) | |||
| JAS | Intact | 0.85 ± 0.09 | 12.50 ± 1.40l |
| (50 µM for 1 h) | Decapped | 0.36 ± 0.18 | 2.02 ± 3.51 |
| CYT-D | Intact | 0.92 ± 0.06 | 66.21 ± 3.44 |
| (10 µM for 2 h) | Decapped | 0.39 ± 0.07 | 38.52 ± 3.86 |
| LAT-B | Intact | 1.22 ± 0.38 | 69.52 ± 3.39 |
| (10 µM for 2 h) | Decapped | 0.31 ± 0.08 | 52.62 ± 3.66 |
| BDM | Intact | 0.88 ± 0.07 | 69.78 ± 2.63 |
| (10−3 M for 4 h) | Decapped | 0.38 ± 0.05 | 48.42 ± 3.21 |
Values are means ± SE, n = 15.
Inhibition of actin turnover and stabilization of F-actin inhibits gravitropism, but does not stop root growth.
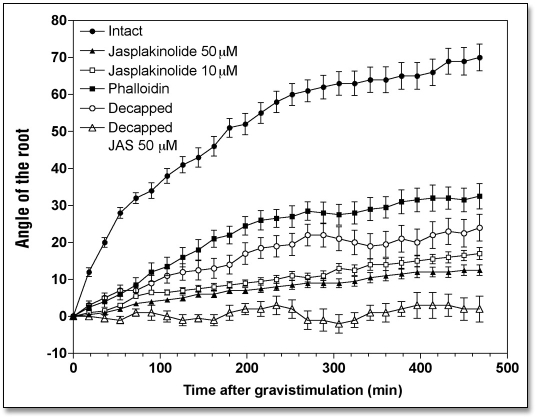
Inhibition of actin turnover and stabilization of F-actin in root cap statocytes due to the exposure of the caps to phalloidin (100 µM for 1 h) considerably inhibited gravitropism of maize root apices (Fig. 1). The angle attained by the root apex after 8 h of treatment was about 30° compared with about 70° reached by control root apices. Phalloidin treatment also inhibited root growth, the control rate of 1.73 mm h−1 falling to 1.10 mm h−1 (Table 1).
Figure 1.
Effects of jasplakinolide (10 and 50 µM for 1 h) and phalloidin (100 µM for 1 h) treatments on the kinetics of the gravibending of intact and decapped maize roots. Values are means ± SE, n = 15.
However, phalloidin has notoriously poor membrane permeability.36,37 To circumvent this feature of phalloidin, we have taken advantage of another F-actin stabilizing drug, jasplakinolide (JP), which is membrane-permeable and effective in inhibition of actin turnover and in stabilization of F-actin in all cell types tested so far, including plant cells.38–40 JP was also applied directly to root caps in order to avoid too much interference with those root growth processes which are dependent on a dynamic actin cytoskeleton.41 Even this selective exposure of root caps to 10 and 50 µM JP (for 1 h) affected the root growth rate substantially during the subsequent 8 h when the control rate of 1.73 mm h−1 decreased to 0.85 mm h−1 (Table 1). This finding indicates that JP, which readily enters plant cells, had an inhibitory effect on cells of the root proper, even though it was applied only to the caps. The JP-treated roots failed to produce any significant gravibending (< 10°) during the 8 h period following JP treatment (Fig. 1), even though the root apices grew an additional 5 mm. This clearly suggests that JP compromises specifically those processes, which are responsible either for gravity sensing or for gravitropism (Table 1).
Disintegration of F-actin slows root growth, but stimulates root gravitropism.
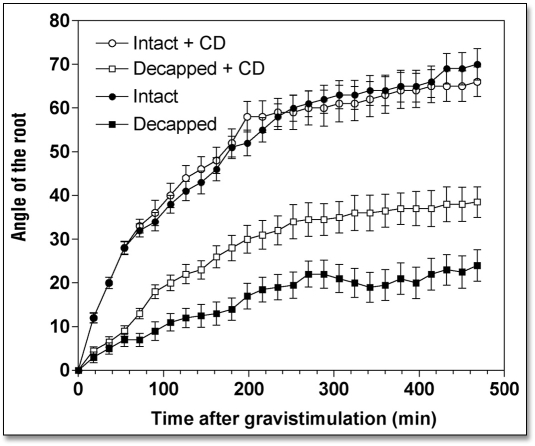
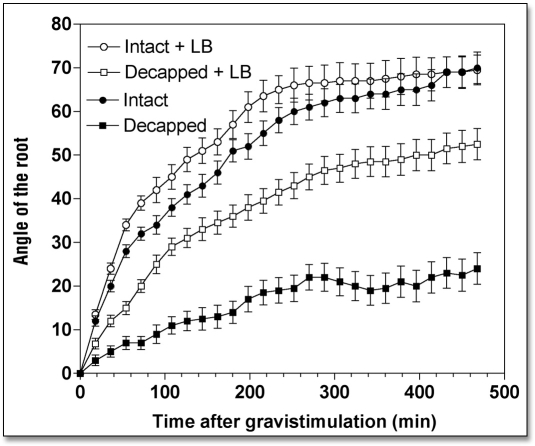
When roots were treated with either cytochalasin D (CD) or latrunculin B (LB), drugs that disintegrate F-actin, root growth rate slowed down considerably while root gravibending was significantly stimulated (Table 1). Specifically, root apices exposed to 10 µM CD (2 h) had a growth rate of 0.92 mm h−1 during the next 8 h, compared to 1.73 mm h−1 of control root apices (Table 1). Similarly, the growth rate of LB-treated (10 µM for 2 h) root apices decreased to 1.22 mm h−1 (Table 1). Despite these significantly decreased root growth rates (by about 45%), gravitropism of root apices either devoid (LB) or depleted (CD) of F-actin was either unaffected (CD) or even slightly stimulated (LB), as shown in Figures 2 and 3. In fact, this means that both CD and LB treatments actually stimulated gravity sensing as evidenced by calculating the rate bending angle in relation to the root growth rate (Table 1).
Figure 2.
Effects of cytochalasin D (10 µM for 2 h) on the kinetics of gravibendings of intact and decapped maize roots. Values are means ± SE, n = 15.
Figure 3.
Effects of Latrunculin B (10 µM for 2 h) on the kinetics of gravibendings of intact and decapped maize roots. Values are means ± SE, n = 15.
Inhibition of myosin motor activities inhibits root growth but stimulates gravitropism.
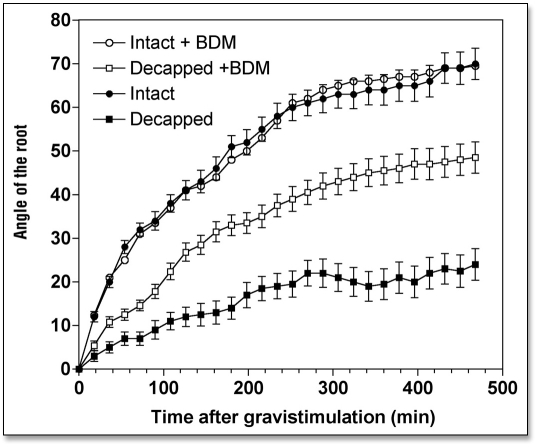
At the concentration used in the present study, 3,4-butanedione monoxime (BDM), is accepted as specific and effective inhibitor of all myosin ATPases42–43. BDM has proved to be effective in plant cells44–47 and hence was used to evaluate the possible importance of myosin-based activities on gravitropism. Exposure of roots to 1 mM BDM for 4 h was found to diminish root growth to 0.88 mm h−1 (Table 1). The gravistimulated root apices attained normal curvatures of approximately 70° during the test period (Fig. 4). Thus, in terms of the angle bent per mm of elongation, BDM treatment stimulated gravitropism by about 50% (Table 1).
Figure 4.
Effects of 2,3-butanedione monoxime (BDM) (1 mM for 4 h) on the kinetics of gravibendings of intact and decapped maize roots. Values are means ± SE, n = 15.
Root apices devoid of root caps grow normally but show only residual gravitropism.
Maize roots are a very useful experimental system owing to the ease with which their root caps can be removed, thereby abolishing root gravitropism without compromising the viability and high growth rates of the root apices.33–35 In our experimental situation, the high growth rate of intact control roots was also maintained by decapped root apices. During a post-operative period of 8 h, decapped root apices grew at 1.69 mm h−1, which is very close to the control root growth rate of 1.73 mm h−1 (Table 1). Despite this high root growth rate, decapped root apices showed little gravitropism. Decapped roots bent a maximum of about 20°, whereas control root apices attained about 70° of root curvature (Fig. 1). Nevertheless, the fact that the decapped root apices show some residual bending strongly suggests that gravity sensing is not restricted to root cap statocytes.
Depolymerization of F-actin and inhibition of myosin-based forces strongly inhibit root growth rates but almost restore a normal gravitropic response to root apices devoid of their root caps.
We tested whether disintegration of F-actin and inhibition of myosin ATPases had an impact on the weak gravitropism of decapped root apices. Our data clearly show that both disintegration of F-actin (Figs. 2 and 3) and inhibition of myosin-based activities (Fig. 4) induced a substantial recovery of gravibending of the decapped root apices. CD increases the curvature of the decapped root apices from about 20° to about 35°, LB from about 20° to about 50°, and BDM raises it from about 20° to about 50°, also.
This substantial restoration of root gravibending occurs despite the clearly negative effects of decapping and drug treatments on root growth rates—due to the more effective penetration of the drugs into decapped root apices (Table 1). In the face of the poor growth rates of such double-treated root apices, their gravity sensing can be predicted to be in fact stimulated when compared with the rapidly growing control root apices (Table 1). Of course, this prediction is in need of experimental tests, which surely will be performed, in future studies.
Discussion
The present data convincingly show that a highly dynamic actin cytoskeleton is essential for the sensing of gravity in root cap statocytes and elsewhere in the root, as well as for the gravitropic response of maize root apices. Firstly, effective inhibition of actin turnover and stabilization of F-actin specifically within root cap cells by either phalloidin or jasplakinolide inhibited and blocked, respectively, gravitropism of root apices. Secondly, decapped root apices treated with two F-actin depolymerizing drugs, cytochalasin D and latrunculin B, each having different mechanisms of action,36,37 recovered their gravitropism. Similarly, inhibition of myosin ATPases activities with 3,4-butanedione monoxime42–47 restored a gravitropic response to decapped roots. The results indicate that actin turnover-regulated gravity-sensing mechanisms are not limited to root cap statocytes but are operative in sites elsewhere in the root.
Gravisensing in root cap statocytes is dependent on high actin turnover and low myosin activity.
A few years ago, we proposed that root apices, besides possessing a “restrained” gravisensing mechanism based on F-actin-mediated tethering of organelles to the plasma membrane, might also possess an “unrestrained” gravity sensing which becomes operative in situations where weakening of actin-based cytoskeletal linkages allows gravity-induced sedimentation of large organelles along the gravity vector.19 This concept was suggested by the unique structural and cytoskeletal organization of root cap statocytes which lack both ER elements22,25–28 and prominent cytoskeletal elements20,21 deep in the cytoplasm. In accordance with the major prediction of the concept of “unrestrained” gravity sensing, disintegration of F-actin was reported to stimulate sedimentation of statoliths in root cap statocytes.11,24,25 A further prediction is that anti-actin drugs should stimulate gravity sensing in root apices. Exactly this has been reported by several groups.13–17 Our present data confirm this phenomenon using two anti-actin drugs, cytochalasin D and latrunculin B, which disintegrate F-actin via different mechanisms.36,37 Importantly, we have also shown that inhibition of actin turnover and stabilization of F-actin within root cap statocytes inhibit or even prevent gravitropism of root apices. Interestingly, both the F-actin-disintegrating and the F-actin-stabilizing drugs affected the root growth rate similarly. These last mentioned findings strongly suggest that these two classes of actin drugs affect the gravibending due to their specific action on a still elusive gravity-sensing process.
A similar conclusion was reached for Arabidopsis hypocotyls where latrunculin B strongly stimulated gravitropism but not phototropism.15 The latter authors concluded that latrunculin B affects early phases (perception and/or transduction) of gravitropism because both gravitropism and phototropism are driven by the same differential growth process. In fact, latrunculin B inhibits growth of roots, hypocotyls, and stems both in maize (this study and ref. 41) and Arabidopsis.15 Considering the inhibited root growth but stimulated root bending, latrunculin B appears to stimulate the elusive gravity sensing. This is at variance with the conclusion taken by Hou et al.17 that F-actin is not related to gravity sensing but rather it is important for downregulation of the gravitropism.
What factors are responsible for the unique status of the actin cytoskeleton in the root cap statocytes? First, one might predict a specific set of actin-binding proteins which would continuously shift the balance between actin polymerization and depolymerization in favor of the latter process. Important factors in these two processes are pH and cytoplasmic calcium. High pH values and increased levels of cytoplasmic calcium are known to stimulate the dynamics of F-actin via actions on profilin, actin depolymerizing factor and villin.48 For instance, the ability of maize profilins to sequester G-actin and thereby further diminish F-actin levels, was reported to be positively correlated with the level of cytoplasmic calcium.49,50 In accordance with this expectations, root cap statocytes are known to contain unusually high levels of cytoplasmic calcium and calmodulin.51,52 Moreover, cytosolic pH increases in root cap statocytes shortly after gravistimulation,53,54 but before any detectable root curvature. The pH increase was necessary for root gravitropism17,54,55 and might contribute to an increased activity of actin depolymerizing factor56 and to higher actin turnover rates allowing more effective gravity sensing.
Despite all the recent progress which clearly highlights a dynamic actin cytoskeleton as a crucial factor in the sensing of gravity in root cap statocytes, there is still a perplexing puzzle that needs urgent solution. How does a high rate of actin turnover allow sensing of gravity? What does the dynamic actin actually do? Magnetophoretic experiments strongly support the essential role of amyloplast-based statoliths for gravity sensing.57,58 In addition, amyloplasts have recently been shown to be susceptors of mechanical vibration.59 All available data seem to converge towards a model viewing the interface between amyloplast/statolith surfaces and adjacent cytoplasmic space as an extremely dynamic environment19,25 where a gravity-mediated mechanical stimulus is transformed into a biological response.60,61 Our present data identifies the high turnover rate of actin as an essential feature for effective gravity sensing. Interestingly in this respect, both cytochalasin D and latrunculin B not only stimulate gravity sensing but also, as already mentioned above, promote sedimentation rates of root cap statoliths.11,24,25
Even in the absence of long actin filaments, the surfaces of statoliths can be expected to be linked to relevant membranes via a dense meshwork of dynamic actin filaments. One should be aware that actin filaments do not need to be long structures—already three G-actin monomers assembled together constitute a unit of F-actin.62 Moreover, these short F-actin units, composed of only few G-actin molecules, can be expected to have an ephemeral life-span and to be organized in all directions in the form of a dense meshwork. A dynamic actin meshwork, composed of ephemeral AFs of minimal length would be mostly below the resolution of the light microscope, and might be expected to resist the full disintegration in response to agents such as latrunculin B and cytochalasin D. Obviously, this dynamic meshwork cannot immobilize and/or move statoliths with their large mass. Statoliths then follow a simple physical principle: they sediment along the gravity vector. Such gravity-accelerated movements of statoliths could have a tremendous mechanical impact on the surrounding actin meshwork. It might well be that dynamic interactions between unstable F-actin elements and sedimenting statoliths is the most important event in the sensing of gravity in plant cells. In fact, selective laser-assisted ablations of root cap cells in Arabidopsis roots revealed that the statocytes which contribute most to root gravitropism have the highest statolith sedimentation rates.63
All the F-actin elements of the dynamic meshwork are interconnected and provide a structural continuum between the statolith surfaces, ER, and the plasma membrane (for ER see ref. 28). One can, therefore, expect that statolith movements which disturb the F-actin meshwork, will have immediate impacts on cytoarchitecture and cell physiology. Such a dynamic meshwork, because it is inherently unstable and interconnected to other cytoskeletal assemblages and organelles, represents an ideal structure for the effective transmission of mechanical signals.19,64–66 The dynamic actin cytoskeleton, closely linked to signalling (for reviews on plants see refs. 48 and 61), can therefore be regarded as both gravisusceptor and gravitransducer.48,61 However, the molecular basis for the processes that gather information regarding the direction of the gravity vector remains to be explored.
The sedimenting statoliths are not fully dissociated from cytoskeletal structures because they perform dynamic movements30,31 which indicates that the system is inherently unstable due to the tendency of the actomyosin-based forces to regain control over the positioning of the statoliths. Normally this is impossible due to the unique cytoloplasmic microenvironment around the statoliths. Our data show that the effectiveness of this unique gravity sensing system can be further enhanced by drugs that disintegrate F-actin and inhibit myosin-based forces. In addition to the increased sedimentation rate of statoliths and gravitropic sensitivity induced by F-actin disintegrating drugs,11,24,25 exposure of plant cells to hypergravity67 encourages additional gravibendings beyond that normally seen at 1g. This would indicate that the system does not usually work at full capacity, and that some potential capacity for gravisensing is held in reserve.
Intuitively, if we accept the above concept that sedimentation of statoliths is permitted only when the F-actin network is ineffective in trapping these organelles, then inhibition of myosin activities should not interfere with gravibending of roots. In fact, less effective myosins might facilitate the release of organelles from any interactions with F-actin, and these would then be free to perform the additional “unrestrained” gravity sensing over and above sensing normally prescribed for statocytes at 1g. Our data strongly support such notion. Root gravitropism was promoted following inhibition of myosin motor activities by exposure of maize root apices to 2,3-butanedione monoxime, a general inhibitor of myosin ATPases (e.g., refs. 42 and 43; for plants see refs. 44, 45 and 47).
Gravisensing outside of the root cap also requires high actin turnover and low myosin activities.
It is easy to remove the caps from maize root tips and such decapped root apices represent an excellent model object to study conditions, which allow gravisensing and gravibending. Although active in growth, decapped maize roots fail to display full gravitropism.33–35 Nevertheless, they show residual gravibending of about 20° (this study). These data agree with other findings, which suggest that gravisensing can occur in regions of the root other than the cap5,68–70 (for reviews see refs. 71–73). Up to 20% of total gravity perception capacity is realized in the transition zone, also known as distal elongation zone, of maize root apices.70,71,73 Interestingly, roots of starch-less Arabidopsis mutants, which lack sedimenting amyloplast statoliths in their statocytes, show similar residual root curvatures of about 10–20° (e.g., refs. 67 and 74–76). Experimental laser-assisted deletion of statocytes in Arabidopsis root caps also revealed a similar residual root gravitropism.63 All these data can be explained with multiple systems for gravisensing in plants.1
Though still elusive, gravity sensing must be operating outside of the root cap to allow a weak gravibending in circumstances when the major gravireceptor is defective. If this “back-up” gravity sensing system were also dependent on high rates of actin turnover, as is the case in the cap statocytes, then a stronger gravibending from decapped root apices should be obtained after their treatment with anti-actin drugs. Exactly this was the case when decapped root apices treated with cytochalasin D, latrunculin B, and 2,3-butanedione monoxime were gravistimulated: their root bending was almost restored to control values.
What about auxin?
Our data pose new questions also to scientists studying the role of auxin in root gravibending. As the decapped roots are able to respond to gravistimulation, it means that auxin transported transcellularly within the root apex, not derived from root caps, is able to accomplish asymmetric redistribution. Polar auxin transport is well accepted to be essential for gravibending of plant organs such as roots hypocotyls and shoots.77 Moreover, the polar transport of auxin is sensitive to F-actin drugs such as cytochalasins and latrunculins.78–80 As classical inhibitors of auxin transport, such as NPA and TIBA, turned-out to be general inhibitors of endocytosis;81 one could expect that F-actin drugs which also inhibits endocytosis will also block auxin transport. But F-actin drugs inhibit auxin transport only partially,79 suggesting that depolymerization of F-actin affects positively an alternative route for transport of mobile auxin molecules. This scenario was confirmed also using DR5:GUS reporter line.17 One attractive possibility is that this other route might be the direct transport of auxin across plasmodesmata. In fact, gravistimulation was shown to selectively open plasmodesmata of peripheral root cap statocytes in Arabidopsis root apices.82
It might be that F-actin-based recycling endosomes and vesicles locally ‘fish-out’ all auxin molecules from the cytoplasm near plasmodesmata orifices, thus preventing their free diffusion through plasmodesmata. As the depolymerization of F-actin is known to open plasmodesmata (reviewed in ref. 82), it can be expected that the loss of intact F-actin will also impair the active ‘vacuum-cleaning’ of free cytoplasmic auxin near plasmodesmata via F-actin based recycling vesicles and endosomes. Cytoplasmic auxin will then be free to traverse plasmodesmata rapidly across the root diameter and to accumulate at the physical underside of gravistimulated roots to locally inhibit the cell growth, resulting in root gravibending.83,84 In this speculative concept, the axial transport of auxin is accomplished via vesicular secretion,85–87 while the lateral auxin transport across the root diameter might rely not only on the vesicular into ‘secretion but also on putative auxin-transporting plasmodesmata.88
Although our data obtained from robust roots of maize, in which the cortex cells are the most relevant one with respect to root bending,83 a similar scenario emerges also for much smaller roots of Arabidopsis in which there is only one layer of cortex cells and in which epidermis cells seems to be the major controller of root growth.84 The latter authors showed that root gravitropism in Arabidopsis requires AUX1-driven auxin transport in postmitotic epidermis cells but not in the root cap (see Fig. 1D and E in ref. 84).
Outlook.
Our results indicate that high rates of actin turnover and weak integrity of the actin cytoskeleton are intimately linked to processes which accomplish gravity sensing in both root cap statocytes as well as in cells of the root body. The immediate prediction is that depolymerization of the actin cytoskeleton allows not only statoliths but also lighter organelles to sediment and thus to act as a gravisensing structures. This indicate that high turnover of actin might be essential for multiple systems of gravity sensing in plants.1 We predict that cellular factors which regulate these extraordinarily rapid assembly and disassembly processes of the dynamic meshwork of actin filaments will turn out to be important in gravity sensing of plant cells. Possible regulators of these sensing processes may be profilin and actin depolymerizing factor, both of which are well-known for their driving of high rates of actin turnover89 and for their inherent linking of the dynamic actin cytoskeleton to diverse signaling pathways and networks (for plant cells see refs. 48 and 61). Interestingly in this respect, actin depolymerization transduces the strength of B-cell receptor stimulation in animal cells,90 resembling the situation with the transduction of gravity signals in root apices. Our future studies should focus on the dynamic actin cytoskeleton as this seems to hold the key for understanding the still elusive sensing and transduction of the gravity signal in higher plants.
Acknowledgements
The authors' research is financially supported by the Deutsches Zentrum für Luft- und Raumfahrt (DLR Köln/Bonn, Germany, Projects 50 WB 9995 and 50 WB 0434), the European Space Agency (ESA/ESTEC, MAP Project AO-99-098) and from the Ente Cassa di Risparmio di Firenze (Italy). F.B. receives partial support from the Slovak Academy of Sciences, Grant Agency VEGA (grant No. 2/5085/25), Bratislava, Slovakia.
Footnotes
Previously published onlinse as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=2432
References
- 1.Barlow PW. Gravity perception in plants: A multiplicity of systems defived by evolution? Plant Cell Environm. 1995;18:951–962. doi: 10.1111/j.1365-3040.1995.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 2.Sievers A, Braun M, Monshausen GB. The root cap: Structure and function. In: Waisel Y, Eshel A, Kafkafi U, editors. Plant Roots: The Hidden Half. 3rd ed. Basel, New York: Marcel Dekker; 2002. pp. 33–47. [Google Scholar]
- 3.Nemec B. Über die Art der Wahrnehmung des Schwerkraftes bei der Pflanzen. Ber Deutsch Bot Ges. 1900;18:241–245. (Ger). [Google Scholar]
- 4.Darwin F. The statolith theory of geotropism. Nature. 1903;67:571–572. [Google Scholar]
- 5.Haberlandt G. Über die Verteilung der geotropischen Sensibilität in der Wurzel. Jahrb Wiss Bot. 1908;45:575–600. (Ger). [Google Scholar]
- 6.Sack FD. Plastids and gravitropic sensing. Planta. 1997;203:S63–S68. doi: 10.1007/pl00008116. [DOI] [PubMed] [Google Scholar]
- 7.Kiss JZ. Mechanisms of the early phases of plant gravitropism. Crit Rev Plant Sci. 2000;19:551–573. doi: 10.1080/07352680091139295. [DOI] [PubMed] [Google Scholar]
- 8.Sievers A, Volkmann D. Gravitropism in single cells. In: Haupt W, Feinleib ME, editors. Encyclopedia of Plant Physiology. Berlin: Springer-Verlag; 1979. pp. 567–572. [Google Scholar]
- 9.Volkmann D, Sievers A. Graviperception in multicellular organs. In: Haupt W, Feinleib ME, editors. Encyclopedia of Plant Physiology. Berlin: Springer-Verlag; 1979. pp. 573–600. [Google Scholar]
- 10.Sievers A, Buchen B, Hodick D. Gravity sensing in tip-growing cells. Trends Plant Sci. 1996;1:273–279. doi: 10.1016/1360-1385(96)10028-5. [DOI] [PubMed] [Google Scholar]
- 11.Sievers A, Kruse S, Kuo-Huang LL, Wendt M. Statoliths and microfilaments in plant cells. Planta. 1989;179:275–278. doi: 10.1007/BF00393699. [DOI] [PubMed] [Google Scholar]
- 12.Sievers A, Buchen B, Volkmann D, Hejnowicz Z. Role of the cytoskeleton in gravity perception. In: Lloyd CW, editor. The Cytoskeletal Basis of Plant Growth and Form. London: Academic Press; 1991. pp. 169–182. [Google Scholar]
- 13.Staves MP, Wayne R, Leopold AC. Cytochalasin D does not inhibit gravitropism in roots. Amer J Bot. 1997;84:1530–1535. [PubMed] [Google Scholar]
- 14.Blancaflor EB, Hasenstein KH. The organization of the actin cytoskeleton in vertical and graviresponding primary roots of maize. Plant Physiol. 1997;113:1447–1455. doi: 10.1104/pp.113.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto K, Kiss JZ. Disruption of the actin cytoskeleton results in the promotion of gravitropism in inflorescence stems and hypocotyls of Arabidopsis. Plant Physiol. 2002;128:669–681. doi: 10.1104/pp.010804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou G, Mohamalawari DR, Blancaflor EB. Enhanced gravitropism of roots with a disrupted cap actin cytoskeleton. Plant Physiol. 2003;113:1360–1373. doi: 10.1104/pp.014423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou G, Kramer VL, Wang YS, Chen R, Perbal G, Gilroy S, Blancaflor EB. The promotion of gravitropism in Arabidopsis roots upon actin disruption is coupled with the extended alkalinization of the columella cytoplasm and a persistent lateral auxin gradient. Plant J. 2004;39:113–125. doi: 10.1111/j.1365-313X.2004.02114.x. [DOI] [PubMed] [Google Scholar]
- 18.Spector I, Braet F, Schochet NR, Bubb MR. New anti-actin drugs in the study of the organization and function of the actin cytoskeleton. Microsc Res Tech. 1999;47:18–37. doi: 10.1002/(SICI)1097-0029(19991001)47:1<18::AID-JEMT3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 19.Baluska F, Hasenstein KH. Root cytoskeleton: Its role in perception of and response to gravity. Planta. 1997;203:S69–S78. doi: 10.1007/pl00008117. [DOI] [PubMed] [Google Scholar]
- 20.Baluska F, Kreibaum A, Vitha S, Parker JS, Barlow PW, Sievers A. Central root cap cells are depleted of endoplasmic microtubules and actin microfilament bundles: Implications for their role as gravity-sensing statocytes. Protoplasma. 1997;196:212–223. doi: 10.1007/BF01279569. [DOI] [PubMed] [Google Scholar]
- 21.Driss-Ecole D, Vassy J, Rembur J, Guivarc'h A, Prouteau M, Dewitte W, Perbal G. Immunolocalization of actin in root cap statocytes of Lens culinaris L. J Exp Bot. 2000;51:521–528. doi: 10.1093/jexbot/51.344.521. [DOI] [PubMed] [Google Scholar]
- 22.Collings DA, Zsuppan G, Allen NS, Blancaflor EB. Demonstration of prominent actin filaments in the root columella. Planta. 2001;212:392–403. doi: 10.1007/s004250000406. [DOI] [PubMed] [Google Scholar]
- 23.White RG, Sack FD. Actin microfilaments in presumptive statocytes of root caps and coleoptiles. Amer J Bot. 1990;77:17–26. [PubMed] [Google Scholar]
- 24.Hensel W. Cytochalasin B affects the structural polarity of statocytes from cress roots (Lepidium sativum L) Protoplasma. 1985;129:178–187. doi: 10.1007/BF01279915. [DOI] [PubMed] [Google Scholar]
- 25.Yoder TL, Zheng HQ, Todd P, Staehelin LA. Amyloplast sedimentation dynamics in maize columella cells support a new model for the gravity-sensing apparatus of roots. Plant Physiol. 2001;125:1045–1060. doi: 10.1104/pp.125.2.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sievers A, Volkmann D. Verursacht differentieler Druck der Amyloplasten auf ein komplexes Endomembransystem die Geoperzeption in Wurzeln? Planta. 1972;102:160–172. doi: 10.1007/BF00384870. (Ger). [DOI] [PubMed] [Google Scholar]
- 27.Barlow PW, Hawes CR, Horne JC. Structure of amyloplasts and endoplasmic reticulum in the root caps of Lepidium sativum and Zea mays observed after selective membrane staining and by high-voltage electron microscopy. Planta. 1984;160:363–371. doi: 10.1007/BF00393418. [DOI] [PubMed] [Google Scholar]
- 28.Zheng HQ, Staehelin LA. Nodal endoplasmic reticulum, a specialized form of endoplasmic reticulum found in gravity-sensing root tip columella cells. Plant Physiol. 2001;125:252–265. doi: 10.1104/pp.125.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liebe S, Menzel D. Actomyosin-based motility of endoplasmic reticulum and chloroplasts in Vallisneria mesophyll cells. Biol Cell. 1995;85:207–222. doi: 10.1016/0248-4900(96)85282-8. [DOI] [PubMed] [Google Scholar]
- 30.Sack FD, Suyemoto MM, Leopold AC. Amyloplast sedimentation and organelle saltation in living corn columella cells. Amer J Bot. 1986;12:1692–1698. [PubMed] [Google Scholar]
- 31.Volkmann D, Baluska F, Lichtscheidl I, Driss-Ecole D, Perbal G. Statoliths motion in gravity-perceiving plant cells: Does actomyosin counteract gravity? FASEB J. 1999;13:S143–S147. doi: 10.1096/fasebj.13.9001.s143. [DOI] [PubMed] [Google Scholar]
- 32.Voigt B, Timmers ACJ, Samaj J, Müller J, Baluska F, Menzel D. GFP-FABD2 fusion construct allows in vivo visualization of the dynamic actin cytoskeleton in all cells of Arabidopsis seedlings. Eur J Cell Biol. 2005;84:595–608. doi: 10.1016/j.ejcb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Barlow PW. Recovery of geotropism after removal of the root cap. J Exp Bot. 1974;25:1137–1146. [Google Scholar]
- 34.Pilet PE. Root cap and georeaction. Nat New Biol. 1971;233:115–116. [Google Scholar]
- 35.Juniper BE, Groves S, Landau-Schachar B, Audus LJ. Root cap and the perception of gravity. Nature. 1966;209:93–94. [Google Scholar]
- 36.Cooper JA. Effects of cytochalasins and phalloidin on actin. J Cell Biol. 1987;105:1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sampath P, Pollard TD. Effects of cytochalasin, phalloidin, and pH on the elongation of actin filaments. Biochemistry. 1991;30:1973–1980. doi: 10.1021/bi00221a034. [DOI] [PubMed] [Google Scholar]
- 38.Bubb MR, Spector I, Beyer BB, Fosen KM. Effects of jasplakinolide on the kinetics of actin polymerization. An explanation for certain in vivo observations. J Biol Chem. 2000;275:5163–5170. doi: 10.1074/jbc.275.7.5163. [DOI] [PubMed] [Google Scholar]
- 39.Holzinger A. Jasplakinolide: An actin-specific reagent that promotes actin polymerization. Meth Mol Biol. 2001;161:109–120. doi: 10.1385/1-59259-051-9:109. [DOI] [PubMed] [Google Scholar]
- 40.Gallo G, Yee HF, Letourneau PC. Actin turnover is required to prevent axon retraction driven by endogenous actomyosin contractility. J Cell Biol. 2002;158:1219–1228. doi: 10.1083/jcb.200204140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baluska F, Jasik J, Edelmann HG, Salajová T, Volkmann D. Latrunculin B induced plant dwarfism: Plant cell elongation is F-actin dependent. Dev Biol. 2001;231:113–129. doi: 10.1006/dbio.2000.0115. [DOI] [PubMed] [Google Scholar]
- 42.Cramer LP, Mitchison TJ. Myosin is involved in postmitotic cell spreading. J Cell Biol. 1995;131:179–189. doi: 10.1083/jcb.131.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herrmann C, Wray J, Travers F, Barman T. Effects of 2,3-butanedione monoxime on myosin and myofibrillar ATPases. An example of an uncompetitive inhibitor. Biochemistry. 1992;31:12227–12232. doi: 10.1021/bi00163a036. [DOI] [PubMed] [Google Scholar]
- 44.Tominaga M, Yokota E, Sonobe S, Shimmen T. Mechanism of inhibition of cytoplasmic streaming by a myosin inhibitor, 2,3-butanediome monoxime. Protoplasma. 2000;213:46–54. [Google Scholar]
- 45.Samaj J, Peters M, Volkmann D, Baluska F. Effects of myosin ATPase inhibitor 2,3-butane-dione 2-monoxime on distribution of myosins, F-actin, microtubules, and cortical endo-plasmic reticulum in maize root apices. Plant Cell Physiol. 2000;41:571–582. doi: 10.1093/pcp/41.5.571. [DOI] [PubMed] [Google Scholar]
- 46.Funaki K, Nagata A, Akimoto Y, Shimada K, Ito K, Yamamoto K. The motility of Chara corallina myosin was inhibited reversibly by 2,3-butanedione monoxime (BDM) Plant Cell Physiol. 2004;45:1342–1345. doi: 10.1093/pcp/pch154. [DOI] [PubMed] [Google Scholar]
- 47.Molchan TM, Valster AH, Hepler PK. Actomyosin promotes cell plate alignment and late lateral expansion in Tradescantia stamen hair cells. Planta. 2002;214:683–693. doi: 10.1007/s004250100672. [DOI] [PubMed] [Google Scholar]
- 48.Staiger CJ. Signaling to the actin cytoskeleton in plants. Ann Rev Plant Physiol Plant Mol Biol. 2000;51:257–288. doi: 10.1146/annurev.arplant.51.1.257. [DOI] [PubMed] [Google Scholar]
- 49.Kovar DR, Drøbak BK, Staiger CJ. Maize profilin isoforms are functionally distinct. Plant Cell. 2000;12:583–598. doi: 10.1105/tpc.12.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snowman BN, Kovar DR, Shevchenko G, Franklin-Tong VE, Staiger CJ. Signal-mediated depolymerization of actin in pollen during the self-incompatibility response. Plant Cell. 2002;14:2613–2626. doi: 10.1105/tpc.002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sinclair W, Oliver I, Maher P, Trewavas A. The role of calmodulin in the gravitropic response of Arabidopsis thaliana agr-3 mutant. Planta. 1996;199:343–351. doi: 10.1007/BF00195725. [DOI] [PubMed] [Google Scholar]
- 52.Chandra S, Chabot J, Morrison G, Leopold A. Localization of Ca2+ in amyloplasts of root cap cells using ion microscopy. Science. 1982;216:1221–1223. doi: 10.1126/science.216.4551.1221. [DOI] [PubMed] [Google Scholar]
- 53.Scott AC, Allen NS. Changes in cytosolic pH within Arabidopsis root columella cells play a key role in the early signaling pathway for root gravitropism. Plant Physiol. 1999;121:1291–1298. doi: 10.1104/pp.121.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fasano JM, Swanson SJ, Blancaflor EB, Dowd PE, Kao TH, Gilroy S. Changes in root cap pH are required for the gravity response of the Arabidopsis root. Plant Cell. 2001;13:907–921. doi: 10.1105/tpc.13.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boonsirichai K, Sedbrook JC, Chen R, Gilroy S, Masson PH. Altered Response to Gravity is a peripheral membrane protein that modulates gravity-induced cytoplasmic alkalinization and lateral auxin transport in plant statocytes. Plant Cell. 2003;15:2612–2625. doi: 10.1105/tpc.015560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gungabissoon RA, Jiang CJ, Drøbak BK, MacIver SK, Hussey PJ. Interaction of maize actin-depolymerising factor with actin and phosphoinositides and its inhibition of plant phospholipase C. Plant J. 1998;16:689–696. [Google Scholar]
- 57.Kuznetsov OA, Hasenstein KH. Magnetophoretic induction of root curvature. Planta. 1996;198:87–94. doi: 10.1007/BF00197590. [DOI] [PubMed] [Google Scholar]
- 58.Kuznetsov OA, Hasenstein KH. Magnetophoretic induction of curvature in coleoptiles and hypocotyls. J Exp Bot. 1997;48:1951–1957. doi: 10.1093/jexbot/48.316.1951. [DOI] [PubMed] [Google Scholar]
- 59.Uchida A, Yamamoto KT. Effects of mechanical vibration on seed germination of Arabidopsis thaliana (L.) Heynh. Plant Cell Physiol. 2002;43:647–651. doi: 10.1093/pcp/pcf079. [DOI] [PubMed] [Google Scholar]
- 60.Volkmann D, Tewinkel M. Gravisensitivity of cress roots: Investigations of threshold values under specific conditions of sensor physiology in microgravity. Plant Cell Environm. 1996;19:1195–1202. doi: 10.1111/j.1365-3040.1996.tb00435.x. [DOI] [PubMed] [Google Scholar]
- 61.Volkmann D, Baluska F. The actin cytoskeleton in plants: From transport networks to signaling networks. Microsc Res Tech. 1999;47:135–154. doi: 10.1002/(SICI)1097-0029(19991015)47:2<135::AID-JEMT6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 62.Carlier MF. Control of actin dynamics. Curr Opin Cell Biol. 1998;10:45–51. doi: 10.1016/s0955-0674(98)80085-9. [DOI] [PubMed] [Google Scholar]
- 63.Blancaflor EB, Fasano JM, Gilroy S. Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol. 1998;115:213–222. doi: 10.1104/pp.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Forgacs G. On the possible role of cytoskeletal filamentous networks in intracellular signaling: An approach based on perlocation. J Cell Sci. 1995;108:2131–2143. doi: 10.1242/jcs.108.6.2131. [DOI] [PubMed] [Google Scholar]
- 65.Ingber D. How cells (might) sense microgravity. FASEB J. 1999;13:S13–S15. doi: 10.1096/fasebj.13.9001.s3. [DOI] [PubMed] [Google Scholar]
- 66.Janmey PA. The cytoskeleton and cell signalling: Component localization and mechanical coupling. Physiol Rev. 1998;78:763–781. doi: 10.1152/physrev.1998.78.3.763. [DOI] [PubMed] [Google Scholar]
- 67.Fitzelle KJ, Kiss JZ. Restoration of gravitropic sensitivity in starch-deficient mutants of Arabidopsis by hypergravity. J Exp Bot. 2001;52:265–275. [PubMed] [Google Scholar]
- 68.Poff KL, Martin HV. Site of graviperception in roots: A reexamination. Physiol Plant. 1989;76:451–455. doi: 10.1111/j.1399-3054.1989.tb06218.x. [DOI] [PubMed] [Google Scholar]
- 69.Ishikawa H, Evans M. Gravity-induced changes in intracellular potentials in elongating cortical cells of mung bean roots. Plant Cell Physiol. 1990;31:457–462. [PubMed] [Google Scholar]
- 70.Wolverton C, Mullen JL, Ishikawa H, Evans ML. Root gravitropism in response to a signal originating outside of the cap. Planta. 2002;215:153–157. doi: 10.1007/s00425-001-0726-9. [DOI] [PubMed] [Google Scholar]
- 71.Wolverton C, Ishikawa H, Evans ML. The kinetics of root gravitropism: Dual motors and sensors. J Plant Growth Regul. 2002;21:102–112. doi: 10.1007/s003440010053. [DOI] [PubMed] [Google Scholar]
- 72.Chen R, Guan C, Boonsirichai K, Masson PH. Complex physiological and molecular processes underlying root gravitropism. Plant Mol Biol. 2002;49:305–317. [PubMed] [Google Scholar]
- 73.Perrin RM, Young LS, Murthy UMN, Harrison BR, Wang Y, Will JL, Masson PH. Gravity signal transduction in primary roots. Ann Bot. 2005;96:737–743. doi: 10.1093/aob/mci227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caspar T, Pickard BG. Gravitropism in a starchless mutant of Arabidopsis. Planta. 1989;177:185–197. [PubMed] [Google Scholar]
- 75.Kiss JZ, Hertel R, Sack FD. Amyloplasts are necessary for full gravitropic sensitivity in roots of Arabidopsis thaliana. Planta. 1989;177:198–206. [PubMed] [Google Scholar]
- 76.Kiss JZ, Wright JB, Caspar T. Gravitropism in roots of intermediate-starch mutants of Arabidopsis. Physiol Plant. 1996;97:237–244. doi: 10.1034/j.1399-3054.1996.970205.x. [DOI] [PubMed] [Google Scholar]
- 77.Friml J. Auxin transport — shaping the plant. Curr Opin Pant Biol. 2003;6:7–12. doi: 10.1016/s1369526602000031. [DOI] [PubMed] [Google Scholar]
- 78.Butler JH, Hu S, Brady SR, Dixon MW, Muday GK. In vitro and in vivo evidence for actin association of the naphthylphthalamic acid-binding protein from zucchini hypocotyls. Plant J. 1998;13:291–301. doi: 10.1046/j.1365-313x.1998.00017.x. [DOI] [PubMed] [Google Scholar]
- 79.Godbole R, Michalke W, Nick P, Hertel R. Cytoskeletal drugs and gravity-induced lateral auxin transport in rice coleoptiles. Plant Biol. 2000;2:176–181. [Google Scholar]
- 80.Sun H, Basu S, Brady SR, Luciano RL, Muday GK. Interactions between auxin transport and the actin cytoskeleton in developmental polarity of Fucus distichus embryos in response to light and gravity. Plant Physiol. 2004;135:266–278. doi: 10.1104/pp.103.034900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Geldner N, Friml J, Stierhof YD, Jurgens G, Palme K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature. 2001;413:425–428. doi: 10.1038/35096571. [DOI] [PubMed] [Google Scholar]
- 82.Samaj J, Chaffey N, Tirlapur U, Jasik J, Hlavacka A, et al. Actin and myosin VIII in plant cell-cell channels. In: Baluska F, Volkmann D, Barlow PW, editors. Cell-Cell Channels. Landes Bioscience; 2006. [Google Scholar]
- 83.Baluska F, Hauskrecht M, Barlow PW, Sievers A. Gravitropism of the primary root of maize: A complex pattern of differential cellular growth in the cortex independent of the microtubular cytoskeleton. Planta. 1996;197:310–318. doi: 10.1007/BF00206258. [DOI] [PubMed] [Google Scholar]
- 84.Swarup R, Kramer EM, Perry P, et al. Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nat Cell Biol. 2005;7:1057–1065. doi: 10.1038/ncb1316. [DOI] [PubMed] [Google Scholar]
- 85.Baluska F, Samaj J, Menzel D. Polar transport of auxin: Carrier-mediated flux across the plasma membrane or neurotransmitter-like secretion? Trends Cell Biol. 2003;13:282–285. doi: 10.1016/s0962-8924(03)00084-9. [DOI] [PubMed] [Google Scholar]
- 86.Baluska F, Volkmann D, Menzel D. Plant synapses: Actin-based adhesion domains for cell-to-cell communication. Trends Plant Sci. 2005;10:106–111. doi: 10.1016/j.tplants.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 87.Baluska F, Hlavacka A. Plant formins come of age: Something special about cross-walls. New Phytol. 2005;168:499–503. doi: 10.1111/j.1469-8137.2005.01595.x. [DOI] [PubMed] [Google Scholar]
- 88.Epel BL, Warmbrodt RP, Bandurski RS. Studies on the longitudinal and lateral transport of IAA in the shoots of etiolated corn seedlings. J Plant Physiol. 1992;140:310–318. doi: 10.1016/s0176-1617(11)81084-9. [DOI] [PubMed] [Google Scholar]
- 89.Didry D, Carlier MF, Pantaloni D. Synergy between actin depolymerizing factor/cofilin and profilin in increasing actin filament turnover. J Biol Chem. 1998;273:25602–25611. doi: 10.1074/jbc.273.40.25602. [DOI] [PubMed] [Google Scholar]
- 90.Hao S, August A. Actin depolymerization transduces the strength of B-cell receptor stimulation. Mol Biol Cell. 2005;16:2275–2284. doi: 10.1091/mbc.E04-10-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]