Abstract
The physiological impact of nonionizing radiation has long been considered negligible. However, here we use a carefully calibrated stimulation system that mimics the characteristics (isotropy and homogeneity) of electromagnetic fields present in the environment to measure changes in a molecular marker (mRNA encoding the stress-related bZIP transcription factor), and show that low amplitude, short duration, 900 MHz EMF evokes the accumulation of this mRNA. Accumulation is rapid (peaking 5–15 min after stimulation) and strong (3.5-fold), and is similar to that evoked by mechanical stimulations.
Key Words: tomato, microwave, non-ionizing radiation, bZIP, MSRC, EMF
Introduction
High frequency nonionizing radiation is becoming increasingly common in the environment because of the exponential use of mobile phone technology and wireless communication devices. While many reports point out its lack of effects on living organisms,1,2 it can be argued that most studies have used unsuitable stimulation devices, primarily TEM-cells,3 custom-made tools4,5 or even commercial cell phones,6 and address the problem at a very general level with little concern for underlying molecular-level events.7 Most stimulation devices are inadequate since they emit a signal as a plane wave with a fixed polarization and incidence. Studies have therefore been undertaken to develop appropriate technology for EMF studies on living systems by adapting reverberating chambers which are widely used in acoustics. This has led to the Mode Stirring Reverberation Chamber (MSRC), a facility specially designed to create isotropic and homogeneous EMF that irradiates the subject from all directions. The reflections of the original signal on the metallic walls of the chamber (that isolates the subject from external radiation) randomize the polarization of the EMF without influencing its amplitude (homogeneity). This mimics Nature, where there are multiple reflections and diffractions of EMF from buildings, mountains and trees. Thus, this equipment is extremely well suited to study the effect of EMF on life.
Two aspects of these biological experiments need special attention: the organism itself and the parameter, preferably a molecular marker,8 to be studied. Plants may be appropriate experimental subjects, in the sense that they are highly sensitive to environmental signals9–11 and plant studies raise less emotional concern than studies on animals or humans. Moreover, radiation from a GSM telephone or from a 105 GHz Gunn oscillator has been shown to have an effect comparable to that of a variety of environmental stimuli (such as manipulation stress, drought, wind and cold shock) in inducing physiological modifications (production of numerous epidermal meristems in the hypocotyls) or modifications of the proteome (pI shift, appearance or disappearance of a spot in 2D electrophoresis) in flax seedlings;12–15 modifications of the proteome have also been observed in Arabidopsis seedlings subjected to stimuli such as cold shock or radiation from a GSM telephone.15 Here, we have studied the effect of mobile-phone intensity microwave radiation on another type of plant (the tomato), taking a particular care to the conditions of irradiation and studying the accumulation of a stress-related transcript (mRNA) that responds very rapidly to even small environmental stimulations.16 We are going to show that such microwave radiation enhances accumulation of the transcript encoding a specific, wound-related transcription factor, LebZIP1.17
Material and Methods
Plant culture and treatment.
Tomato plants (Lycopersicon esculentum cv VFN-8) were germinated in the greenhouse and transferred to an EMF-permeable culture chamber and grown under controlled conditions (Light/Dark 16 h/8 h 26°C/21°C, light intensity of 175 µmols−1m−2 at plant level) for three weeks, until the 4th terminal leaf had formed. The culture chamber containing the plants was transferred to the stimulation chamber at least 18h before treatment. Stimulation was made using the MSRC (Fig. 1A). This facility is a large room (8.4 × 6.7 × 3.5 m, about 195 m3) enclosed in double-layered metal walls which act as a Faraday cage to protect the experiment from external (environmental) EMF background. For plant stimulation, a single frequency (900 MHz) was produced by a signal synthesizer (Anritsu model 68147C), verified with a signal analyser (Anritsu model MS2665C) and emitted into the chamber with a log-periodic emission antenna. A rotary stirrer was used to create different patterns of multiple reflections on the chamber walls, thus randomizing the polarization of the electromagnetic waves. The resulting electromagnetic field is statistically isotropic and homogeneous within a defined “working” volume. The stimulation (5 V/m, 10 min) was given to the plant in the middle of the light period. The 4th terminal leaf was collected at various times after the end of the stimulation and immediately frozen in liquid nitrogen. Control plants were collected before stimulation. Because of the limited size of the culture chamber, only one plant could be used for each time point. For some experiments, the culture chamber was shielded in a polymer mesh covered with an aluminium layer that causes a 45 dB signal attenuation at 900 MHz (more than 87%).
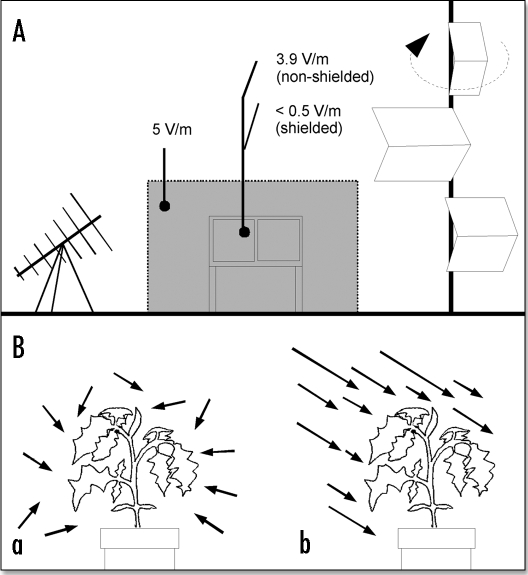
Figure 1.
(A) The mode stirring reverberation chamber. This is a large room with metal walls (dark lines) to exclude external EMF, an antenna (lower left) to emit tunable EMF, a rotary stirrer to make the EMF homogeneous (right side) and a plant culture chamber placed within the working volume (gray area). (B) Schematic representation of EMF types. a, non-polarized (isotropic) and homogeneous field, where the field components align in all possible directions and the field has the same amplitude at all points. b, polarized, nonhomogeneous field, where the field components align in a single direction while the amplitude varies (heterogeneity).
RNA isolation and quantitative PCR.
RNA was isolated from frozen tissue using Tri-Reagent (Sigma) and total RNA (1 µg) was used to drive cDNA synthesis (Advantage RT for PCR, BD Bioscience) for 1 h at 42°C both according to the manufacturers' instructions. The cDNA was diluted 5-fold and used as DNA template for quantitative PCR analysis (Two step qPCR Mastermix Plus for SYBR-Green, Eurogentech). The chosen primers (sense: 5′-GGGATGGAGAAGTTTGGTGGTGG3′ Anti-sense:5′-CTTCGACCAAGGGATGGTGTAGC-3′) amplify just LebZIP1 cDNA17 (Genbank accession number AF176641). The reactions were performed and analysed using the 2-ΔΔCt method18 with actin as internal control, and values calculated relative to the nonexposed control plants. Actin and LebZIP1 fragments amplify with the same efficiency in our experimental conditions (data not shown).
Results and Discussion
Experiments were conducted inside the MSRC within the working volume (Fig. 1A, grey area) containing the plant culture chamber, where the EMF was statistically isotropic and homogeneous (Fig. 1B, a) in contrast to a polarized, nonhomogeneous EMF (Fig. 1B, b). These characteristics were determined by measuring the values of the standard deviations (σx, σy and σz) of the three spatial components of the electric field in 8 locations within the culture chamber, and the σxyz calculated from these 24 components.
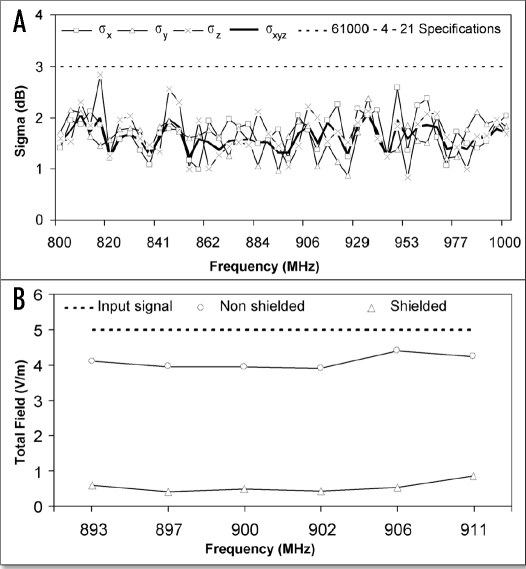
To verify the isotropy and homogeneity of the EMF, we excited the chamber with single frequency signals varying from 800 to 1000 MHz and made 18 measurements of the generated field per stirrer rotation for each tested frequency and the results are shown in Figure 2A. The standard deviation for each of the three spatial components (σx, σy and σz) and of the combined (σxyz) never exceeded the 3 dB limit listed in the 61000-4-21 specification,19 proving that the field quality is not affected by interactions with the culture chamber walls. However, they do cause a signal attenuation of about 20%, since the input amplitude was 5 V/m (Fig. 2B dashed line), whereas the measured amplitude inside the chamber was 3.9 V/m (Fig. 2B, “non-shielded”). Furthermore, the shielding material decreased the field amplitude by 87% inside the chamber 0.5 V/m (45 dB attenuation) at 900 MHz (Fig. 2B, “shielded”). In the unshielded culture chamber, the plants were therefore exposed to an EMF similar to that occurring in the natural environment in terms of amplitude, isotropy and homogeneity, indicating that the MSRC is an outstanding facility to generate such conditions.
Figure 2.
Field characteristics in the plant culture chamber. The tunable antenna was used to generate EMF of 5 Vm−1 from 800–1000 MHz (A), or 893–911 MHz (B). (A) Standard deviation of amplitude for the total field (σxyz, bold line) and for each of the Cartesian axes (σx, σy, σz); dashed line: 3dB limit specified by the IEC 61000-4-21 standard. (B) Effective values measured within the nonshielded (○) and shielded (Δ) culture chamber. Dashed line: 5 V/m input signal.
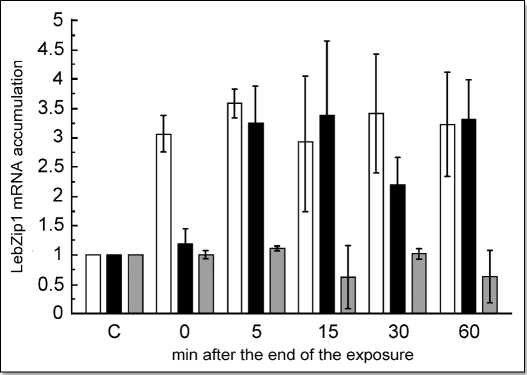
Exposure of the plant to EMF induced a rapid (maximum after 5–15 min) and strong (3.5-fold) accumulation of the stress-related LebZIP1 mRNA in the 4th terminal leaf (Fig. 3). In some experiments, the accumulation began immediately after the end of the stimulation (Fig. 3, white bars), while in others there was a short delay (Fig. 3, black bars). In all cases, the response was maximal at 5–15 min after the end of stimulation, in some cases (black bars) declined somewhat at 30 min, but generally remained at high levels until 60 min. Since, space limitations within the MSRC precluded the use of more than 1 plant (1 leaf) per time point, and since each experiment used a different batch of plants, we are amazed at the relative consistency of the results. When plants were placed in the shielded culture chamber, no significant accumulation of LebZIP1 transcript was seen (Fig. 3, gray). These results indicate that: (1) the cellular responses are directly linked to exposure of plants to the EMF; and (2) that the remaining EMF (0.5 V/m) present in the shielded culture chamber is insufficient to evoke LebZIP1 mRNA accumulation. Although the treated tissue did not display any apparent damage, the rapidity and amplitude of the response are comparable to those observed after strong stimulation such as flaming.17
Figure 3.
Accumulation of LebZIP1 transcript after EMF-stimulation in the nonshielded culture chamber. Plant show either an immediate response (white bars) or a 5 min delayed response (black bars). Plants stimulated in the shielded culture chamber (gray bars). Each value is expressed relative to the nonexposed control (C) and normalized to the actin mRNA and is the average of at least 3 independent repetitions ± the standard error.
These results are quite surprising and strongly question the mechanism of interaction between the plant and the EMF. The energy associated with the EMF radiation is extremely low20 and insufficient to evoke plant defence mechanisms involving the genesis of free radicals or molecule ionization. Variations of cytosolic Ca2+ concentration have been implicated after EMF stimulation13 and might constitute the initial signal that evokes the observed molecular responses. Thermal effects21 are unlikely to arise considering the frequency (900 MHz) and the very low power (0.1 W) dissipated in the large volume of the MSRC. The response is therefore triggered by a mechanism which is unlikely to be based on a simple energy transfer from the wave to the plant. The amplitude (3.9 Vm−1) of the signal that evoked this rapid stress-related response is within the range used for mobile phone communication or to the EMF background present in an urban environment.
The major points arising from this study are that high frequency low amplitude EMF cause enhanced expression of at least one plant-wound gene. This response is reproducible and exceedingly rapid, in all instances peaking within 15 min following the end of exposure. This rapid response and its suppression by an EMF-proof shield allows us to make a formal link between the EMF stimulation and the accumulation of the bZIP mRNA.
Acknowledgements
The authors wish to thank the French Ministry of Education and Research for the grant RTM 0005 “effets biologiques de la radiotéléphonie mobile” awarded to G.L.
Abbreviations
- LebZIP1
Lycopersicon esculentum basic leucine zipper
- MSRC
Mode Stirring Reverberation Chamber
- TEM cell
transverse electromagnetic cell
- EMF
electromagnetic field
Footnotes
Previously published onlinse as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=2434
References
- 1.De Seze R, Fabro-Peray P, Miro L. GSM radiocellular telephones do not disturb the secretion of antepituitary hormones in humans. Bioelectromagnetics. 1998;19:271–278. doi: 10.1002/(sici)1521-186x(1998)19:5<271::aid-bem1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Hietanen M, Hämäläinen AM, Husman T. Hypersensitivity symptoms associated with exposure to cellular telephones: No causal link. Bioelectromagnetics. 2002;23:264–270. doi: 10.1002/bem.10016. [DOI] [PubMed] [Google Scholar]
- 3.Nikoloski N, Fröhlich J, Samaras T, Schuderer J, Kuster N. Reevaluation and improved design of the TEM cell in vitro exposure unit for replication studies. Bioelectromagnetics. 2005;26:215–224. doi: 10.1002/bem.20067. [DOI] [PubMed] [Google Scholar]
- 4.Laval L, Leveque P, Jecko B. A new in vitro exposure device for the mobile frequency of 900 MHz. Bioelectromagnetics. 2000;21:255–263. doi: 10.1002/(sici)1521-186x(200005)21:4<255::aid-bem2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 5.Rao RR, Kisaalita WS. A single magnetic field exposure system for sequential investigation of real time and downstream cellular responses. Bioelectromagnetics. 2004;25:27–32. doi: 10.1002/bem.10151. [DOI] [PubMed] [Google Scholar]
- 6.Besset A, Espa F, Dauvilliers Y, Billard M, De Seze R. No effect on cognitive function from daily mobile phone use. Bioelectromagnetics. 2005;26:102–108. doi: 10.1002/bem.20053. [DOI] [PubMed] [Google Scholar]
- 7.Sienkiewicz ZJ, Blackwell RP, Haylock RGE, Saunders RD, Cobb BL. Low-level exposure to pulsed 900 Mhz microwave radiation does not cause deficits in the performances of a spacial learning task in mice. Bioelectromagnetics. 2000;21:151–158. doi: 10.1002/(sici)1521-186x(200004)21:3<151::aid-bem1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 8.De Mattei M, Gagliano N, Moscheni C, Dellavia C, Calastrini C, Pellati A, Gioia M, Caruso A, Stabellini G. Changes in polyamines, v-myc and c-fos gene expression in osteoblast-like cells exposed to pulsed electromagnetic fields. Bioelectromagnetics. 2005;26:207–214. doi: 10.1002/bem.20068. [DOI] [PubMed] [Google Scholar]
- 9.Depège N, Thonat C, Coutand C, Julien JL, Boyer N. Morphological responses and molecular modifications in tomato plants after mechanical stimulation. Plant Cell Physiol. 1997;38:1127–1134. doi: 10.1093/oxfordjournals.pcp.a029097. [DOI] [PubMed] [Google Scholar]
- 10.Verdus MC, Thellier M, Ripoll C. Storage of environmental signals in flax: Their morphogenetic effect as enabled by a transient depletion of calcium. Plant J. 1997;12:1399–1410. [Google Scholar]
- 11.Vian A, Henry-Vian C, Davies E. Rapid and systemic accumulation of chloroplast mRNA binding protein transcripts after flame stimulus in tomato. Plant Physiol. 1999;121:517–524. doi: 10.1104/pp.121.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tafforeau M, Verdus MC, Charlionet R, Cabin-Flaman A, Ripoll C. Two-dimensional electrophoresis investigation of short term response of flax seedlings to cold shock. Electrophoresis. 2002;23:2534–2540. doi: 10.1002/1522-2683(200208)23:15<2534::AID-ELPS2534>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 13.Tafforeau M, Verdus MC, Norris V, White G, Demarty M, Thellier M, Ripoll C. SIMS study of the calcium-deprivation step related to epidermal meristem production induced in flax by cold shock or radiation from a GSM telephone. J Trace Microprobe Techn. 2002b;20:611–623. [Google Scholar]
- 14.Tafforeau M, Verdus MC, Norris V, White GJ, Cole M, Demarty M, Thellier M, Ripoll C. Plant sensitivity to low intensity 105 GHz electromagnetic radiation. Bioelectromagnetics. 2004;25:403–407. doi: 10.1002/bem.10205. [DOI] [PubMed] [Google Scholar]
- 15.Tafforeau M, Verdus MC, Norris V, Ripoll C, Thellier M. Memory processes in the response of plants to environmental signals. Plant Sign Behav. 2006;1:9–14. doi: 10.4161/psb.1.1.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vian A, Henry-Vian C, Schantz R, Ledoigt G, Frachisse JM, Desbiez MO, Julien JL. Is membrane potential involved in calmodulin gene expression after external stimulation in plants? FEBS Lett. 1996;380:93–96. doi: 10.1016/0014-5793(96)00015-4. [DOI] [PubMed] [Google Scholar]
- 17.Stankovic B, Vian A, Henry-Vian C, Davies E. Molecular cloning and characterization of a tomato cDNA encoding a systematically wound-inducible bZIP DNA-Binding protein. Planta. 2000;212:60–66. doi: 10.1007/s004250000362. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.IEC: International Electrotechnical Commission, author. Electromagnetic compatibility. 2002. (Part 4 Section 21, Reverberation Chamber Test Methods, Standard IEC 61000-4-21, Draft CD2) [Google Scholar]
- 20.Challis LJ. Mechanisms for interaction between RF fields and biological tissues. Bioelectromagnetics supp. 2005;26:S98–S106. doi: 10.1002/bem.20119. [DOI] [PubMed] [Google Scholar]
- 21.Blank M, Goodman R. A biological guide for electromagnetic safety: The stress response. Bioelectromagnetics. 2004;25:642–646. doi: 10.1002/bem.20061. [DOI] [PubMed] [Google Scholar]