Abstract
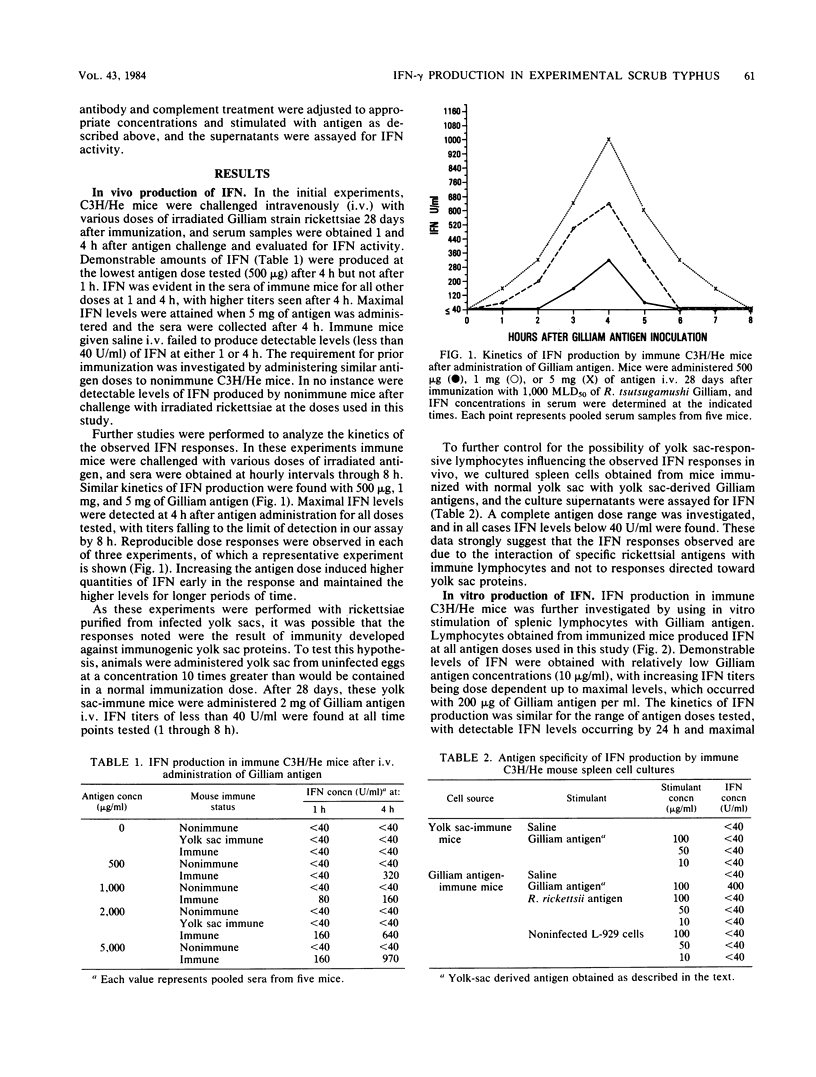
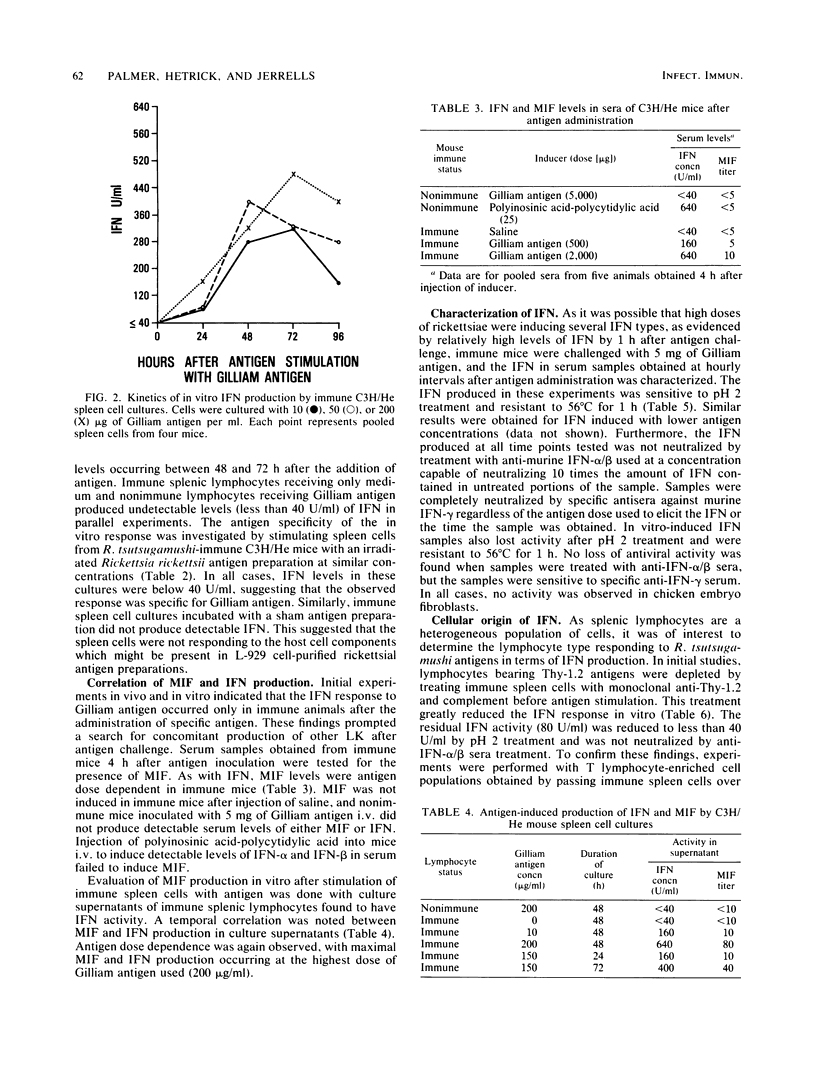
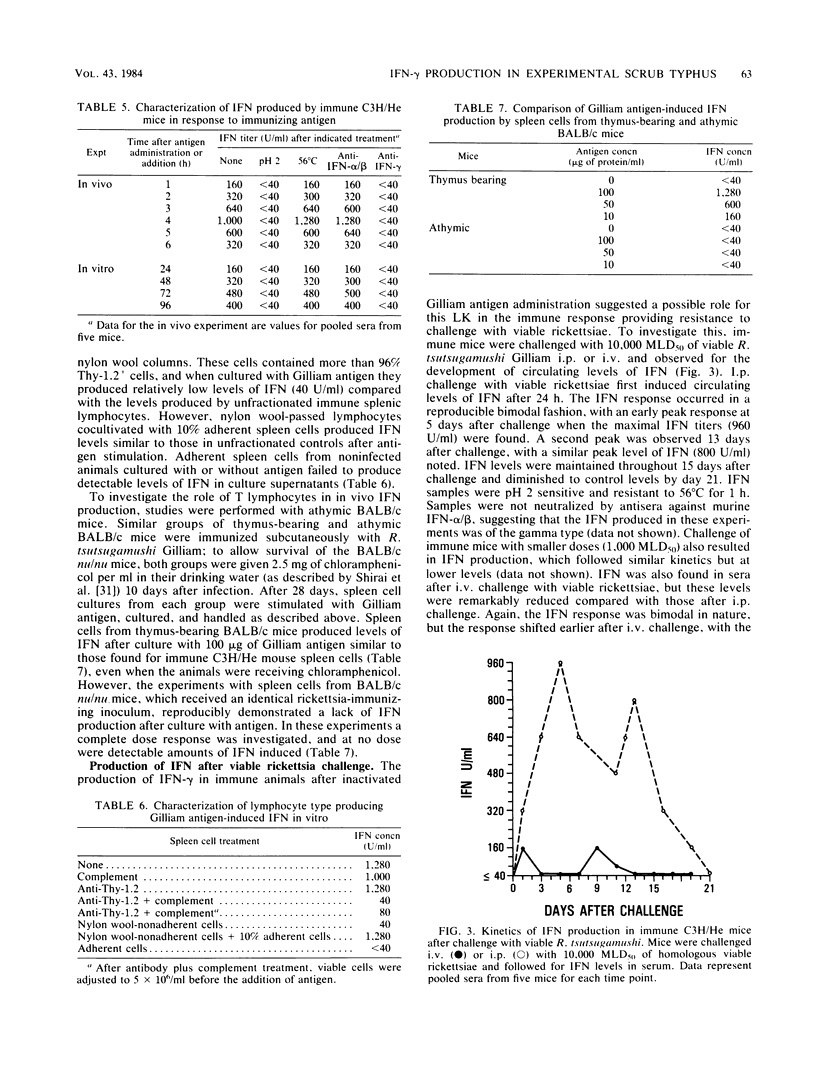
C3H/He mice immunized by subcutaneous infection with Rickettsia tsutsugamushi Gilliam were examined for the production of immune interferon after intravenous administration of irradiated strain Gilliam antigen, in supernatants of immune lymphocytes stimulated with specific antigen, and after a secondary challenge with viable rickettsiae. Mice administered various doses of irradiated whole-organism antigen 28 days after immunization showed circulating levels of interferon which peaked 4 h after inoculation and were antigen dose dependent. The interferon produced was pH 2 sensitive and stable at 56 degrees C for 1 h and was neutralized by antiserum directed against immune, but not against alpha/beta, interferon. The production of another lymphokine, macrophage migration inhibition factor, paralleled that of interferon. The interferon produced by cultures of spleen cells obtained from immune animals was antigen specific and dose dependent. Peak levels were obtained 48 to 72 h after the addition of antigen. The interferon produced by spleen cell cultures after stimulation with Gilliam antigen was characterized as immune interferon by the same physical and antigenic criteria used for serum interferon. Interferon was produced in vitro by the Thy-1.2+ lymphocyte and required the presence of a spleen-adherent cell population. Immune mice produced high circulating levels of immune interferon after intraperitoneal challenge with viable rickettsiae, which suggested a possible role for interferon in the resistance of immune mice to rechallenge with R. tsutsugamushi.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blalock J. E., Georgiades J. A., Langford M. P., Johnson H. M. Purified human immune interferon has more potent anticellular activity than fibroblast or leukocyte interferon. Cell Immunol. 1980 Feb;49(2):390–394. doi: 10.1016/0008-8749(80)90041-6. [DOI] [PubMed] [Google Scholar]
- Blalock J. E., Georgiades J., Johnson H. M. Immune-type interferon-induced transfer of viral resistance. J Immunol. 1979 Mar;122(3):1018–1021. [PubMed] [Google Scholar]
- Eisemann C. S., Osterman J. V. Proteins of typhus and spotted fever group rickettsiae. Infect Immun. 1976 Jul;14(1):155–162. doi: 10.1128/iai.14.1.155-162.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L. B., Kreth H. W., Herzenberg L. A. Fluorescence-activated cell sorting of human T and B lymphocytes. II. Identification of the cell type responsible for interferon production and cell proliferation in response to mitogens. Cell Immunol. 1974 Jun;12(3):407–421. doi: 10.1016/0008-8749(74)90097-5. [DOI] [PubMed] [Google Scholar]
- Evans S. R., Johnson H. M. The induction of at least two distinct types of interferon in mouse spleen cell cultures by Corynebacterium parvum. Cell Immunol. 1981 Oct;64(1):64–72. doi: 10.1016/0008-8749(81)90458-5. [DOI] [PubMed] [Google Scholar]
- Ewing E. P., Jr, Takeuchi A., Shirai A., Osterman J. V. Experimental infection of mouse peritoneal mesothelium with scrub typhus rickettsiae: an ultrastructural study. Infect Immun. 1978 Mar;19(3):1068–1075. doi: 10.1128/iai.19.3.1068-1075.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann W. R., Jr Potentiation of the direct anticellular activity of mouse interferons: mutual synergism and interferon concentration dependence. Cancer Res. 1982 Mar;42(3):869–875. [PubMed] [Google Scholar]
- Interferon nomenclature. J Immunol. 1980 Nov;125(5):2353–2353. [PubMed] [Google Scholar]
- Jerrells T. R., Mallavia L. P., Hinrichs D. J. Detection of long-term cellular immunity to Coxiella burneti as assayed by lymphocyte transformation. Infect Immun. 1975 Feb;11(2):280–286. doi: 10.1128/iai.11.2.280-286.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Osterman J. V. Development of specific and cross-reactive lymphocyte proliferative responses during chronic immunizing infections with Rickettsia tsutsugamushi. Infect Immun. 1983 Apr;40(1):147–156. doi: 10.1128/iai.40.1.147-156.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Osterman J. V. Host defenses in experimental scrub typhus: delayed-type hypersensitivity responses of inbred mice. Infect Immun. 1982 Jan;35(1):117–123. doi: 10.1128/iai.35.1.117-123.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Osterman J. V. Host defenses in experimental scrub typhus: inflammatory response of congenic C3H mice differing at the Ric gene. Infect Immun. 1981 Mar;31(3):1014–1022. doi: 10.1128/iai.31.3.1014-1022.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Osterman J. V. Role of macrophages in innate and acquired host resistance to experimental scrub typhus infection of inbred mice. Infect Immun. 1982 Sep;37(3):1066–1073. doi: 10.1128/iai.37.3.1066-1073.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Palmer B. A., Osterman J. V. Gamma-irradiated scrub typhus immunogens: development of cell-mediated immunity after vaccination of inbred mice. Infect Immun. 1983 Jan;39(1):262–269. doi: 10.1128/iai.39.1.262-269.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H. M., Baron S. The nature of the suppressive effect of interferon and interferon inducers on the in vitro immune response. Cell Immunol. 1976 Jul;25(1):106–115. doi: 10.1016/0008-8749(76)90100-3. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Stanton G. J., Baron S. Relative ability of mitogens to stimulate production of interferon by lymphoid cells and to induce suppression of the in vitro immune response. Proc Soc Exp Biol Med. 1977 Jan;154(1):138–141. [PubMed] [Google Scholar]
- Juhlin L., Cantell K. Interferon response of lymphocytes and resistance to infections. Lancet. 1978 Mar 25;1(8065):667–668. doi: 10.1016/s0140-6736(78)91179-0. [DOI] [PubMed] [Google Scholar]
- Kazár J. Interferon-like inhibitor in mouse sera induced by rickettsiae. Acta Virol. 1966 May;10(3):277–277. [PubMed] [Google Scholar]
- Kono S., Kohase M., Sakata H., Shimizu Y., Hikita M. Production of interferon in primary chick embryonic cells infected with Rickettsia mooseri. J Immunol. 1970 Dec;105(6):1553–1558. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nacy C. A., Meltzer M. S. Macrophages in resistance to rickettsial infection: macrophage activation in vitro for killing of Rickettsia tsutsugamushi. J Immunol. 1979 Dec;123(6):2544–2549. [PubMed] [Google Scholar]
- Nacy C. A., Osterman J. V. Host defenses in experimental scrub typhus: role of normal and activated macrophages. Infect Immun. 1979 Nov;26(2):744–750. doi: 10.1128/iai.26.2.744-750.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta R., Salvin S. B. In vivo release of lymphokines in different strains of mice. Cell Immunol. 1980 Apr;51(1):173–178. doi: 10.1016/0008-8749(80)90247-6. [DOI] [PubMed] [Google Scholar]
- Oaks S. C., Jr, Osterman J. V., Hetrick F. M. Plaque assay and cloning of scrub typhus rickettsiae in irradiated L-929 cells. J Clin Microbiol. 1977 Jul;6(1):76–80. doi: 10.1128/jcm.6.1.76-80.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne L. C., Georgiades J. A., Johnson H. M. Classification of interferons with antibody to immune interferon. Cell Immunol. 1980 Jul 15;53(1):65–70. doi: 10.1016/0008-8749(80)90426-8. [DOI] [PubMed] [Google Scholar]
- Padarathsingh M. L., Dean J. H., McCoy J. L., Lewis D. D., Northing J. W., Natori T., Law L. W. Cell-mediated immunity against particulate and solubilized tumor-associated antigens of murine plasmacytomas detected by macrophage migration inhibition assays. Int J Cancer. 1977 Oct 15;20(4):624–631. doi: 10.1002/ijc.2910200421. [DOI] [PubMed] [Google Scholar]
- Salvin S. B., Youngner J. S., Lederer W. H. Migration inhibitory factor and interferon in the circulation of mice with delayed hypersensitivity. Infect Immun. 1973 Jan;7(1):68–75. doi: 10.1128/iai.7.1.68-75.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai A., Catanzaro P. J., Eisenberg G. H., Jr, Osterman J. V. Host defenses in experimental scrub typhus: effect of chloramphenicol. Infect Immun. 1977 Nov;18(2):324–329. doi: 10.1128/iai.18.2.324-329.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai A., Catanzaro P. J., Phillips S. M., Osterman J. V. Host defenses in experimental scrub typhus: role of cellular immunity in heterologous protection. Infect Immun. 1976 Jul;14(1):39–46. doi: 10.1128/iai.14.1.39-46.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenfeld G., Mandel A. D., Merigan T. C. The immunosuppressive effect of type II mouse interferon preparations on antibody production. Cell Immunol. 1977 Dec;34(2):193–206. doi: 10.1016/0008-8749(77)90243-x. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld G., Mandel A. D., Merigan T. C. Time and dosage dependence of immunoenhancement by murine type II interferon preparations. Cell Immunol. 1978 Oct;40(2):285–293. doi: 10.1016/0008-8749(78)90336-2. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld G., Merigan T. C. A regulatory role for interferon in immunity. Ann N Y Acad Sci. 1979;332:345–355. doi: 10.1111/j.1749-6632.1979.tb47128.x. [DOI] [PubMed] [Google Scholar]
- Steeg P. S., Moore R. N., Johnson H. M., Oppenheim J. J. Regulation of murine macrophage Ia antigen expression by a lymphokine with immune interferon activity. J Exp Med. 1982 Dec 1;156(6):1780–1793. doi: 10.1084/jem.156.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. N., Weedon L. L., Moore R. N., Rosenstreich D. L. Correction of defective macrophage differentiation in C3H/HeJ mice by an interferon-like molecule. J Immunol. 1982 Jan;128(1):380–387. [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock E. F. Interferon-like virus-inhibitor induced in human leukocytes by phytohemagglutinin. Science. 1965 Jul 16;149(3681):310–311. [PubMed] [Google Scholar]


